Abstract
OBJECTIVE
Obesity and type 2 diabetes are national and worldwide epidemics. Because currently available antiobesity and antidiabetic drugs have limited efficacy and/or safety concerns, identifying new medicinal agents, such as ginsenoside Rb1 (Rb1) as reported here, offers exciting possibilities for future development of successful antiobesity and antidiabetic therapies.
RESEARCH DESIGN AND METHODS
Changes in feeding behavior after acute intraperitoneal administration of Rb1 and the effects of intraperitoneal Rb1 for 4 weeks on body weight, energy expenditure, and glucose tolerance in high-fat diet (HFD)-induced obese rats were assessed. We also examined the effects of Rb1 on signaling pathways and neuropeptides in the hypothalamus.
RESULTS
Acute intraperitoneal Rb1 dose-dependently suppressed food intake without eliciting signs of toxicity. This inhibitory effect on feeding may be mediated by central mechanisms because Rb1 stimulated c-Fos expression in brain areas involved in energy homeostasis. Consistent with this, Rb1 activated the phosphatidylinositol 3-kinase/Akt signaling pathway and inhibited NPY gene expression in the hypothalamus. Four-week administration of Rb1 significantly reduced food intake, body weight gain, and body fat content and increased energy expenditure in HFD-induced obese rats. Rb1 also significantly decreased fasting blood glucose and improved glucose tolerance, and these effects were greater than those observed in pair-fed rats, suggesting that although Rb1's antihyperglycemic effect is partially attributable to reduced food intake and body weight; there may be additional effects of Rb1 on glucose homeostasis.
CONCLUSIONS
These results identify Rb1 as an antiobesity and antihyperglycemic agent.
Obesity has reached epidemic proportions and created serious public health problems in the U.S. Obesity is a major risk factor for type 2 diabetes, cardiovascular problems, and some forms of cancer. Although efforts to address the environmental and genetic factors responsible for the “epidemic” must continue, and because currently available antiobesity and antidiabetic drugs have limited efficacy and/or safety concerns, developing safe and effective medicinal agents, particularly with the dual properties of controlling body weight and reducing blood glucose, offers exciting possibilities for developing successful therapies.
Panax ginseng is a highly valued herb in much of the world, and the root of the ginseng plant has been used as an herbal remedy in Eastern Asia for over 2000 years. The word “panax” is derived from “panacea,” which means cure-all and longevity. Ginseng and its constituents have historically been ascribed as general tonics and adaptogens to maintain homeostasis and the body's resistance to adverse factors. The efficacy of ginseng was recognized in the West by the 18th century, and now ginseng is one of the most popular and top-selling herbals in the U.S. (1,2). Modern pharmacological studies indicate that ginseng extracts possess a wide range of effects, including enhancing learning and memory, reducing anxiety, reducing cancer risk, and improving immunomodulation (3–6).
Chronic administration of an extract of ginseng root significantly reduced body weight, fat mass, and serum lipids in diet-induced obese rats (7,8), and extracted ginseng has also been reported to ameliorate hyperglycemia in animals and humans (9–12). In one report, both diabetic and nondiabetic subjects taking an extract of ginseng had stabilized postprandial glycemia, suggesting that ginseng may also benefit healthy individuals (13). A shortcoming of the existing literature is that the specific compound(s) within extracted ginseng that have beneficial actions are unknown.
Although the components in ginseng root vary across sources and species, ginsenoside Rb1 (Rb1) is considered the most abundant and most important active factor (14). Rb1 has been well characterized chemically (15); it has the structure of a tetracyclic triterpenoid with a molecular formula of C54H92O23 and molecular weight of 1,109.26 (16). Rb1 has been reported to have diverse biological activities, including facilitating acquisition and retrieval of memory (17), scavenging free radicals (18), blocking calcium over-influx into neurons (19), and preserving the structural integrity of the neurons (20). Rb1 also increased glucose uptake and suppressed lipid accumulation in 3T3-L1 adipocytes and enhanced insulin secretion from pancreatic Min6 cells (21–23). Based on these reports, we hypothesized that Rb1 has potent antiobesity and antidiabetic effects.
RESEARCH DESIGN AND METHODS
Animals.
