Abstract
OBJECTIVE
To test the graft-promoting effects of mesenchymal stem cells (MSCs) in a cynomolgus monkey model of islet/bone marrow transplantation.
RESEARCH DESIGN AND METHODS
Cynomolgus MSCs were obtained from iliac crest aspirate and characterized through passage 11 for phenotype, gene expression, differentiation potential, and karyotype. Allogeneic donor MSCs were cotransplanted intraportally with islets on postoperative day (POD) 0 and intravenously with donor marrow on PODs 5 and 11. Recipients were followed for stabilization of blood glucose levels, reduction of exogenous insulin requirement (EIR), C-peptide levels, changes in peripheral blood T regulatory cells, and chimerism. Destabilization of glycemia and increases in EIR were used as signs of rejection; additional intravenous MSCs were administered to test the effect on reversal of rejection.
RESULTS
MSC phenotype and a normal karyotype were observed through passage 11. IL-6, IL-10, vascular endothelial growth factor, TGF-β, hepatocyte growth factor, and galectin-1 gene expression levels varied among donors. MSC treatment significantly enhanced islet engraftment and function at 1 month posttransplant (n = 8), as compared with animals that received islets without MSCs (n = 3). Additional infusions of donor or third-party MSCs resulted in reversal of rejection episodes and prolongation of islet function in two animals. Stable islet allograft function was associated with increased numbers of regulatory T-cells in peripheral blood.
CONCLUSIONS
MSCs may provide an important approach for enhancement of islet engraftment, thereby decreasing the numbers of islets needed to achieve insulin independence. Furthermore, MSCs may serve as a new, safe, and effective antirejection therapy.
Multipotent mesenchymal stem cells (MSCs) (1,2) can deliver immunomodulatory signals (3–7) that inhibit allogeneic T-cell responses through downregulation of the proinflammatory cytokines TNF-α and IFN-γ and production of the regulatory cytokines/molecules IL-10, hepatocyte growth factor (HGF), TGF-β, vascular endothelial growth factor (VEGF), indoleamine 2,3-dioxygenase, galectin-1, prostaglandin E2, nitric oxide, and matrix metalloproteinase-2 and -9 (3,8–12). Inflammatory signals, such as IFN-γ, can activate and upregulate MSC suppressive activities (9,13). These cells are able to migrate to sites of injury after intravenous injection (14,15). Their use in clinical trials and experimental models is based on their immunomodulatory and regenerative properties (1,7,16). Clinically, MSCs have been observed to enhance donor bone marrow cell (DBMC) engraftment and chimerism (17,18). Therefore, cotransplantation of MSCs that secrete immunomodulatory cytokines and growth factors might enhance islet survival and function. In experimental mouse models, intravenously infused MSCs are capable of migrating to pancreatic islets (19,20). Systemic infusion of MSCs in murine models of diabetes was accompanied by delayed onset of diabetes, improved glycemic levels, reduced pancreatic insulitis, and pancreatic tissue regeneration (19,21–25), as well as prevention of autoimmune destruction of β-cells via induction of regulatory T-cells (Tregs) (26). Cotransplantation of syngeneic MSCs with a marginal mass of allogeneic islets under the kidney capsule of streptozotocin (STZ)-induced diabetic mice resulted in prolonged normoglycemia (11). Cotransplantation of syngeneic MSC with a marginal mass of allogeneic islets has been performed in the omentum (27) and kidney capsule (28) of STZ-induced diabetic rats, with enhanced islet graft survival as compared with animals receiving islets alone. In this study, cynomolgus monkey MSCs were characterized and donor MSCs were examined for the ability to promote intraportal islet engraftment as well as chimerism in recipients of islet/DBMC transplants. In addition, we tested the use of donor or third-party MSCs to reverse episodes of islet allograft rejection.
RESEARCH DESIGN AND METHODS
Donor and recipient cynomolgus monkeys (>4 and >2 years of age, respectively) were obtained from Charles River BRF, Inc. (Houston, TX) or Alpha Genesis, Inc. (Yemassee, SC) and were negative for TB, Herpes B, SRV, SIV, and STLV-1. Each donor–recipient pair was tissue typed retrospectively and demonstrated to be fully or partially mismatched for major histocompatibility complex (MHC) class II alleles identified using microsatellite analysis as previously described (29–31). All study transplant protocols were approved by The Institutional Animal Care and Use Committee of The University of Miami.
Diabetes induction and management, islet preparation, and transplantation.
Diabetes was induced with STZ (1,250 mg/m2 i.v.) (32) and defined as fasting C-peptide <0.2 and stimulated C-peptide <0.3 ng/ml in response to a glucagon challenge undertaken four weeks after STZ (32). Blood glucose levels were monitored 2–3 times daily via heel stick. Subcutaneous insulin was administered as needed, on the basis of an individualized sliding scale, to maintain the following plasma glucose levels: 250–350 for the first 2 weeks after STZ; 175–250 for the third and fourth weeks; and 75–150 mg/dl before and after transplantation. Using established methods, the donor pancreas was recovered, islets were isolated and cultured for 39–42 h, and islets were collected, washed, and counted for transplantation into the liver as previously described (32). For group 1 (Table 1), islets were infused first, followed immediately by infusion of DBMCs. For group 2 (Table 2), MSCs were added to the islet preparation 15–20 min before intraportal infusion.
TABLE 1.
Intraportal cotransplant of allogeneic DBMCs and islets
| Group 1 ID no. | MHC class II match | IEQ/kg | Days off insulina | Decrease in functionb | Days C-peptide positive/POD necropsy | Dose DBMCsc/kg × 109 | Dose CD34/kg × 106 | POD chimerism | Peak level (%) of chimerism |
|---|---|---|---|---|---|---|---|---|---|
| 58 Y | MM | 8,000 | 0 | 25 | 326/348 | 0.64 | 107 | ND | ND |
| 93-343 | MM | 9,724 | 9 | 25 | 45/45 | 0.48 | 62.2 | 1, 5 | ∼2 |
| 105-111 | MM | 11,598 | 12 | 21 | 46/61 | 0.43 | 71.5 | 4 | 0.37 |
Animals received induction treatment with four doses of 10 mg/kg thymoglobulin given in the week prior to transplant and four doses of 50 mg/m2 total of fludarabine. IM rapamycin was initiated 1–5 days prior to intraportal islet/DBMC transplant on POD 0, with a goal of achieving trough levels of 15–20 ng/ml. DBMCs were debulked with a CD11b bead-based method. Chimerism was measured using 6 class II based primer and probe sets for donor DNA; the lower limit of detection ranged from 0.03 to 0.1%, depending on the primer probe set.
aNot necessarily consecutive to islet transplant.
bOn the basis of blood glucose levels, insulin requirement, and/or C-peptide.
cTotal DBMC dose; DBMCs were given intraportally on POD 0 and intravenously on PODs 5 and 11. ND, not determined; MM, recipient and donor are MHC class II mismatched.
