Abstract
OBJECTIVE
To identify physiological and clinical variables associated with development of type 2 diabetes up to 12 years after pregnancies complicated by gestational diabetes.
RESEARCH DESIGN AND METHODS
Seventy-two islet cell antibody–negative nondiabetic Hispanic women had oral (oGTT) and intravenous (ivGTT) glucose tolerance tests, glucose clamps, and body composition assessed between 15 and 30 months after pregnancies complicated by gestational diabetes mellitus (GDM). They returned for oGTTs at 15-month intervals until they dropped out, developed diabetes, or reached 12 years postpartum. Cox regression analysis was used to identify baseline predictors and changes during follow-up that were associated with development of type 2 diabetes.
RESULTS
At baseline, relatively low insulin sensitivity, insulin response, and β-cell compensation for insulin resistance were independently associated with development of diabetes. During follow-up, weight and fat gain and rates of decline in β-cell compensation were significantly associated with diabetes, while additional pregnancy and use of progestin-only contraception were marginally associated with diabetes risk.
CONCLUSIONS
In Hispanic women, GDM represents detection of a chronic disease process characterized by falling β-cell compensation for chronic insulin resistance. Women who are farthest along at diagnosis and/or deteriorating most rapidly are most likely to develop type 2 diabetes within 12 years after the index pregnancy. Weight gain, additional pregnancy, and progestin-only contraception are potential modifiable factors that increase diabetes risk.
The diagnosis of gestational diabetes mellitus (GDM) identifies relatively young women without known diabetes who have circulating glucose concentrations in the upper end of the population distribution during pregnancy. Those women have a 20–60% risk of developing diabetes, especially type 2 diabetes, in the 5–10 years after the index pregnancy (1). Thus, they provide an opportunity to study diabetes in evolution to determine what clinical and physiological factors predict and/or attend the development of diabetes.
Cross-sectional studies of glucose regulation conducted during and after pregnancy (2–4) indicate that women with GDM have, on average, more insulin resistance and less pancreatic β-cell compensation for that resistance than to women who maintain normal glucose levels in pregnancy. Longitudinal studies are relatively few in number and limited to relatively short times after the index pregnancy. For example, our group has reported that, in Hispanic women, β-cell compensation for chronic insulin resistance falls progressively during the first 5 years after the index pregnancy (5).
Glucose levels rise quite slowly until compensation reaches low levels (e.g., ∼15% of normal for acute insulin secretion), at which time glucose may rise very quickly and into the diabetic range as β-cell compensation falls further. Predictors of diabetes in this context could reflect the greatest degree of deterioration at baseline testing and/or the greatest rate of deterioration thereafter. In the present report, we examine the relative contribution of such factors during what we believe to be the longest detailed study of glucose regulation following pregnancies complicated by GDM.
RESEARCH DESIGN AND METHODS
Subjects were islet cell antibody–negative women who participated in a longitudinal study of the pathogenesis of type 2 diabetes following GDM. Selection of the original cohort has been described in detail (6). Briefly, all Latino women referred to Los Angeles County Women's Hospital for management of GDM between August 1993 and March 1995 were asked to participate if they met all of the following criteria: 1) gestational age between 28 and 34 weeks, 2) no current or prior insulin therapy, 3) all fasting serum glucose concentrations <130 mg/dl (7.2 mmol/l) during pregnancy, 4) otherwise uncomplicated singleton pregnancy, and 5) both parents and at least three of four grandparents were from Mexico, Guatemala, or El Salvador. All women had detailed metabolic testing during the third trimester (6). They were asked to return for a 75-g oral glucose tolerance test (oGTT) 6 months postpartum and then for an oGTT, intravenous glucose tolerance test (ivGTT), and glucose clamp at ∼15 months postpartum. oGTTs and ivGTTs were scheduled every 15 months thereafter. Height, weight, and information on contraceptive use and pregnancies were collected at each visit. Bioelectrical impedance was measured at each oGTT visit to assess body composition. At the time of diagnosis of impaired glucose tolerance or diabetes, subjects met with a dietitian and received advice on nutrition and daily walking. Subjects remained in follow-up until they withdrew consent, were lost to follow-up, developed a fasting plasma glucose concentration >140 mg/dl, or reached the final scheduled study visit 12 years postpartum. Women who were pregnant at the time of a scheduled battery of tests were studied at least 4 months after pregnancy and at least 1 month after completion of breastfeeding.