Adult male Long-Evans rats (Harlan, Indianapolis, IN) were individually housed in a temperature-controlled vivarium on a 12/12-h light/dark cycle (lights on at 0000 h, lights off at 1200 h). Laboratory chow (Purina 5001, Hudson, NH) was provided ad libitum except where noted. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Materials.
Rb1 purified by high-performance liquid chromatography (HPLC) was purchased from Jilin University in China. High-performance liquid chromatography (Shimadzu, Kyoto, Japan) analysis was performed in our laboratory and confirmed that the Rb1 had a purity of ≥98% using an Rb1 standard obtained from LKT laboratories (St. Paul, MN). The antibody against c-Fos was from Chemicon (Millipore, Billerica, MA), and antibodies for p-Akt and Akt were from Cell Signaling Technology (Beverly, MA).
Food intake in ad libitum–fed and fasted rats.
Two studies were conducted to determine the effects of acute administration of Rb1 on food intake. In the first study, ad libitum–fed rats received intraperitoneal Rb1 in saline at doses of 0, 2.5, 5, 10, or 20 mg/kg; in the second study, 24-h–fasted rats received intraperitoneal Rb1 at a dose of 10 mg/kg. The Rb1 or saline was administered at the beginning of dark, and food intake was recorded after 2, 4, 6, 8, 10, and 24 h.
Conditioned taste aversion assessment.
To determine whether Rb1 elicits visceral illness, we used a standard two-bottle preference paradigm (24,25). Rats (n = 24) were adapted to 2-h daily access to water (available in two bottles) for 5 days. On day 6, rats were given 0.15% saccharin in water in each bottle for the 2-h period instead of water and then were injected with either Rb1 (40 mg/kg i.p.) or vehicle (n = 8/group). A third group (n = 8) received LiCl as a positive control (0.15 mol/l; 127 mg/kg i.p.). On day 7, animals had water in both bottles during the 2-h session, and on the test day (day 8), rats were presented with one bottle containing saccharin and one containing water for 24 h. Saccharin preference ratio was calculated as the amount of saccharin consumed divided by the total consumption of both liquids over 2 and 24 h.
c-Fos expression in the brain.
After a 4-h light-phase food restriction, animals received intraperitoneal Rb1 (10 mg/kg) or saline at the onset of dark. No food was provided after injection. Rats were anesthetized and perfused with 4% paraformaldehyde for c-Fos immunostaining 2 h after the injections using a previously published method (26). Free-floating sections (30 μm) were cut through the regions of the brainstem and the hypothalamus (27) and incubated with primary rabbit anti–c-Fos antibody (1:10,000, overnight at 4°C), biotinylated donkey anti-rabbit antibody (Vector, 1:300, 2 h), and avidin-biotin-peroxidase complex (Vector, 2 h) with PBS wash in-between. The sections were then developed by diaminobenzidine with nickel sulfate to produce a dark-purple reaction product. Specific staining was abolished by omission of primary antiserum.
The number of c-Fos–positive cells was quantified by a blind observer in the following areas: nucleus of the solitary tract (NTS) (−13.80 from bregma) and the hypothalamic arcuate (ARC) (−2.30 from bregma), ventromedial (VMH) (−2.56 from bregma), and lateral nuclei (LH) (−1.88 from bregma). For each animal, the number of c-Fos–positive cells was an average value of four to five sections. Data for c-Fos activation in all of these areas were bilaterally assessed under a light microscope with low magnification (×40) and were presented as the total number per section.
High-fat diet–induced obese rats.
A total of 36 rats were fed with a high-fat diet (HFD) containing 20 g fat (19 g butter oil and 1 g soybean oil)/100 g diet prepared by Research Diets (category number D03082706; New Brunswick, NJ) for 13 weeks, as described previously (28). The rats became obese (mean body weight 596 g) and were divided into three groups of comparable body weight. Groups 1 and 2 received intraperitoneal saline or Rb1 (10 mg/kg), respectively, daily for 28 days and had free access to the same HFD. Because Rb1-treated rats had reduced food intake, an HFD pair-fed group was included as a control. These rats were given the HFD but with an amount limited to the average daily consumption of Rb1-treated rats. Food intake and body weight were recorded daily, and energy expenditure and glucose tolerance were assessed at specified times. At the end of the experiment, the rats were killed after a 6-h fast, and blood and tissues were collected and stored at −80°C for further analyses as detailed below.