TABLE 2.
Intraportal cotransplant of allogeneic donor MSCs + islets
| Group 2 ID no. | MHC class II matchb | IEQ/kg | Days off insulinc | Decrease in functiond | Days C-peptide positive/POD necropsy | Dose DBMCsf/kg × 109 | Dose CD34/kg × 106 | POD 0 MSCs/kg × 106 | IV MSCsg/kg × 106 | POD chimh | Peak level (%) chim |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 105-131a | haplo | 3,000 | 3 | 94 | 354/354 | 0.17 | 19.3 | 1 | 3.4 | 38 | <0.06 |
| 26-20a | MM | 3,928 | 0 | 64 | 202/202 | 0.20 | 23.9 | 1.6 | 5.3 | UD | UD |
| 105-117 | haplo | 4,581 | 0 | 60 | 60/89 | 0.31 | 63.0 | 1.5 | 6.2 | UD | UD |
| 93-108 | MM | 5,432 | 5 | 60 | 95/137 | 0.27 | 57.0 | 1.2 | 5.5 | 31 | 0.08 |
| CW1Ha | haplo | 8,185 | 0 | 103 | 251/251 | 0.11 | 12.2 | 1.1 | 5.1 | UD | UD |
| 105-71 | haplo; hmz | 9,192 | 89 | 90 | 97/97 | 0.25 | 35.3 | 1.2 | 5 | UD | UD |
| 35-493 | MM; hmz | 10,978 | 106 | 106 | 181/181 | 0.30 | 60.4 | 1.4 | 5.8 | UD | UD |
| 105-99 | MM | 14,000 | 59 | 68 | 75/76e | 0.33 | 59.8 | 1.6 | 6.5 | 31 | <0.06 |
Animals received induction treatment with four doses of 10 mg/kg thymoglobulin given in the week prior to transplant, and four doses of 50 mg/m2 fludarabine. IM rapamycin were initiated 1–5 days prior to intraportal islet/donor MSC transplant on POD 0, with a goal of achieving trough levels of 15–20 ng/ml in all animals, except for 105-131, 26-20, and CW1H, in which rapamycin treatment started on POD 14. These three animals were also treated with anti-CD154. Chimerism was measured using 6 class II based primer and probe sets for donor DNA.
aAnti-CD154 rx.
bMM, recipient and donor are MHC class II mismatched; haplo, MHC class II haploidentical; hmz, homozygous recipient.
cNot necessarily consecutive to islet transplant.
dOn the basis of blood glucose levels, insulin requirement, and/or C-peptide.
eAnimal expired CMV positive.
fDBMCs were debulked with a CD11b bead-based method and given intravenously on PODs 4 or 5 and 11.
gMSCs given intravenously on PODs 5 and 11.
hChimerism, monitored twice per month, determined using 6 class II based primer probe sets for donor DNA; the lower limit of detection ranged from 0.03 to 0.1%, depending on the primer probe set. UD, undetectable.
Isolation of donor hematopoietic stem cells.
Donor vertebral bodies were harvested and processed to obtain DBMCs as previously described (33). DBMCs were depleted of CD11b positive cells using EasySep magnetic cell separation (Stem Cell Technologies, Vancouver, BC), cryopreserved in cryoMACS freezing bags (Miltenyi, Auburn, CA) at a concentration of 500 × 106 cells per bag, and stored until infusion.
Isolation, culture, and expansion of MSCs.
Bone marrow aspirates were harvested from the iliac crest of donor or third-party monkeys and processed with Ficoll Paque Plus to obtain mononuclear cells. Cells (P0, bone marrow aspirate) were plated at a density of 5 × 107 cells per 185 cm2 Nunclon Delta Solo flask (VWR, West Chester, PA) in culture media consisting of Minimum Essential Medium Alpha Media (Invitrogen) supplemented with 20% fetal bovine serum (FBS) (Hyclone, Logan, UT), 1% penicillin-streptomycin (Invitrogen), and 1% l-glutamine (Mediatech). Cells were kept in culture at 37°C, 5% CO2, with two media changes weekly. Once cells reached confluency, the adherent cells were removed using 0.25% Trypsin-EDTA (Invitrogen) (37°C for 5 min). P1 and P2 cells (after first and second trypsinization, respectively) were plated at a concentration of 1 × 106 cells per flask; subsequent passages were plated at 107 cells/ml; cells were cryopreserved in 90% FBS and 10% dimethlysulfoxide (Sigma).
Characterization of MSCs
Differentiation.
Following manufacturer's instructions (Human Mesenchymal Stem Cell Functional Identification Kit, R & D Systems, Minneapolis, MN), MSCs were characterized for osteogenic and adipogenic differentiation.
Immunomodulatory capacity.
P2 MSCs (5 × 104 cells) were allowed to adhere for 24 h to a U-bottomed 96-well plate (Corning, NY), followed by addition of responding peripheral blood mononuclear cell (PBMC) (1 × 105/well) and phytohemaglutinin (PHA) at a final concentration of 10 μg/well, in 0.2 ml of culture media. Cultures were incubated at 37°C, 5% CO2 for 5 days, and T-cell proliferation was determined by addition of [3H]thymidine (GE Healthcare) at 1 μCi/well for the last 18 h of culture, harvesting onto fiberglass filters, and counting.
Karyotyping.
Adherent MSCs were treated with Colcemid (0.1 μg/ml) for 2 h prior to cell harvesting. After mitotic arrest, cells were processed in accordance with standard cytogenetics laboratory procedures at the University of Pittsburgh Cancer Institute Cytogenetics Facility (Pittsburgh, PA).
Gene expression.
Total RNA was isolated from MSCs using an RNeasy kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized using “SuperScript III First-Strand Synthesis SuperMix for qRT-PCR” kit according to manufacturer's instructions (Invitrogen). Gene expression levels for IL-6, IL-10, HGF, VEGF, TGF-β, and galectin-1 were determined by using Taqman assays (Applied Biosystems, Foster City, CA) in the LightCycler PCR system (model 1.2, Roche). Taqman assay IDs for the genes are lL6, Hs00985639_m1; IL-10, Hs00174086_m1; HGF, Hs00300159_m1; VEGF, Rh02621759_m1; TGF-β, Hs00171257_m1; galectin-1, Hs00169327_m1; and 18S, Hs99999901_s1. Amplification of each sample was performed in a 20-μl reaction mixture containing 1X LightCycler FastStart DNA Master Hybridization Probe (Roche Diagnostics, Mannheim, Germany), 1X Taqman assay, 4 mmol/l MgCl2, and 2 μl cDNA sample. PCR amplification consisted of 95°C for 10 min, followed by 40 cycles at 95°C for 10 s and at 60°C for 1 min. DNA fragments from each target gene (from copy number 109 to 102) were used to construct the standard curve in each PCR amplification. Results are expressed as the ratio of the copy number of the target gene to the copy number of 18S.
Flow cytometry analyses
MSC.