All subjects gave written, informed consent for participation in the study, which was approved by the institutional review board of the University of Southern California and the Los Angeles County and the University of Southern California Medical Center.
End point for present analysis.
For the present report, which is focused on physiological changes associated with development of diabetes from the first postpartum visit onward, we analyzed data from all subjects who 1) had baseline oGTT, ivGTT, glucose clamp, and body composition studies without diabetes within 30 months postpartum and 2) returned for at least one additional oGTT to determine diabetes status. Follow-up data were used up to earlier of either the diagnosis of diabetes by American Diabetes Association criteria (fasting ≥126 mg/dl or 2 h ≥200 mg/dl) or the last visit without diabetes.
Testing protocols.
For the baseline battery of oGTT, ivGTT, glucose clamp, and body composition, subjects came to the general clinical research center on 3 separate days, at least 48 h apart, after 8–12 h overnight fasts and at least 3 days on an unrestricted diet. The order of ivGTTs and clamps was alternated among individuals.
On one day, bioelectrical impedance (BIA) was measured immediately prior to an oGTT. For BIA, subjects lay supine while plastic electrodes were placed on their right hand and foot and a trained technician took dual resistance and reactance readings with a Quantom Impedance Meter (RJL Systems, Clinton Township, MI). For oGTTs, subjects drank 75 g of dextrose. Blood was obtained from an antecubital venous catheter before and 15, 30, 60, 90, 120, and 180 min after the glucose ingestion, placed on ice, and plasma was separated within 20 min and stored at −80°C.
On a separate day, an ivGTT was performed starting between 0700 and 1000 h. Dextrose (300 mg/kg) was injected over 1 min, followed in 20 min by a 5-min infusion of crystalline human insulin (0.03 units/kg). Arterialized venous blood was drawn into iced tubes before (n = 2) and for 240 min after (n = 32) the dextrose injection. Plasma was separated within 20 min and stored at −80°C.
On a third day, a glucose clamp was performed starting between 0600 and 0630 h. A primed (0.035 mmol/kg body wt), continuous (2.5 × 10−4 mmol/min/kg) infusion of 6,6 2H2 d-glucose (“tracer”) was administered through an antecubital vein for 360 min. A nonprimed infusion of crystalline human insulin (40 mU/min per m2 body surface area) was administered during the final 180 min of the tracer infusion. Dextrose (20% wt/vol in water), containing dideutero-glucose (0.021 mmol/cc) to minimize changes in plasma tracer enrichment, was given to maintain arterialized venous plasma glucose concentrations at ∼88 mg/dl during the insulin infusion. Blood samples for measurement of tracer, hormone/ and metabolite concentrations were drawn into ice-cold tubes at −90, −50, −30, −10, 30, 60, 90, 120, 160, and 180 min relative to the start of the insulin infusion. Plasma was separated within 20 min and stored at −80°C.
Laboratory analysis.
Glucose was measured by glucose oxidase (Beckman Glucose Analyzer II; Beckman, Brea, CA). Insulin was measured by a radioimmunoassay (Novo Pharmaceuticals, Danbury, CT) that measured insulin and proinsulin. Plasma free fatty acids (FFAs) were measured by an enzymatic colorimetric method (WAKO Chemicals, Richmond, VA). Plasma adiponectin and leptin levels were measured using radioimmunoassay kits from Linco Research. Plasma C-reactive protein (CRP) and interleukin (IL)-6 were measured using CRP enzyme-linked immunosorbent assays and ultrasensitive IL-6 enzyme-linked immunosorbent assays kits from ALPCO Diagnostics. 6,6, 2H2-glucose concentrations in infusates and perchloroacetate (PCA) supernatants of plasma were measured by gas chromatography and mass spectrometry after conversion of glucose to its aldonitrile penta-acetate derivative. Anti–pancreatic islet cell antibodies in plasma were measured in the laboratory of Dr. Jerry Palmer by an indirect immunofluorescence assay using human pancreas.
Data analysis.