Measurement of intestinal fat absorption.
A noninvasive method was used to measure intestinal fat absorption (29). Feces were isolated from three rats in each group on day 24 of Rb1 treatment, and the fatty acids in the feces were analyzed and quantified with a heptadecanoic acid standard by gas chromatography (29).
Measurement of plasma samples.
Plasma triglyceride and cholesterol levels were measured using Infinity reagent (Thermo, Middletown, VA). Free fatty acid content was measured using NEFA reagent (Wako, Richmond, VA). Leptin was measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Millipore, St. Charles, MO). The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine were assessed with VITROS Chemistry Products Calibrator Kit 3 and VITROS Chemistry Systems (Ortho-Clinical Diagnostics, Buckinghamshire, U.K.).
Indirect calorimetry.
Energy expenditure was assessed with a Physioscan Open Circuit Calorimeter System (Accuscan Instruments, Columbus, OH). The rats were maintained on their respective diet regimens and placed in individual metabolic chambers for 24 h (24). Data were sampled every 60 s.
Body fat/lean composition determination.
Body fat was assessed by nuclear magnetic resonance (NMR) (EchoMRI; EchoMedical Systems, Houston, TX). Unanesthetized rats were placed in a restraint tube and inserted into the nuclear magnetic resonance, providing estimates of total lean tissue, fat tissue, and water.
Histological analysis and morphometry.
Adipose tissue was fixed in 10% formalin for 1 month and then embedded with paraffin. Tissue sections (10 μm) were cut with a microtome (Leica, Germany) and mounted on superfrost/plus microscope slides. After being air-dried, they were stained with hematoxylin and eosin and photographed at 100× magnification. At least two fields per slice and six slices per fat mass were analyzed for the purpose of quantifying adipocyte size.
Intraperitoneal glucose tolerance test.
On day 28 of treatment, rats were fasted overnight and given intraperitoneal glucose (1 g/kg, at 1000 h). Blood samples were taken from the tail vein, and glucose was assessed with a glucometer (Freestyle; Abbott Diabetes Care, Alameda, CA) at 0 (fasting), 15, 30, 60, 120, and 180 min after glucose administration. Plasma insulin was measured in the same samples with a rat insulin ELISA kit (Millipore).
Assessment of the phosphatidylinositol 3-kinase signaling pathway.
Primary neuronal cells, prepared from hypothalami of embryos and cultured as previously described (30), were treated with different concentrations of Rb1 (0.1–100 μmol/l) for 4 h, or with 10 μmol/l Rb1 for a range of times (0–4 h). The proteins from the cultured neuronal cells, as well as the proteins from the hypothalamus of HFD-induced obese rats with different treatments, were then extracted for measuring phosphorylated Akt (p-Akt) and total Akt levels by Western immunoblotting as described previously (30).
qPCR for neuropeptide mRNA measurement.
Total RNA (100 ng), extracted from hypothalami of HFD-induced obese rats with different treatments using Tri Reagent (Molecular Research Center, Cincinnati, OH), was reverse-transcribed to first-strand complementary DNA (GE Healthcare Bio-Sciences, Piscataway, NJ). Quantitative real-time PCR (qPCR) was performed in a 25-μl final reaction volume with an iCycler iQ Detection System using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). qPCR conditions and sequences of the primers for measuring mRNA levels of the neuropeptides, including proopiomelanocortin (POMC), neuropeptide Y (NPY), and agouti-related protein (AgRP), were described previously (24).
Statistical analysis.
Data were analyzed using parametric statistics (Sigma Stat version 3.1). Two-way ANOVA and two-way repeated-measures ANOVA followed by the Student-Newman test were used for analysis of food intake, body weight, energy expenditure, blood glucose, and plasma insulin levels. For other comparisons, one-way ANOVA was followed by the Student-Newman test. Data are presented as means ± SE. Values of P < 0.05 were considered significant.
RESULTS
Acute Rb1 administration inhibited food intake.