Cells (105) were labeled with conjugated monoclonal antibodies (mAbs) specific for CD14, CD29, CD56, CD90, HLA class II (Beckman Coulter, Fullerton, CA), CD11c, CD34, CD44, CD45, CD73, CD166, HLA class I (BD PharMingen, San Diego, CA), CD31 (Ebioscience, San Diego, CA), and CD105 (Fitzgerald Industries International, Concord, MA).
Whole blood.
EDTA blood (100 μl) was labeled with a combination of mAbs specific for CD3, CD4, CD45 (BD Pharmingen), CD25 (eBioscience), CD8, CD11b, CD16, CD20, CD56, CD69, CD127, and HLA class II (Beckman Coulter); 7AAD was included for viability assessment. Erythrocytes were lysed using an ImmunoPrep Reagent System and a Q-Prep Workstation (Beckman Coulter).
Foxp3.
Following manufacturer's instructions, cynomolgus PBMCs were stained intracellularly for Foxp3, clone PCH101 (eBioscience). All samples were analyzed on a Coulter Cytomics FC500.
Experimental design, immunosuppressive regimen, and drug levels.
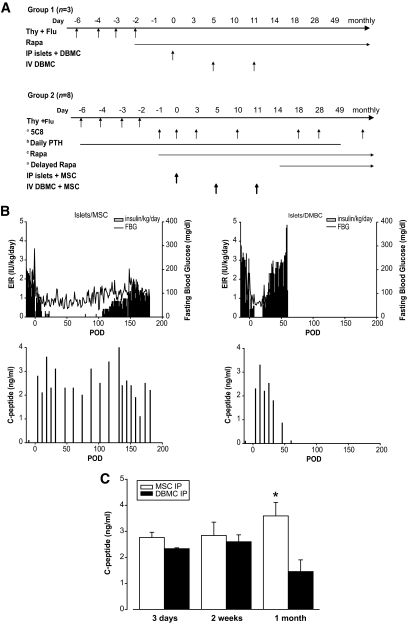
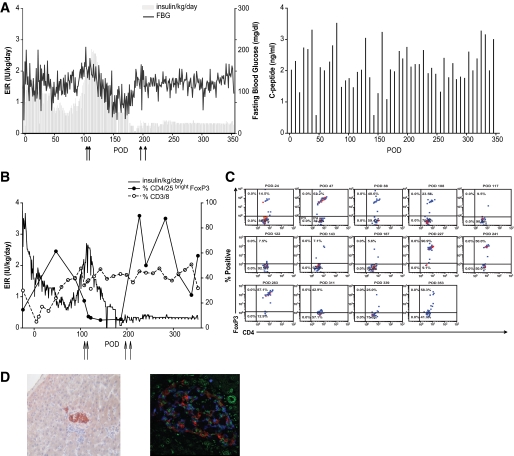
Fig. 4A is a schematic of the design used to test the effect of intraportal codelivery of islets with DBMCs (group 1; Table 1) or with donor MSCs (group 2, Table 2) on chimerism and islet allograft survival. We depleted CD11b positive vertebral body bone marrow cells to debulk the marrow and remove fragile myeloid cells. Myeloid cell death results in DNA release, clumping, and decreased overall cell viability (34). A total of three animals in group 1 received induction therapy consisting of four 10 mg/kg doses of thymoglobulin and four doses (50 mg/m2 total) of fludarabine on postoperative days (PODs) −6, −4, −3, and −2. Intramuscular (IM) rapamycin was initiated on POD −2 to achieve and maintain trough levels of 15–20 ng/ml. In this group, we examined the effect of intraportal codelivery of islets and DBMCs on POD 0, followed by intravenous infusions of DBMCs on PODs 4 or 5 and 11. The timing of DBMC infusion was based on previous clinical studies, in which delayed marrow infusion was found to be optimal in the setting of solid organ or islet transplantation (35). Group 2 had eight animals that received the same induction therapy with thymoglobulin and fludarabine. In five of eight animals, IM rapamycin was initiated on POD −1 to achieve and maintain trough levels of 15–20 ng/ml. Rapamycin was delayed (POD 14) for the remaining three animals (105-131, 26-20, and CW1H), which were also treated with 20 mg/kg human/mouse chimeric anti-CD154 specific mAb, derived from the 5c8 clone (NCRR Nonhuman Primate Reagent Resource), on PODs −1, 0, 3, 10, 18, 28, and monthly thereafter. The noticeable lack of chimerism observed after the first two animals prompted the addition of parathyroid hormone (PTH) to augment chimerism in the subsequent six animals (Forteo, 5 μg/kg from POD −7 or −6 until POD 49) (36). All animals in group 2 were used to examine the effect of intraportal codelivery of islets and MSCs on POD 0, followed by intravenous infusions of CD 11b depleted DBMC + MSC on PODs 5 and 11.
FIG. 4.
A: Schematic of the design used to test the effect of intraportal codelivery of islets with DBMCs (group 1) or with donor MSCs (group 2) on chimerism and islet graft survival in two groups of animals. Animals in group 1 received induction therapy consisting of four doses of thymoglobulin (Thy) and four doses of fludarabine (Flu) on PODs −6, −4, −3, and −2. IM rapamycin (Rapa) was initiated on POD −2. Islets were cotransplanted with DBMCs intraportally on POD 0, followed by intravenous (IV) infusions of DBMCs on PODs 5 and 11. Animals in group 2 received the same induction therapy with Thy and Flu. aanti-CD154 (5C8) on PODs −1, 0, 3, 10, 18, 28, and monthly thereafter, and rapamycin was initiated on POD 14 (n = 3). bPTH from POD −7 or −6 until POD 49 (n = 6). cRapamycin was initiated on POD −1 (n = 5). B: EIR, fasting blood glucose (FBG) (upper panel) and fasting C-peptide (lower panel) for representative animals from group 1 and group 2 that received similar numbers of islets. Both animals received induction therapy with thymoglobulin and fludarabine in the week prior to transplant and IM rapamycin starting on POD −1. The animal from group 1 (105-111, panel on the right) was transplanted with 11,598 IEQ/kg and 0.1 × 109 DBMC/kg from a mismatched donor into the liver on POD 0, followed by intravenous DBMCs (0.33 × 109 cells/kg) on PODs 5 and 11. The animal from group 2 (35-493, panel on the left) was transplanted with 10,978 IEQ/kg and 1.4 × 106 MSCs/kg from a mismatched donor into the liver on POD 0, followed by IV donor MSCs (5.8 × 106 cells/kg) together with CD11b depleted DBMCs (0.30 × 109 cells/kg) on PODs 5 and 11. C: Fasting C-peptide levels for recipients of allogeneic islets in the liver at 3 days, 2 weeks, and 1 month posttransplant. Empty bars represent recipients of islet + MSC codelivery in the liver and delayed IV DBMC + MSC infusion (n = 8; group 2, Table 2). Black bars represent recipients of islet + DBMC codelivery in the liver and delayed IV DBMC infusion (n = 3; group 1, Table 1); *P = 0.043 at 1 month posttransplant.