BMI was calculated as weight in kilograms divided by the square of height in meters. Diabetes was diagnosed by oGTTs using the American Diabetes Association criteria (7). ivGTT results were analyzed using the MINMOD program (8) to obtain measures of fractional glucose disappearance due to an increase in insulin above basal (insulin sensitivity; SI). The acute insulin response to intravenous glucose (AIRg) was calculated by the trapezoid rule as the incremental area under the insulin curve during the first 10 min after the glucose injection. The product of SI and AIRg (the disposition index; DI) was calculated as a measure of acute pancreatic β-cell compensation for insulin resistance (8). Body fat and fat-free mass were calculated by the formula of Kotler et al. (9) using height, weight, and bioelectrical impedance analysis measurements.
Follow-up data were used up to the diagnosis of diabetes or the last visit without diabetes, whichever occurred first. Cumulative diabetes incidence rates were estimated using life-table methodology and displayed using Kaplan-Meier plots. Cox proportional hazard regression was used to test for associations between baseline and/or follow-up variables and time to diabetes development. Baseline variables were treated as fixed covariates and follow-up variables were treated as time-dependent covariates. The baseline measures included all variables listed in Table 1. Follow-up measures included changes from baseline in body weight and fat and disposition index, the presence or absence of additional pregnancies, and use of hormonal contraception. The proportional hazard assumption was evaluated by testing for interaction between the covariate and time in the model; no significant violation was found for the tested variables.
TABLE 1.
Baseline characteristics
| Variable | Median (interquartile range) |
|---|---|
| Age (years) | 32.2 (28.2–36.4) |
| BMI (kg/m2) | 30.7 (27.8–32.8) |
| Systolic blood pressure (mmHg) | 111 (104–119) |
| Diastolic blood pressure (mmHg) | 69 (64–74) |
| Body percent fat (%)* | 44.0 (40.7–48.4) |
| Fasting total cholesterol (mg/dl) | 169 (155–202) |
| Fasting HDL cholesterol (mg/dl) | 38 (32–44) |
| Fasting LDL cholesterol (mg/dl) | 110 (93–129) |
| Fasting triglycerides (mg/dl) | 127 (82–170) |
| Fasting free fatty acid (μmol/l) | 468 (382–570) |
| Fasting adiponectin (ng/ml) | 6,034 (4,584–7,362) |
| Fasting CRP (ng/ml) | 28.9 (14.2–57.3) |
| Fasting leptin (ng/ml) | 11.3 (8.7–13.7) |
| Fasting IL-6 (pg/ml) | 2.4 (1.5–3.2) |
| oGTT† | |
| Fasting glucose (mg/dl) | 95.0 (90.5–104) |
| 2-h glucose (mg/dl) | 142.5 (119.5–166) |
| Glucose total area (AUC: mg/dl per min/1,000) | 25.0 (22.4–28.6) |
| Fasting insulin (μU/ml) | 17.5 (12.5–25) |
| 2-h insulin (μU/ml) | 110 (77–178) |
| Insulin total area (μU/ml per min/1,000) | 18.1 (13.2–25.3) |
| 30 Δinsulin (μU/ml)‡ | 80.5 (54.5–110) |
| ivGTT | |
| Insulin sensitivity (SI; min/μU/ml × 10−4)§ | 1.27 (0.95–2.0) |
| Acute insulin response (AIRg, μU/ml × min)‖ | 509 (304–843) |
| Disposition index (DI: SI × AIRg)¶ | 834 (423–1,320) |
| Hyperinsulinemic-euglycemic clamp** | |
| Basal glucose production (HGO: mmol/min/m2) | 0.38 (0.33–0.42) |
| Basal glucose clearance (Clrglu: 100 × ml/min/m2) | 7.11 (6.43–7.79) |
| Steady-state glucose infusion rate (Ginf:mmol/min/m2) | 0.70 (0.57–0.79) |
| Steady-state ΔHGO (mmol/min/m2)†† | −0.27 (−0.23 to −0.30) |
| Steady-state ΔClrglu (100 × ml/min/m2)†† | 10.1 (7.6–12.9) |
To convert the glucose in unit of mg/dl to the SI unit of mmol/l, multiply the number in the table by 0.0555.
*Estimated by bioelectrical impedance.
†During 75-g oGTTs.
‡Calculated as 30 min insulin − fasting insulin during oGTT.
§Calculated by minimal model analysis of ivGTT insulin and glucose data.
‖Incremental insulin area during first 10 min of ivGTT.
¶A measure of β-cell compensation for insulin resistance.
**Basal values are the means of data collected during the last 90 min of the 3-h basal tracer infusion period, and steady-state values are the means of data collected during the final 30 min of the 3-h euglycemic insulin infusion period.