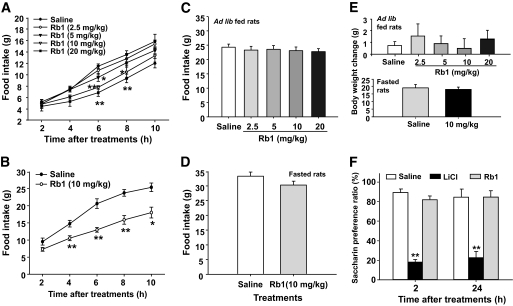
Intraperitoneal Rb1 at doses ≥5 mg/kg significantly reduced food intake in ad libitum–fed rats (Fig. 1A). In a subsequent experiment, intraperitoneal Rb1 (10 mg/kg) significantly decreased food intake in 24-h–fasted rats (Fig. 1B). This effect was present at 4 h and lasted for at least 6 h more. There were no significant changes of food intake or body weight by 24 h after Rb1 treatment in any condition (Fig. 1C, D, and E).
FIG. 1.
Intraperitoneal administration of Rb1 dose-dependently reduced food intake in ad libitum–fed rats (A). In 24-h–fasted rats, Rb1 (10 mg/kg i.p.) significantly reduced food intake (B). No significant differences in food intake (C and D) or body weight change (E) were found in either ad libitum–fed or fasted rats after Rb1 treatment. Intraperitoneal administered LiCl, but not Rb1 (40 mg/kg i.p.), caused a conditioned taste aversion assessment in rats (F). Values are expressed as means ± SE; n = 6–8. *P < 0.05, **P < 0.01 vs. saline controls.
Reduction of food intake induced by Rb1 was not associated with illness.
Rats that received saccharin paired with Rb1 (40 mg/kg i.p.) had comparable preference for saccharin as saline controls at both 2 and 24 h after treatments. In contrast, animals that received LiCl consumed significantly less saccharin than controls (Fig. 1F).
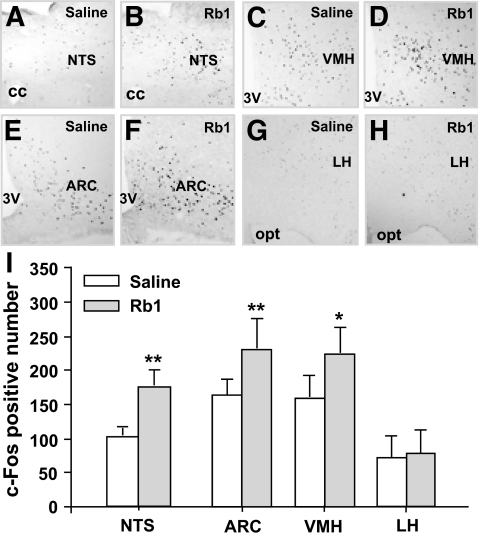
Rb1 induced brain c-Fos expression.
Rb1 significantly induced c-Fos expression in the hindbrain NTS and the ARC and VMH in the hypothalamus, but not in the LH (Fig. 2).
FIG. 2.
Rb1 (10 mg/kg i.p.) significantly increased c-Fos expression in the cells of NTS, VMH, and ARC, but not LH, areas. A–H: Images of representative brain microsections. I: Fos-like immunoreactivity counts (mean ± SE) in difference brain sections; n = 4–5, *P < 0.05, **P < 0.01 vs. saline controls. 3V, third ventricle; cc, central canal; opt, optic tract.
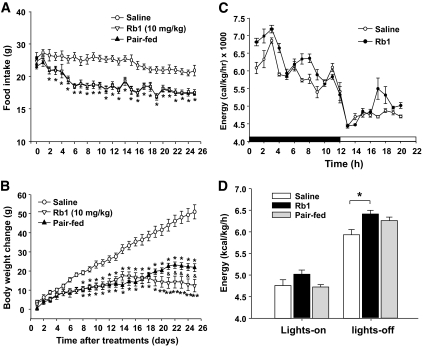
Rb1 reduced food intake and body weight gain in HFD-induced obese rats.