Chimerism.
The levels of donor DNA in a recipient peripheral blood sample were determined twice per month using LightCycler PCR as previously described (37). Chimerism results were reported as donor percentages, and each sample was analyzed in duplicate.
Histopathology.
Tissues fixed in 10% neutral buffered formalin and embedded in paraffin were sectioned (5 μm) and stained with hematoxylin and eosin. Insulin expression in the islets was assessed by immunohistochemistry using a guinea pig anti-porcine insulin polyclonal antibody (Dako, Carpinteria, CA) and a biotinylated donkey anti-guinea pig immunoglobulin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), followed by streptavidin-horseradish peroxidase and revealed by aminoethylcarbazole (Invitrogen, Carlsbad, CA). For immunofluorescence microscopy, sections were stained with anti-insulin (AbCam, Cambridge, MA), anti-glucagon (Sigma, St. Louis, MO), and anti-CD31 (AbCam), anti-CD34 (BioGenex, San Ramon, CA), and anti-actin (AbCam) for blood vessels followed by Alexa Fluor conjugated secondary antibodies (Molecular Probes, Carlsbad, CA) as previously described (38).
Statistics.
All data represent means ± SEM. A repeated-measures ANOVA was used to evaluate within-group C-peptide values at different time points posttransplant, as well as gene expression levels at different MSC passages. The between-group comparison of C-peptide values at each time point posttransplant was evaluated using a t test. In all appropriate cases, post hoc least significant difference (LSD) test was used. All analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL) and P values <0.05 were considered statistically significant.
RESULTS
Isolation, expansion, and characterization of cynomolgus monkey MSCs.
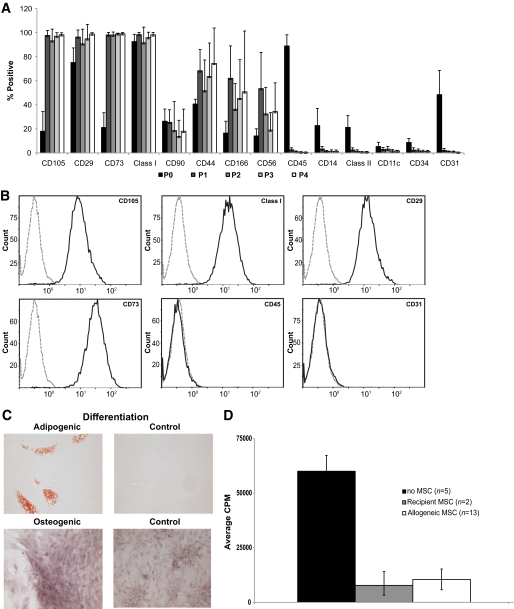
Vertebral body marrow and iliac crest aspirate were evaluated as the MSC source, and iliac crest aspirate was optimal with regards to cell yield. Cells were characterized to verify absence of cell surface markers associated with leukocytes (CD11c, 14, 45, 56), endothelial and hematopoietic stem cells (CD31, 34), and HLA class II and presence of cell surface markers associated with MSCs in published studies, including CD29, 44, 73, 90, 105, and 166, as well as class I. The percentage of non-MSC associated markers was highest in the source material, much lower in P1, and dropped to <1% thereafter, with a corresponding increase in the percentage of CD105, 29, and 73 cells to nearly 100% (Fig. 1A and B). Markers reported on MSCs from other species (CD90, 44, 166), as well as CD56, were variably positive. Differentiation of cells to fat and bone verified MSC identity (Fig. 1C). Addition of MSCs to cynomolgus mixed lymphocyte reactions resulted in variable suppression of proliferation (data not shown). In contrast, addition of either autologous (n = 2) or allogeneic (n = 13) MSCs to PHA-stimulated PBMCs significantly inhibited proliferation by 85% (Fig. 1D, P < 0.009).
FIG. 1.
A: Phenotypic characterization of MSCs isolated from cynomolgus bone marrow. P0, aspirate; P1–P4, after first, second, third, and fourth trypsinization, respectively. Values represent mean ± SD, number of different donors for each passage in parentheses. P0 (n = 29); P1 (n = 25); P2 (n = 24); P3 (n = 14); and P4 (n = 9). B: Representative flow cytometric analysis of P2-cultured MSCs. Compared with isotype control (dashed lines), cynomolgus bone marrow MSCs stained positive for CD105, MHC class I, CD29, and CD 73 and negative for CD45 and CD31. Histograms represent consistent findings in 24 different donors. C: Adipogenic and osteogenic differentiation of MSCs (passage 2) isolated from cynomolgus bone marrow aspirates. D: Effect of MSCs on PHA-induced stimulation of PBMC proliferation. Allogeneic MSCs significantly inhibited PBMC proliferation by 85% (P < 0.009); results for autologous cells were similar, but too few donors were tested to assess statistical significance. CPM, counts per minute. (A high-quality color representation of this figure is available in the online issue.)
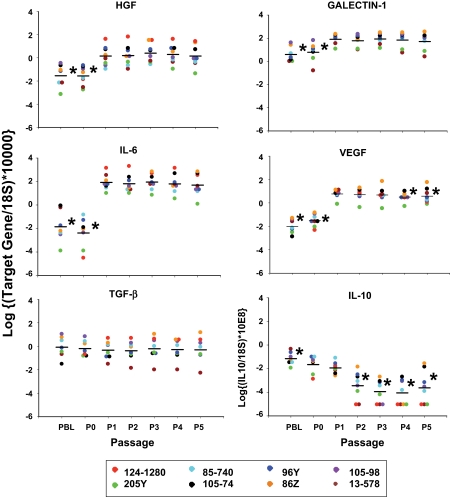
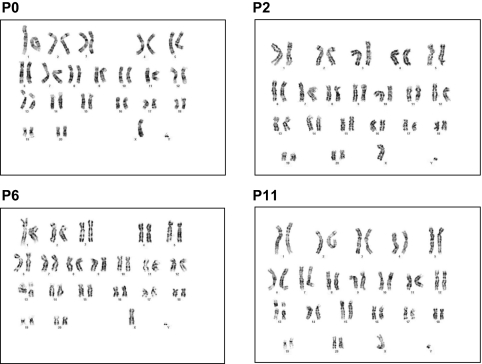
We assessed gene expression levels for IL-6, IL-10, VEGF, TGF-β, HGF, and galectin-1 over several passages of MSC from the same donor, with peripheral blood as a control; 8 different donors were studied through passage 5 (Fig. 2), and four were studied through passage 11 (data not shown). Levels of TGF-β remained stable through passage 8 and were similar to peripheral blood (PBL) and P0 material; significant decreases were noted after P8 for those cells followed further out. HGF, galectin-1, IL-6, and VEGF expression levels were significantly higher in MSCs as compared with PBL and marrow and remained relatively stable through P5, with VEGF dropping off at P4, HGF and galectin-1 dropping off at P7, and IL-6 dropping off at P9. In contrast, IL-10 expression levels were extremely low, relative to control, in all passages. Variability in expression levels was observed among different donors. MSCs from four of these animals were also characterized for cytogenetic stability at passages 0, 2, 6, and 11. Results revealed normal karyotypes, without clonal numerical or structural aberrations. The cells maintained clonal chromosomal stability between passages 0 and 11 (Fig. 3).