††Calculated as steady state-basal.
All statistical tests were two-sided, and statistical significance was defined as P ≤ 0.05.
RESULTS
Baseline characteristics.
A total of 72 women met the inclusion criteria for this report. Sixty women had their baseline visits at 15 months postpartum and 12 had their baseline visits at 30 months. At baseline, 26 women had normal glucose levels, 8 had impaired fasting alone, 19 had impaired 2-h glucose alone, and 19 had impaired fasting and 2-h glucose levels using American Diabetes Association criteria (7). Baseline characteristics are summarized in Table 1.
Follow-up.
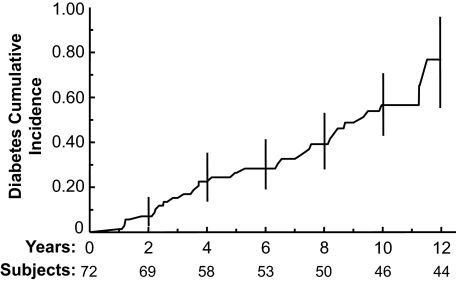
The average annual rate of loss to follow-up was 6.5%. During a median follow-up of 72 months (minimum 12 months; maximum 142 months), 31 (43%) of the women developed type 2 diabetes. The average annual incidence rate of diabetes, calculated by person-years, was 7.2%. The annual diabetes incidence rates were 4.1, 6.9, 7.0, and 14.8% in women whose oGTT values at baseline were, respectively, normal, impaired fasting alone, impaired 2-h alone, and impaired fasting and 2-h glucose together. The Kaplan-Meier plot of diabetes cumulative incidence rates appears in Fig. 1.
FIG. 1.
Kaplan-Meier plot of diabetes cumulative incidence rate in 72 women without diabetes at entry and with at least one follow-up oGTT. Vertical lines are 95% CIs. The numbers given by the Subjects line included subjects who developed diabetes and who were under follow-up without diabetes by the corresponding follow-up years.
Weight and glucose increased significantly during follow-up, while acute insulin secretion (AIRg) and the disposition index (DI) fell significantly (Table 2). Twenty women experienced a total of 23 pregnancies. Nine women used combination oral contraceptives exclusively for a median of 21 months (range 2–67). Eight women used progestin-only contraception exclusively for a median of 15 months (3–40). One woman started with a progestin-only contraceptive, was off from any hormonal contraception, and then switched to combination oral contraceptives.
TABLE 2.
Rates of change during follow-up*
| Variables | Median (interquartile range) |
|---|---|
| Weight (kg/year) | 0.69 (0.18–1.25)† |
| Fasting glucose (mg/dl/year) | 1.3 (0.3–3.4)† |
| oGTT 2-h glucose (mg/dl/year) | 4.6 (1.7–12.9)† |
| ivGTT SI (unit/year) | 0.0 (−0.12 to 0.08) |
| ivGTT AIRg (unit/year) | −24.3 (−72.8 to 0.0)† |
| ivGTT DI (unit/year) | −41.6 (−106 to 0.0)† |
To convert the glucose in unit of mg/dl to the SI unit of mmol/l, multiply the number in the table by 0.0555.
*Variables are defined in Table 1.
†P < 0.0001 vs. zero using random coefficient mixed-effects regression models. SI, AIRg, and DI were log transformed for statistical testing.
Factors associated with development of diabetes.
Univariate Cox regression analysis using each of the baseline variables presented in Table 1 revealed that relatively high glucose, relatively low insulin sensitivity, relatively low insulin responses, and relatively low β-cell compensation for insulin resistance were associated with development of diabetes (Table 3). Multivariate Cox regression analysis using all baseline variables revealed four that were significantly associated with development of diabetes (Table 4). The strongest was β-cell compensation for insulin resistance, expressed as the disposition index from ivGTTs. Women with the lowest disposition index had the highest risk of developing diabetes. The other baseline predictors identified from multivariate analysis were insulin sensitivity, measured as incremental glucose clearance during hyperinsulinemic clamps (low = increased risk), total glucose area during oGTTs (high = increased risk), and the 30-min increment in insulin on oGTTs (low = increased risk).
TABLE 3.