When Rb1 (10 mg/kg) was administered intraperitoneally in HFD-induced obese rats, it gradually and significantly decreased food intake during the first week, and the reduced food intake was apparent throughout the 25-day observation period (Fig. 3A). Body weight gain was significantly suppressed beginning on day 8 and continued until the end of the experiment (Fig. 3B). Body weight change was comparable between Rb1-treated rats and HFD pair-fed rats during the first 20 days of treatment. However, beginning after the third week, the weight of the Rb1-treated rats became significantly reduced relative to that of the pair-fed rats (Fig. 3B). No side effects, such as diarrhea, were observed. In addition, HFD-fed rats, with or without Rb1, had similar levels of AST, ALT, and creatinine (Table 1).
FIG. 3.
Chronic treatment of Rb1 in HFD-induced obese rats significantly reduced food intake (A), reduced body weight gain (B), and increased energy expenditure (C), especially during the dark phase (D), when compared with saline administration. Food intake after Rb1 treatment was significantly reduced compared with that after saline treatment since day 2, and the changes of body weight in Rb1-treated rats were significantly lower than that in saline controls since day 8. Rb1-treated rats also had significantly less body weight gain versus rats under pair-feeding since day 21. In addition, caloric restriction also has elevated energy expenditure, but the difference between saline and pair-fed group is not as prominent as the saline and Rb1 group. Data are means ± SE; n = 12. *P < 0.05, **P < 0.01 vs. saline controls; &P < 0.05, Rb1-treated vs. pair-fed rats.
TABLE 1.
Plasma levels of AST, ALT, and creatinine, and body fat and lean composition and lipid profiles in HFD-induced obese rats with or without Rb1 treatment
| Saline | Rb1 | Pair-fed | |
|---|---|---|---|
| AST (U/l) | 119.1 ± 13.7 | 125.0 ± 10.9 | 102.8 ± 10.6 |
| ALT (U/l) | 40.0 ± 5.8 | 37.1 ± 3.6 | 33.7 ± 3.5 |
| Creatinine (mg/dl) | 0.395 ± 0.04 | 0.404 ± 0.04 | 0.392 ± 0.04 |
| Fat mass (g) | 166.3 ± 7.8 | 133.6 ± 6.1* | 140.7 ± 6.2† |
| Lean mass (g) | 377.6 ± 9.3 | 382.9 ± 4.9 | 379.2 ± 7.6 |
| Brown adipose tissue (g) | 0.94 ± 0.07 | 0.92 ± 0.08 | 0.84 ± 0.05 |
| Water (g) | 338.1 ± 8.27 | 343.1 ± 4.47 | 339.8 ± 6.81 |
| Triglyceride (mg/dl) | 125.1 ± 12.71 | 92.4 ± 7.02† | 77.8 ± 7.57* |
| Cholesterol (mg/dl) | 100.6 ± 7.11 | 72.5 ± 6.0† | 80.8 ± 3.52 |
| Nonesterified fatty acids (mEq/l) | 0.442 ± 0.035 | 0.439 ± 0.023 | 0.371 ± 0.021 |
Data are means ± SE.
*P < 0.01,
†P < 0.05 vs. HFD obese rats treated with saline.
Rb1 increased energy expenditure in HFD-induced obese rats.
Energy expenditure measured by indirect calorimetry on day 26 of Rb1 treatment was significantly higher during the dark phase relative to saline controls (Fig. 3C and D). No difference in respiratory quotient occurred among the three groups (data not shown).
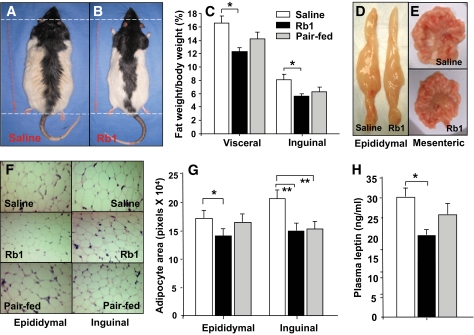
Rb1 reduced body fat in HFD-induced obese rats.