FIG. 2.
Gene expression levels for IL-6, IL-10, VEGF, TGF-β, HGF, and galectin-1 for sequential passages from eight cynomolgus monkey donors. All results were expressed as the log ratio of the copy number of the target gene to the copy number of 18S (used as the endogenous control gene). Gene expression levels for PBL, P0, and P2–P5 were compared with levels expressed in P1 MSCs. n = 8, *P < 0.05 versus P1.
FIG. 3.
Trypsin-Giemsa banded karyotypes for MSCs from animal 105-74 from passages 0, 2, 6, and 11 showing a 42,XY normal male Pearson classification (52).
Effect of MSCs on islet engraftment/function and chimerism.
Fig. 4A shows a schematic of the design used to test the effect of intraportal islet/DBMC (group 1) and intraportal islet/MSC (group 2) transplant on chimerism and allogeneic islet engraftment. Islet function was detected for all recipients, as evidenced by stabilization of blood glucose levels, decreased exogenous insulin requirements, and positive fasting C-peptide. Attainment and duration of insulin independence was associated with islet dose (Tables 1 and 2).
Three animals in group 1 received intraportal islet/DBMC cotransplants on POD 0 (Table 1) and intravenous DBMCs on PODs 4 or 5 and 11. None of the group 1 animals experienced long-term graft survival, with all recipients manifesting decreased graft function on PODs 21–25. Insulin independence was transiently observed for the two monkeys that received a dose near the 10,000 IEQ/kg required for reproducible insulin independence in this model. Donor-derived cells were only detected in peripheral blood in relation to DBMC infusion (PODs 1–11).
Group 2 animals (Table 2) were treated with MSCs, delivered both intraportally with the islets on POD 0 and intravenously with the delayed DBMC infusions. All animals in group 2 received five million intraportal MSCs on POD 0, but due to the broad range of IEQ transplanted, this resulted in islet cell/MSC ratios from 3.7 to 13.4 (based on an IEQ of 1,500 cells per islet). In contrast to group 1, decreased graft function was not evident until ≥POD 60 for group 2 (as compared with PODs 21–25 for group 1), with five of eight animals C-peptide positive at necropsy. Mean duration of function prior to destabilization was 81 ± 20 days for group 2 versus 24 ± 2 for group 1 (P < 0.001). This is illustrated in Fig. 4B, which shows fasting blood glucose, exogenous insulin requirements, and fasting C-peptide levels for one animal from group 1 (105-111; 11,598 IEQ/kg) and one animal from group 2 (35-493; 10,978 IEQ/kg) that received a comparable number of islets. Extremely low levels of chimerism, not associated with DBMC infusion, were observed transiently in three of eight group 2 monkeys at ∼1 month after transplant.
There was no significant difference in the mean number of islets transplanted in recipients from group 1 (9,774 ± 1,039 IEQ/kg; n = 3) versus recipients from group 2 (7,412 ± 1,359 IEQ/kg; n = 8). However, we observed a striking early increase in islet function in recipients of intraportal islet/MSC cotransplants and chose three time points to compare islet function between animals in group 1 (intraportal islets/DBMC) and those in group 2 (intraportal islets/MSC): 3 days, 2 weeks, and 1 month posttransplant. We first determined that, within the eight animals in group 2, there was no significant difference in the fasting C-peptide values between the ones treated with delayed rapamycin and anti-CD154 (n = 3) and those that began treatment with rapamycin before transplant (n = 5), or between the animals that received PTH (n = 6) and those that did not (n = 2), or between the animals that were MHC class II mismatched (n = 4) or haploidentical (n = 4), at any of the three time points (data not shown). All eight animals in group 2 were therefore used for subsequent analysis. The results for the comparison of fasting C-peptide levels at 3 days, 2 weeks, and 1 month posttransplant between animals in group 1 and group 2 are shown in Fig. 4C. Analysis of fasting C-peptide within each group as a function of time showed a trend toward an increase in function (P = 0.07) over the first posttransplant month only in the animals that received intraportal islet/MSC. Values for intraportal islet/DBMC recipients (group 1) did not change significantly in this same time frame. Comparison of C-peptide values between the two groups at 1 month posttransplant showed a significantly higher C-peptide value (more than double) for recipients of islet/MSC cotransplants (3.6 ± 0.5 ng/ml vs. 1.4 ± 0.4 ng/ml, P = 0.043; Fig. 4C).
Treatment of rejection with intravenous MSCs and immunological changes in MSC-treated recipients.
We used donor and/or third-party MSCs to treat the rejection process after intraportal islet/MSC transplant in five of the animals in group 2 (93-108, 105-71, CW1H, 26-20, 105-131). Two animals received additional donor MSCs (up to 2 × 106 MSCs/kg) > 10 days after graft dysfunction (93-108 received one dose and CW1H received two doses administered 11 days apart), and one animal received a single dose of MHC class II mismatched third party MSCs (105-71) at the first sign of rejection without success. Our first evidence of efficacy in amelioration of rejection was obtained for an animal that experienced a decrease in function on POD 64 (animal 26-20, Table 2; Fig. 5). This animal was treated with islet donor MSCs (2 × 106/kg, MHC class II mismatched to the recipient) on PODs 64 and 68, but the exogenous insulin requirement (EIR) continued to rise. Infusion of third-party MSCs (2 × 106 cells/kg, MHC class II haploidentical to the recipient) on PODs 71, 77, 86, and 91 resulted in EIR reduction and increased C-peptide, although the effect was short lived. Additional third-party MSCs were given on PODs 155 and 160, and at the time of necropsy, both fasting blood glucose and EIR were decreasing.
FIG. 5.