Univariate significant baseline predictors of diabetes
| Variables* | HR (95% CI)† | P value |
|---|---|---|
| Glucose | ||
| oGTT fasting | 1.49 (1.03–2.17) | 0.03 |
| oGTT 2-h | 1.82 (1.24–2.67) | 0.002 |
| oGTT total area | 2.23 (1.52–3.27) | <0.0001 |
| Insulin sensitivity | ||
| ivGTT SI‡ | 0.61 (0.38–0.99) | 0.047 |
| Clamp Ginf‡ | 0.53 (0.36–0.77) | 0.001 |
| Clamp ΔClrglu | 0.50 (0.32–0.77) | 0.002 |
| β-Cell function | ||
| ivGTT AIRg‡ | 0.38 (0.24–0.61) | <0.0001 |
| ivGTT DI‡ | 0.27 (0.16–0.46) | <0.0001 |
*Variables and units are defined in Table 1.
†By Cox regression, expressed as per 1-SD unit increase.
‡Variables were log transformed; SDs were calculated using log-transformed data.
TABLE 4.
Multivariate significant baseline predictors of diabetes*
| Variables† | Adjusted HR‡ 95% CI | Adjusted P value |
|---|---|---|
| ivGTT DI§ | 0.41 (0.24–0.71) | 0.001 |
| Clamp ΔClrglu | 0.50 (0.29–0.84) | 0.01 |
| oGTT total area | 1.68 (1.05–2.66) | 0.028 |
| oGTT 30 Δinsulin§ | 0.60 (0.38–0.96) | 0.034 |
*P < 0.05 on multivariate Cox regression analysis considering all baseline variables listed in Table 1.
†Variables and units are defined in Table 1.
‡By Cox regression adjusting for other variables in the table, expressed per 1-SD unit increase.
§Variables were log transformed; SDs were calculated using log-transformed data.
Once the effects of these baseline variables were considered, three clinical variables assessed during follow-up provided additional and independent information about the risk of diabetes (Table 5; baseline and follow-up variables in final multivariate-adjusted model). Weight change (gain = increased risk, adjusted P = 0.021) was significantly associated with diabetes risk. Change in body fat, assessed by bioelectrical impedance, gave results that were very similar to change in body weight (hazard ratio [HR] 1.68, adjusted P = 0.04). Additional pregnancy (increased risk, adjusted P = 0.085) and use of progesterone-only contraception (increased risk versus other hormonal and nonhormonal forms, adjusted P = 0.068) were marginally associated with diabetes risk despite the limited statistical power associated with the infrequency of these events.
TABLE 5.
Multivariate assessment of significant baseline and clinical variables during follow-up in relation to development of diabetes*
| Variables | Adjusted HR (95% CI)† | Adjusted P value |
|---|---|---|
| Baseline‡ | ||
| ivGTT DI§ | 0.31 (0.17–0.54) | <0.0001 |
| Clamp ΔClrglu | 0.50 (0.28–0.89) | 0.018 |
| oGTT total area | 2.03 (1.21–3.39) | 0.007 |
| oGTT 30 Δinsulin§ | 0.61 (0.37–1.02) | 0.059 |
| Follow-up‖ | ||
| Weight change (per 5 kg) | 1.67 (1.08–2.57) | 0.021 |
| Additional pregnancy (yes/no) | 1.77 (0.92–3.44) | 0.085 |
| Progestin-only method (yes/no) | 4.28 (0.90–20.4) | 0.068 |
*Using multivariate Cox regression analysis where baseline variables from Table 4 and follow-up clinical variables were included in the model.
†By Cox regression adjusting for other variables in the table; for baseline variables, they were expressed per 1-SD unit increase; for follow-up variables, they were expressed per 5-kg increase for weight change and yes/no for additional pregnancy and progestin contraception use.
‡Variables and units are defined in Table 1.
§Variables were log transformed; SDs were calculated using log-transformed data.
‖All three follow-up variables were treated as time-dependent variables in the Cox regression analysis.
We also examined change from baseline in β-cell compensation (DI) from ivGTTs for association with development of diabetes. Change in disposition index on the log scale was significantly associated with diabetes development (HR 0.73 [95% CI 0.61–0.88], P = 0.001). Adjustment for baseline disposition index decreased the level of association, but the association remained statistically significant (adjusted HR 0.83 [95% CI 0.69–0.99], P = 0.045). Thus, the rate of decline in disposition index had an impact on diabetes risk beyond the risk associated with low disposition index at baseline. Further adjustment for the other three significant baseline variables had almost no impact on the relative hazard estimate for change in disposition index. Adjustment for weight change during follow-up reduced the HR to 0.94 (95% CI 0.77–1.14, P = 0.51).