Consistent with the reduced body weight, Rb1-treated rats had reduced body fat compared with saline controls (Fig. 4B versus 4A). Whereas fat composition was slightly reduced in HFD pair-fed rats, only Rb1-treated rats had a significantly lower percentage of fat compared with controls. There were no differences in lean tissue mass, body water, or brown adipose tissue mass among groups (Table 1). To determine whether there was regionally specific fat loss, selected fat depots were excised and weighed. Rb1 treatment, but not pair-feeding, significantly decreased visceral fat, including epididymal, retroperitoneal, and mesenteric fat, as well as inguinal fat compared with saline controls (Fig. 4C, D, and E). Rb1-induced reduction of adipose tissue weight was mainly due to decreased adipocyte size, with adipocytes from epididymal and inguinal fat pads being significantly smaller in Rb1-treated rats than in the saline controls (Fig. 4F and G). The reduced body fat was mirrored by a concomitant fall in plasma leptin (Fig. 4H). Rb1 treatment also significantly reduced the levels of plasma triglyceride and cholesterol, but not nonesterified fatty acids (Table 1). There were no differences in these parameters between Rb1-treated and HFD pair-fed rats.
FIG. 4.
Effect of chronic administration of Rb1 on body fat and plasma leptin levels in HFD-induced obese rats. Whole body weight (A and B); visceral fat tissues, including epididymal, retroperitoneal, and mesenteric fat; and inguinal fat were significantly decreased after Rb1 treatment (C). D and E: Photos of epididymal and mesenteric fat in rats treated with Rb1 or saline. F: Hematoxylin and eosin staining of epididymal and inguinal fat tissues. The size of adipocytes was calculated and represented by total adipocyte area (n = 6). Rb1 treatment resulted in reduced average adipocyte size (G). Rb1 decreased plasma leptin (H). *P < 0.05; **P < 0.01 vs. saline controls. (A high-quality digital representation of this figure is available in the online issue.)
Rb1 did not affect lipid absorption in HFD-obese rats.
No significant differences in lipid content (%) of the feces were found between Rb1 and saline-treated rats (data not shown), indicating that Rb1 did not affect lipid absorption.
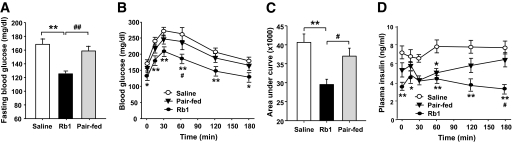
Rb1 ameliorated hyperglycemia and improved glucose tolerance in HFD-induced obese rats.
After 4 weeks of 10 mg/kg Rb1 treatment, fasting plasma glucose in HFD-induced obese rats was reduced (Fig. 5A), and glucose levels during the intraperitoneal glucose tolerance test were reduced by Rb1 all time points from 15 to 180 min (Fig. 5B). The area under the curve in Rb1-treated rats was decreased by 30% compared with vehicle controls (Fig. 5C). Hence, peripheral Rb1 is efficacious in ameliorating diabetic symptoms associated with obesity. There was also significantly lower fasting insulin in Rb1-treated rats, compared with saline controls (Fig. 5D). Fasting glucose levels and glucose tolerance were improved to a greater extent by Rb1 than by pair-feeding (Fig. 5A and C).
FIG. 5.
Rb1 reduced fasting blood glucose and improved glucose tolerance in HFD-induced obese rats. A: Basal hyperglycemia was significantly reduced in Rb1-treated obese rats, compared with both saline controls and pair-fed rats. B: After intraperitoneal administration of glucose (1 g/kg), the glucose incremental area under the curve was significantly lower in the Rb1 group than in the saline group. C and D: Rb1 treatment also reduced fasting insulin level and the insulin AUC after intraperitoneal glucose. Data are means ± SE; n = 12. *P < 0.05; **P < 0.01 vs. the saline group; #P < 0.05; ##P < 0.01 vs. the pair-fed group.
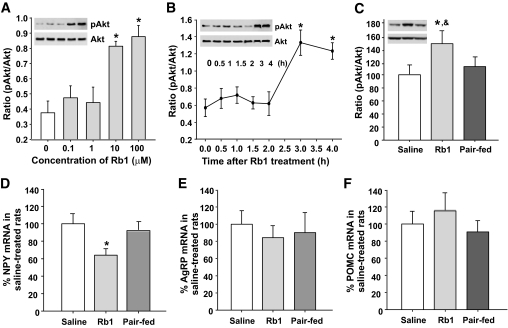
Rb1 activated the phosphatidylinositol 3-kinase/Akt signaling pathway in hypothalamus.