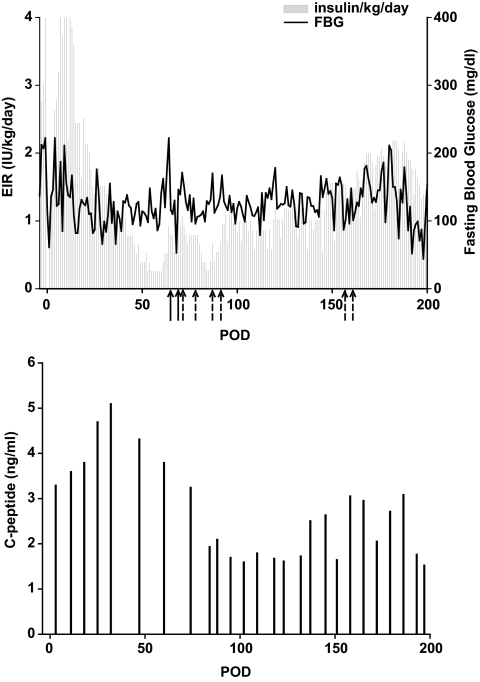
EIR and fasting blood glucose (FBG) (upper panel) and fasting C-peptide (lower panel) for animal 26-20. This animal received induction therapy with thymoglobulin and fludarabine in the week prior to transplant, and IM rapamycin was initiated on POD 14 to achieve and maintain trough levels of 15–20 ng/ml. In addition, treatment with anti-CD154 (20 mg/kg) started on POD −1, with five doses in the first month posttransplant and monthly thereafter until POD 168. On POD 0, 3,928 IEQ/kg and 1.6 × 106 MSCs/kg from a mismatched donor were transplanted into the liver. IV donor MSC (5.3 × 106 cells/kg), together with CD11b depleted donor bone marrow cells (0.20 × 109 cells/kg) were given on PODs 5 and 11. Additional donor MSCs (full arrow, 2 × 106 cells/kg) were given on PODs 64 and 68, and third-party MSCs from a haploidentical third party (dashed arrow, 2 × 106 cells/kg) were given on PODs 71, 77, 86, 91,155, and 160.
Therapy of an additional recipient with islet donor MSCs, haploidentical to the recipient, with greater duration of follow-up after MSC infusion, revealed clear reversal of rejection (105-131, Table 2; Fig. 6). Fig. 6A–D illustrates key points related to graft function, MSC infusion, Tregs, and T-effector cells. This animal received a very marginal mass of 3,000 IEQ/kg. Posttransplant, a gradual reduction in insulin/kg was eventually offset by increased insulin requirements and destabilization of blood glucose on POD 94, accompanied by decreased fasting C-peptide (Fig. 6A, lower panel). Additional islet donor MSCs were given intravenously at 2 × 106/kg on PODs 105, 110, 196, and 207. Of note is the gradual decline in insulin requirement after MSC infusion on PODs 105 and 110 and the ultimate recovery of fasting C-peptide levels; additional infusions were given on PODs 196 and 207 with the rationale that repeated doses would have an additive effect. Exogenous insulin requirements, fasting blood glucose, and the day of additional MSC infusion (arrows) are shown in Fig. 6A. Graft destabilization on POD 94 was associated with increased CD3/8 effector cells and decreased FoxP3 Tregs (Fig. 6B). In addition, MSC infusion after resolution of graft dysfunction resulted in an increase in Tregs (Fig. 6B and C). Subsequent to graft recovery and additional intravenous MSC infusion, Tregs increased in percentage and absolute numbers to levels that were higher than those observed before rejection. Results from histological examination of tissues after necropsy are shown in Fig. 6D. Examination of liver sections using immunohistochemistry, as well as immunofluorescence staining, revealed insulin-positive, highly vascularized scattered islets. Consistent with our previous observations in STZ-induced diabetic monkeys, islets in the pancreas were negative for insulin staining.
FIG. 6.
A: EIR and fasting blood glucose (FBG) (left panel) and fasting C-peptide (right panel) for animal 105-131. This animal received induction therapy with thymoglobulin and fludarabine in the week prior to transplant, and IM rapamycin was initiated on POD 14 to achieve and maintain trough levels of 15–20 ng/ml. In addition, treatment with anti-CD154 (20 mg/kg) started on POD −1, with five doses in the first month posttransplant and monthly thereafter. On POD 0, 3,000 IEQ/kg and 1.0 × 106 MSCs/kg from a haploidentical donor were transplanted into the liver. IV donor MSCs (3.4 × 106 cells/kg), together with CD11b depleted DBMCs (0.17 × 109 cells/kg) were given on PODs 5 and 11. Additional donor MSCs (full arrow, 2 × 106 cells/kg) were given on PODs 105, 110, 196, and 207. B: EIR (line), % CD4/25 bright FoxP3 T-cells (filled circles), and %CD3/8 positive T-cells (empty circles) for animal 105-131. Arrows indicate donor MSCs (2 × 106 cells/kg) given on PODs 105, 110, 196, and 207. C: Frequency of FoxP3 positive T-cells in peripheral blood of animal 105-131 gated for CD3+CD4+CD25bright lymphocytes. D: Histopathology of liver sections after necropsy. Left panel shows immunohistochemistry staining of an islet with hematoxylin and eosin and positive signal for insulin (brown) (×200). Right panel shows a representative confocal microscopy analysis with immunofluorescence for insulin (red), glucagon (blue), and blood vessels (green) (×400). (A high-quality digital representation of this figure is available in the online issue.)
We measured the frequency of Tregs in five animals in group 2. Representative data for two animals, shown in Fig. 6B and C (105-131) and supplementary Fig. S1 (35-493), available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0136/DC1, illustrate that stable islet allograft function was associated with an increased number of Tregs in the periphery, while a decrease in Tregs occurred after graft dysfunction.
DISCUSSION
The International Society for Cellular Therapy criteria for human MSCs include fibroblast-like morphology, adherence to plastic, phenotypic characteristics, in vitro potential for trilineage differentiation, and inhibition of proliferation of allo- or mitogen-activated lymphocytes (39). Rhesus (40) and cynomolgus macaque (41) and baboon (42) MSCs appear to be phenotypically and functionally similar to their human counterparts. Our study depicts the first thorough characterization of cynomolgus MSC phenotypic markers, gene expression levels, and karyotype through several passages. Gene expression levels for IL-6, IL-10, VEGF, TGF-β, HGF, and galectin-1 vary over several passages of MSCs from the same donor (as well as between donors), indicating that the actual functional capacity of the cells might vary between donors and between passages. These findings could impact clinical outcomes and suggest a need for additional functional criteria in the standardization of MSC products for cell therapies.
The cytogenetic stability of these cells, studied up to passage 11, appears to be in agreement with human studies by Bernardo and colleagues (43), in which cells from 10 human donors, followed to senescence or passage 25, showed no cytogenetic abnormalities or malignant transformation. While small in numbers, our study demonstrates the feasibility of using cynomolgus macaque MSCs up to passage 11 for therapeutic efficacy in future studies.