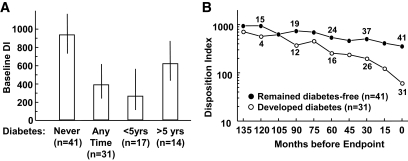
The left panel of Fig. 2 depicts disposition index at baseline, independent of time before diabetes. Baseline disposition index in women who developed diabetes at any time during follow-up was only 41% of disposition index in women who remained diabetes free (geometric means [95% CIs] 384 [239–616] versus 931 [737–1,173]; P = 0.002). Among women who developed diabetes, baseline disposition index was lowest in those whom diabetes developed within 5 years of the index pregnancy (261 [119–569]) and intermediate in those whom diabetes developed more than 5 years after the index pregnancy (615 [436–886]). The right panel of Fig. 2 depicts the course of the disposition index relative to the study end point of diabetes or, in women who remained diabetes-free, completion of follow-up. This format is important to allow visualization of patterns of change with minimal impact of the degree of deterioration at baseline. It is analogous to assessing changes in disposition index by biological rather than chronological age. The plot demonstrates that disposition index fell more rapidly in women who developed diabetes (Fig. 2, right). Note that the number of people in the “developed diabetes” group (Fig. 2, right) is relatively small on the left of the graph because only a few individuals who developed diabetes took the full 120–135 months to do so.
FIG. 2.
A: Baseline disposition index and 95% CIs according to final diabetes status during the entire follow-up (left two bars) or within 5 years and >5 years after the index pregnancy (right two bars). B: Disposition index during follow-up in women who developed diabetes and in women who remained diabetes free. Data are plotted relative to end points of diabetes or final visit without diabetes. Numbers shown above/below the lines are the corresponding sample sizes. Log scale is depicted to reflect that data were log transformed prior to all data analysis; geometric means are presented.
DISCUSSION
This study provides the longest follow-up of which we are aware that used detailed physiological measurements to characterize the natural history of glucose regulation following pregnancies complicated by GDM. Two general types of physiological variables were associated with development of type 2 diabetes: the degree of metabolic deterioration at baseline (low insulin sensitivity and β-cell function, high glucose levels) and the rate of deterioration thereafter (falling β-cell compensation for insulin resistance). To our knowledge, this is the first demonstration that both the degree of deterioration after pregnancy and the rate of deterioration thereafter contribute to the risk of diabetes. Our findings are consistent with the concept (4) that GDM most commonly represents detection of a chronic condition (i.e., low and falling β-cell compensation for chronic insulin resistance) rather than development of an acute condition (i.e., inability to compensate for acquired insulin resistance) during pregnancy. That concept is strongly supported by serial studies of insulin resistance and β-cell compensation in women who develop GDM. Those studies reveal that the large majority of the β-cell defect observed during the third trimester is present before (2) and after (3,4) pregnancy. Moreover, women with GDM increase insulin secretion in parallel to normal women during pregnancy (4), but they do so along a sensitivity-section curve that is characterized by inappropriately low insulin secretion for any degree of insulin resistance. Thus, from the physiological standpoint, it appears that GDM is most often a chronic disease characterized by insulin resistance and falling β-cell compensation that is simply detected by routine glucose screening during pregnancy.
In addition to the physiological variables, three clinical variables provided information about the risk of diabetes after GDM. Weight gain was the strongest, consistent with prior clinical observations of shorter duration (10). Weight gain is a known risk factor for diabetes; it can worsen insulin resistance and β-cell function as demonstrated previously (11–13). Indeed, adjustment for weight gain explained part of the association between falling β-cell compensation and diabetes risk in this study, suggesting that the impact of falling compensation on diabetes risk may have been mediated at least in part through weight gain. Gain in body fat gave similar results to gain in weight, suggesting that increased adiposity is the important component of weight gain accentuating diabetes risk. Evidence for association between diabetes and additional pregnancy or progesterone-only contraception was statistically marginal in this study, where only 20 women had one or more additional pregnancies and 8 women used progesterone-only contraception. However, the point estimates for risk were substantial (1.77 for additional pregnancy and 4.28 for progestin-only contraception). Moreover, we have previously found both events to be significantly associated with diabetes risk in a much larger clinical cohort of women with prior GDM (10,14,15). The present report adds validity to those prior findings and demonstrates that the risks occur independently of baseline glucose levels, insulin resistance, β-cell function, and weight gain. These three variables represent potentially modifiable risk factors for diabetes after GDM.