Phosphorylation of Akt (pAkt) in primary cultured neurons was stimulated by Rb1 at doses of ≥10 μmol/l (Fig. 6A), with the acute effect of Rb1 on phosphorylation of Akt beginning after 2 h (Fig. 6B). This effect was confirmed in HFD-induced obese rats, i.e., chronic Rb1 treatment, but not pair-feeding, significantly increased hypothalamic pAkt levels (Fig. 6C).
FIG. 6.
Effects of Rb1 on phosphorylation of Akt in cultured primary hypothalamic neurons and in the hypothalamus of HFD-induced obese rats. A: Cells were treated with different concentrations of Rb1. B: Cells were treated with Rb1 (10 μmol/l) for the indicated times. n = 3–4. C: Hypothalamic tissues were dissected from HFD-induced obese rats. D and E: Effect of chronic Rb1 treatment on neuropeptide gene expression in HFD-induced obese rats. n = 6–7. Data are means ± SE. *P < 0.05 vs. before treatment (in vitro) or saline controls (in vivo); &P < 0.05 vs. pair-fed rats.
Rb1 reduced NPY gene expression in HFD-induced obese rats.
Rb1 significantly reduced hypothalamic NPY mRNA (Fig. 6D), but not AgRP mRNA (Fig. 6E), levels compared with saline controls. Although there was a trend toward increased POMC gene expression after Rb1 treatment, it was not significant (Fig. 6F).
DISCUSSION
Ginseng root has historically been ascribed to be a general tonic or adaptogen to maintain the body's resistance to adverse factors and homeostasis. Modern studies have demonstrated that most of the pharmacological actions of extracted ginseng are attributed to ginsenosides (31), and >30 of them have been isolated (32). As a class of compounds, ginsenosides have been demonstrated to be useful in the control of body weight (7,8,11) and blood glucose (9–12,33–36). However, the complications caused by the multiple known and unknown ingredients in ginseng prevent its application to clinical usage as an alternative medicine to treat obesity and diabetes. The present studies extend previous observations and indicate that ginsenoside Rb1 may prove particularly important in treating obesity and diabetes.
We have demonstrated for the first time that acute intraperitoneal administration of Rb1 significantly suppresses food intake. This effect is dose dependent and does not elicit malaise, in that an effective dose of Rb1 did not cause a conditioned taste aversion. Hence, visceral illness is likely not a factor in the feeding suppressive actions of peripherally administered Rb1.
c-Fos expression has been used as an index of neuronal activity in rats and mice (37,38). Using c-Fos immunohistochemistry (39), we observed that Rb1, at an effective dose (10 mg/kg) in reducing food intake, significantly induced c-Fos expression in the hindbrain NTS and in the ARC and VMH of the hypothalamus, but not in the LH. Many endogenous factors influence satiation via signals passing from abdominal viscera to the NTS through the vagus nerve, with the signals then being relayed anteriorly to the hypothalamus and other brain areas (40–42). Therefore, one possible explanation of the effect of intraperitoneal Rb1 on food intake is via alteration of peripheral afferent neural activity. There is also a previous report that food intake was reduced by acute intra-third ventricular (i3vt) administration of Rb1 (43), implying that central neural circuits are sensitive to Rb1 and that peripherally administered Rb1 may penetrate the brain reaching local circuits that influence food intake and body weight. Because the blood-brain barrier is a major controlling site for the entry of substances into the brain (44,45), evaluating the capability of Rb1 to penetrate the blood-brain barrier will be an important question for future research. Since ginseng and its extract are generally consumed orally, the implication is that its active ingredients are able to penetrate membranes.
Epidemiological studies have identified a positive correlation between average dietary fat intake and the incidence of obesity. To determine the effect of chronic Rb1 treatment on obesity, we used an HFD-induced obese rat model (28). Those studies confirmed Rb1's effect to reduce food intake, especially during the early period of treatment, and to be maintained throughout the observation period, such that Rb1's anorectic capacity was still evident with sustained administration.