MSCs have been shown to enhance DBMC engraftment (17,18). Studies in rodent models suggest that an intraportal route of antigen delivery may be tolerogenic (44,45). Despite intraportal infusion of MSCs or DBMCs with islets, we did not observe enhancement of donor marrow engraftment, chimerism, or tolerance. We did observe significant enhancement of islet engraftment and function at 1 month posttransplant in recipients of intraportal MSCs as compared with animals that received islets plus DBMCs. To further define the significance of the engraftment data with MSCs, we compared 1-month posttransplant fasting C-peptide values from the animals that received intraportal islet/MSC (3.6 ± 0.5 ng/ml) versus two additional historical, published groups. The first group consisted of three recipients of intraportal islets alone, treated with thymoglobulin, fludarabine, and rapamycin, plus samarium lexidronam and delayed intravenous DBMCs on PODs 5 and 11 (1.3 ± 0.2 ng/ml) (46). The second group comprised 19 recipients of intraportal islets alone under the cover of steroid-free immune suppression (1.6 ± 0.2 ng/ml) (38). Taken together, and including animals from group 1 in the present study, it is striking that recipients of islets/MSC had engraftment values of more than double compared with 27 animals that did not get MSCs, regardless of the conditioning regimen (P < 0.01). This suggests that, compared to other regimens, MSCs provide a distinctive advantage in promoting early islet engraftment. Marrow MSCs have been shown to express chemokine receptors that mediate migration of these cells to pancreatic islets (20); after intracardiac infusion of MSCs in diabetic mice, cells homed selectively to pancreatic islets and glomeruli (21) and intravenous infusion of MSC was accompanied by improved glycemic levels, reduced pancreatic insulitis, and pancreatic tissue regeneration (19,21–23,25). Although this data may suggest that the mechanism behind the engraftment effect of MSCs observed in our NHP islet allograft model may involve migration of MSCs to the pancreas and pancreatic islet regeneration, we did not observe any insulin-positive islets in histological analysis of pancreas tissue after necropsy. We can hypothesize that MSCs enhance islet engraftment by staying in proximity to the islets at the time of cotransplant, providing immunomodulatory, revascularization, and regenerative signals. However, understanding the mechanism of action of MSCs requires knowing where the MSCs localize after intraportal or intravenous infusion. In an attempt to track the fate of the implanted MSCs, we performed pilot experiments in which each of two monkeys was transplanted with intraportal islets/green fluorescent protein (GFP)-tagged MSCs on POD 0 under the cover of anti-CD154. One month posttransplant, the animals were killed for histological examination of the tissues. Using immunohistochemistry, as well as measuring GFP DNA, we could not find any traces of the GFP-tagged MSCs in any of the tissues of one of the animals, but we could detect some GFP-tagged MSCs in the liver and in the lungs of the other recipient (data not shown). Further experiments, including tracking tagged MSCs after shorter periods posttransplant, will be needed to elucidate the fate and potential mechanisms of action for intraportally implanted MSCs in our NHP model of islet transplantation.
Intraportal islet engraftment and stable function were accompanied by an increase in the percentage of Tregs in the circulation. The immunomodulatory effect of MSCs has been shown to involve generation of Tregs in in vitro studies (47,48), as well as in the prevention of autoimmune diabetes in NOD mice (26); however, our data are the first evidence of in vivo Treg generation in the context of MSC-augmented allogeneic islet grafts in a preclinical model with close proximity to humans.
To the best of our knowledge, this is the first time that intravenous infusion of either islet donor or third-party marrow MSCs were used to reverse episodes of islet graft rejection in the context of allogeneic islet transplantation. The most striking result was obtained after intravenous infusion of two doses of 2 × 106 islet donor MSC/kg spaced 4 days apart, given after the EIR had reached levels similar to those previous to transplantation of a marginal islet mass. The resolution of graft dysfunction was accompanied by an increase in the circulating Tregs. Timing appeared to be an important factor because MSCs given several days after graft destabilization were ineffective. These findings suggest that the inflammatory process during rejection may provide activating cytokines or chemokines that enhance efficacy of MSC suppression. In another in vivo model of T-cell-mediated tissue destruction, IFN-γ was critical for suppression of graft versus host disease by MSCs (49).
The use of a histocompatibility matched, autologous, or a mismatched source of MSCs when treating autoimmune disorders is still in debate. Fiorina et al. (24) reported delayed onset of diabetes as well as reversal of hyperglycemia in NOD mice treated with allogeneic but not autologous MSCs, while Solari et al. (27) observed prolonged survival of allogeneic islets in STZ-induced diabetic rats with autologous but not with allogeneic MSCs. The immunogenicity and functional integrity of MSCs from subjects with autoimmune diseases is also not clear. Transplantation of autologous MSCs in NOD mice was accompanied by development of soft tissue and visceral tumors, which were not observed with the transfer of allogeneic MSCs (24). A concern with MSCs from patients with autoimmune disease has been the potential for decreased immunomodulatory capacity, although recent studies reported that MSCs from patients with multiple sclerosis and rheumatoid arthritis had normal cell surface and molecular phenotype and ability to support hematopoiesis (50,51). It is not clear whether the capacity of these MSCs is as robust as allogeneic MSCs for immunomodulation or whether these cells require additional manipulation ex vivo to augment their immunomodulatory capacity. Our results, obtained in a preclinical model, justify the clinical investigation of MSC as both a feasible approach for enhancement of islet engraftment and as a safe and effective antirejection therapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health U19-AI-051728. Tissue typing was supported by NIAID contract number HHSN266200400088C/N01-A1-30061. Gene expression and karyotype studies were supported by the Diabetes Research Institute Foundation. The anti-CD154 antibody used was provided by the NIH Nonhuman Primate Reagent Resource (RR-016001, AI-040101). The cytogenetic studies were carried out in the University of Pittsburgh Cancer Institute Cytogenetics Facility, supported in part by P30-CA-47904 to R. B. Herberman/S. M. Gollin.
A.M.B. is an employee of the University of Illinois at Chicago College of Medicine, reviewer for NY stem cell organization, and California Institute of Regenerative Medicine; she received monetary compensation for consultant agreement with Cleveland Bio Labs, and received grant/research support from NIH/NIAID. No other potential conflicts of interest relevant to this article were reported.
D.M.B. and N.S.K. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. M.A.W. and N.M.K. researched data, contributed to the discussion, and reviewed/edited the manuscript. D.H.O. reviewed/edited the manuscript. G.K. and A.M.B. contributed to the discussion and reviewed/edited the manuscript. O.C., J.A.K., R.W.W., and D.H. researched data and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the TERMIS-NA 2008 Conference and Exposition, San Diego, California, 7–12 December 2008; and at the American Transplant Conference, Boston, Massachusetts, 30 May–3 June 2009.