Taken together, our findings in Hispanic women suggest the following scenario for development of diabetes after GDM. Chronic glucose intolerance is detected by routine glucose screening during pregnancy. Women who have that intolerance are at different stages in progression toward diabetes, in part because they are deteriorating at different rates. Women who are the most insulin resistant also have the worst β-cell function and highest glucose levels. They are closest to diabetes and develop it soonest, as indicated by the Cox regression analysis for baseline predictors of diabetes. Faster deterioration is also associated with diabetes, even after adjustment for where women are at baseline. Gaining weight, becoming pregnant again, and using progesterone-only contraception can accelerate development of diabetes after GDM. Whether there is a common mechanism underlying the effects of these factors on β-cell compensation remains to be determined. They are all modifiable and they represent, along with amelioration of insulin resistance (16–17), potential clinical approaches to reducing diabetes risk after GDM.
In summary, using the longest physiological follow-up of women with prior GDM available to date, we found important differences in both degrees and rates of metabolic deterioration in our exclusively Hispanic cohort. The differences, along with the occurrence of weight gain, an additional pregnancy, and use of progestin-only contraception, had important associations with the risk of developing diabetes during more than a decade of follow-up. Our findings provide three potentially modifiable clinical risk factors for diabetes in this high-risk group. They also suggest that genetic and environmental determinants of rates of change in β-cell compensation for chronic insulin resistance should be an important focus of research to understand the pathophysiology of both GDM and type 2 diabetes that so often follows it.
ACKNOWLEDGMENTS
This work was supported by grants R01-DK-46374 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); M01-RR-43 from the Division of Clinical Research, National Center for Research Resources, NIH; and a Distinguished Clinical Scientist Award from the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
A.H.X. researched data, wrote the manuscript, and contributed to the discussion. S.L.K. reviewed the manuscript. M.T. researched data. E.T. collected and researched data. T.A.B. researched data, edited the manuscript, and contributed to the discussion.
The authors acknowledge the following individuals from the University of Southern California: Susie Nakao, Carmen Martinez and thank the staff of the General Clinical Research Center for assistance with metabolic studies and Lilit Barolikian for performance of insulin and glucose assays.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 2. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 3. Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab 2001;86:568–573 [DOI] [PubMed] [Google Scholar]
- 4. Buchanan TA, Xiang AH, Kjos SL, Watanabe RM. What is gestational diabetes? Diabetes Care 30(Suppl. 2):S105–S111, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 6. Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes mellitus. Diabetes 1999;48:848–854 [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- 8. Bergman RN. Toward a physiological understanding of glucose tolerance: minimal model approach. Diabetes 1989;38:1512–1528 [DOI] [PubMed] [Google Scholar]
- 9. Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr 1996;64(Suppl. 3):S489–S497 [DOI] [PubMed] [Google Scholar]
- 10. Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of a single pregnancy in women with prior gestational diabetes mellitus. Lancet 1996;347:227–230 [DOI] [PubMed] [Google Scholar]
- 11. Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining β-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weyer C, Hanson K, Bogardus C, Pratley R. Long-term changes in insulin action and insulin secretion associated with weight gain, loss, regain and maintenance of body weight. Diabetologia 2000;43:36–46 [DOI] [PubMed] [Google Scholar]
- 13. Kriketos AD, Carey DG, Jenkins AB, Chisholm DJ, Furler SM, Campbell LV. Central fat predicts deterioration of insulin secretion index and fasting glycemia: 6-year follow-up of subjects at varying risk of type 2 diabetes. Diabet Med 2003;294–300 [DOI] [PubMed] [Google Scholar]
- 14. Kjos SL, Peters RK, Xiang A, Thomas D, Schafer U, Buchanan TA. Oral contraception and the risk of type 2 diabetes in Latino women with prior gestational diabetes. JAMA 1998;280:533–538 [DOI] [PubMed] [Google Scholar]
- 15. Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006;29:613–617 [DOI] [PubMed] [Google Scholar]
- 16. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic B-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 17. Xiang A, Peters RK, Kjos SL, Marroqiun A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]