The authors thank Waldo Diaz and James Geary for their care of the monkeys used in the transplant studies; Alex Rabassa for his assistance with islet isolation, immunohistochemistry of histological sections, and animal care; and Kevin Johnson for his assistance with sample processing.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736 [DOI] [PubMed] [Google Scholar]
- 2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 3. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–48 [DOI] [PubMed] [Google Scholar]
- 4. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–3843 [DOI] [PubMed] [Google Scholar]
- 5. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722–3729 [DOI] [PubMed] [Google Scholar]
- 6. Bartholomew A, Polchert D, Szilagyi E, Douglas GW, Kenyon N. Mesenchymal stem cells in the induction of transplantation tolerance. Transplantation 2009;87 (Suppl. 9):S55–57 [DOI] [PubMed] [Google Scholar]
- 7. Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 2008;57:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadri T, Lataillade JJ, Doucet C, Marie A, Ernou I, Bourin P, Joubert-Caron R, Caron M, Lutomski D. Proteomic study of Galectin-1 expression in human mesenchymal stem cells. Stem Cells Dev 2005;14:204–212 [DOI] [PubMed] [Google Scholar]
- 9. Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 2007;149:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004;103:4619–4621 [DOI] [PubMed] [Google Scholar]
- 11. Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 2009;58:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007;109:228–234 [DOI] [PubMed] [Google Scholar]
- 13. Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006;24:386–398 [DOI] [PubMed] [Google Scholar]
- 14. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003;108:863–868 [DOI] [PubMed] [Google Scholar]
- 15. Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol 2007;137:491–502 [DOI] [PubMed] [Google Scholar]
- 16. Caplan AI. Why are MSCs therapeutic? New data: new insight. The Journal of Pathology 2009;217:318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DH, Yoo KH, Yim YS, Choi J, Lee SH, Jung HL, Sung KW, Yang SE, Oh WI, Yang YS, Kim SH, Choi SY, Koo HH. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci 2006;21:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lönnies H, Nava S, Ringdén O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007;21:1733–1738 [DOI] [PubMed] [Google Scholar]
- 19. Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631–640 [DOI] [PubMed] [Google Scholar]
- 20. Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005;106:419–427 [DOI] [PubMed] [Google Scholar]
- 21. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 2006;103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ende N, Chen R, Reddi AS. Effect of human umbilical cord blood cells on glycemia and insulitis in type 1 diabetic mice. Biochem Biophys Res Commun 2004;325:665–669 [DOI] [PubMed] [Google Scholar]
- 23. Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003;21:763–770 [DOI] [PubMed] [Google Scholar]
- 24. Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009;183:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, Uher F. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 2008;26:244–253 [DOI] [PubMed] [Google Scholar]
- 26. Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A, Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 2009;52:1391–1399 [DOI] [PubMed] [Google Scholar]
- 27. Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun 2009;32:116–124 [DOI] [PubMed] [Google Scholar]
- 28. Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc 2009;41:1797–1800 [DOI] [PubMed] [Google Scholar]
- 29. O'Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O'Connor DH. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics 2007;59:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiseman RW, Wojcechowskyj JA, Greene JM, Blasky AJ, Gopon T, Soma T, Friedrich TC, O'Connor SL, O'Connor DH. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J Virol 2007;81:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karl JA, Wiseman RW, Campbell KJ, Blasky AJ, Hughes AL, Ferguson B, Read DS, O'Connor DH. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 2008;60:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, Picha KM, Soderman AR, Jordan RE, Bugelski PJ, Horninger D, Lark M, Davis JE, Alejandro R, Berggren PO, Zimmerman M, O'Neil JJ, Ricordi C, Kenyon NS. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation 2007;84:308–315 [DOI] [PubMed] [Google Scholar]
- 33. Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han D, Wagner JL, Ruiz P, Berho M, Inverardi L, Alejandro R, Mintz DH, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes 1999;48:1473–1481 [DOI] [PubMed] [Google Scholar]
- 34. Kenyon N, Zwerner R, Gribben J, Nadler L, Ricordi C, Russell T. High Density Particles: A Novel, Highly Efficient Cell Separation Technology. New York, Marcel Dekker, Inc., 1997 [Google Scholar]
- 35. Kenyon NS, Chatzipetrou M, Tzakis A, Miller J, Alejandro R, Ricordi C. Allogeneic hematopoietic stem cell transplantation in recipients of cellular or solid organ allografts. Cancer Treat Res 1999;101:109–132 [DOI] [PubMed] [Google Scholar]
- 36. Reina E, Genrich K, Moadsiri A, Polchert D, Pundy T, Setty S, Bartholomew A. Manipulation of the bone marrow microenvironment with low dose parathyroid hormone pulse therapy increases allogeneic hematopoietic stem cell engraftment in non-human primates. Am J Transplantation 2006;6:219 [Google Scholar]
- 37. Han D, Berman DM, Kenyon NS. Sequence-specific analysis of microchimerism by real-time quantitative polymerase chain reaction in same-sex nonhuman primates after islet and bone marrow transplantation. Transplantation 2007;84:1677–1685 [DOI] [PubMed] [Google Scholar]
- 38. Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009;9:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317 [DOI] [PubMed] [Google Scholar]
- 40. Lee CC, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev 2006;15:209–220 [DOI] [PubMed] [Google Scholar]
- 41. Ke H, Wang P, Yu W, Liu X, Liu C, Yang F, Mao FF, Zhang L, Zhang X, Lahn BT, Xiang AP. Derivation, characterization and gene modification of cynomolgus monkey mesenchymal stem cells. Differentiation 2009;77:256–262 [DOI] [PubMed] [Google Scholar]
- 42. Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001;29:244–255 [DOI] [PubMed] [Google Scholar]
- 43. Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007;67:9142–9149 [DOI] [PubMed] [Google Scholar]
- 44. Morsiani E, Rozga J, Dellagiacoma G, Demetriou AA. Repeated intraportal injections of subtherapeutic islet cell isografts restore normoglycemia in streptozotocin-diabetic rats. Cell Transplant 1997;6:17–22 [DOI] [PubMed] [Google Scholar]
- 45. Yasunami Y, Ryu S, Ueki M, Arima T, Kamei T, Tanaka M, Ikeda S. Donor-specific unresponsiveness induced by intraportal grafting and FK506 in rat islet allografts: importance of low temperature culture and transplant site on induction and maintenance. Cell Transplant 1994;3:75–82 [DOI] [PubMed] [Google Scholar]
- 46. Berman DM, Willman M, Han D, Inverardi L, Ricordi C, Kenyon NM, Kenyon NS. Targeted radioisotope therapy with Samarium-Lexidronam (Sm-Lex) increases the occurrence of chimerism in cynomolgus monkey models of allogeneic islet-bone marrow transplantation. Am J Transplant 2009;9:659 [Google Scholar]
- 47. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008;26:212–222 [DOI] [PubMed] [Google Scholar]
- 48. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 49. Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 2008;38:1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos GD, Jorgensen C, Charbord P, Häupl T, Boumpas DT, Papadaki HA. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis 2008;67:741–749 [DOI] [PubMed] [Google Scholar]
- 51. Papadaki HA, Tsagournisakis M, Mastorodemos V, Pontikoglou C, Damianaki A, Pyrovolaki K, Stamatopoulos K, Fassas A, Plaitakis A, Eliopoulos GD. Normal bone marrow hematopoietic stem cell reserves and normal stromal cell function support the use of autologous stem cell transplantation in patients with multiple sclerosis. Bone Marrow Transplant 2005;36:1053–1063 [DOI] [PubMed] [Google Scholar]
- 52. Pearson PL, Roderick TH, Davisson MT, Garver JJ, Warburton D, Lalley PA, O'Brien SJ. Report of the committee on comparative mapping. Cytogenet Cell Genet 1979;25:82–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.