Rb1-treated obese rats behaved normally with no obvious health problems (i.e., no side effects, such as diarrhea were observed during the period of Rb1 treatment). To evaluate whether chronic Rb1 had nonspecific toxic effects that could complicate the interpretation of the results, plasma levels of ALT, AST, and creatinine were assessed at the end of the experiment. AST and ALT are enzymes that become elevated with liver disease or injury. Creatinine is a byproduct of muscle metabolism and is excreted by the kidneys, and elevated levels can indicate kidney disease, urinary tract obstruction, or dehydration. Animals fed HFD, with or without Rb1, had similar levels of AST, ALT, and creatinine (Table 1), indicating that chronic treatment with Rb1 did not have any appreciable toxic effect on the liver or kidney.
In parallel with the reduction of food intake, there was a significant decrease in body weight in rats treated with Rb1, compared with vehicle controls. More importantly, the reduced body weight was mainly due to a selective reduction of body fat, leaving lean mass unchanged. Pair-fed rats weighed more than the rats treated with Rb1 after the first 3 weeks of treatment, implying that Rb1's effect on body weight is not simply secondary to a reduction of food intake. This is consistent with our observation that chronic treatment with Rb1 significantly increased energy expenditure relative to the saline control group. Although the difference in energy expenditure between Rb1 and pair-fed groups did not reach statistical significance during the 1-day test, a slight but persistent increase in energy expenditure induced by Rb1 may explain why Rb1-treated rats had less body weight gain than HFD pair-fed rats beginning after 21 days when food intake was the same for both groups. These observations suggest that attenuated weight gain associated with Rb1 treatment is due to a combination of reduced food intake and increased energy expenditure.
One possible explanation for the reduced fat mass could be reduced fat absorption induced by Rb1. However, this seems unlikely because there was no significant difference in fecal lipid excretion between Rb1 and saline-treated rats, implying that Rb1 did not significantly affect lipid absorption.
Obese subjects are often hyperglycemic and glucose intolerant, and this could be due to increased insulin resistance and/or defects in insulin secretion. Chronic Rb1 treatment significantly reduced fasting glucose and improved impaired glucose tolerance. More importantly, these effects were greater than those observed in pair-fed controls, implying that there are effects of Rb1 on glucose homeostasis beyond those due to decreased food intake and body weight. One way to improve insulin sensitivity is through an increase of tissue glucose uptake, and this possibility is supported by previous in vitro studies demonstrating that Rb1 significantly stimulated basal and insulin-mediated glucose uptake in a time- and dose-dependent manner in both 3T3-L1 adipocytes and C2C12 myotubes (21). The effect was mediated through increased gene expression of GLUT4 and increased GLUT4 translocation to the cell surface (21). Although Rb1-induced reduction of fasting blood glucose was not accompanied by increased plasma insulin in HFD-obese rats, it cannot be ruled out that Rb1 may stimulate insulin secretion because the improved insulin sensitivity requires less insulin to maintain blood glucose level. This possibility is supported by a report that Rb1 promoted glucose-stimulated insulin secretion from Min6 cells, a mouse insulinoma cell line (23).
Phosphatidylinositol 3-kinase is an intracellular signaling pathway that plays a pivotal role in many essential cellular processes. Using primary cultured neuronal cells, prepared from hypothalami of embryos (30), we found that Rb1 significantly and dose-dependently (0.1–100 μmol/l) activates the phosphatidylinositol 3-kinase signaling pathway beginning after 2 h of treatment. This effect was confirmed in HFD-induced obese rats (i.e., chronic Rb1 treatment, but not pair-feeding, significantly increased hypothalamic pAkt levels, indicating that Rb1 may influence metabolic parameters, at least in part, by regulating this signaling pathway in the brain).
Central nervous system circuits involved in food intake can be conceptually divided into anabolic and catabolic systems. NPY, AgRP, and POMC are key peptides in these systems, respectively (46), and it is the balance between these systems that ultimately determines the animal's ingestive behavior and energy expenditure (46,47). Chronic Rb1 treatment significantly reduced NPY, but not AgRP, mRNA in the hypothalamus of HFD-obese rats, implying that Rb1 exerts its anorectic action, at least partially, by inhibiting the activity of the NPY system. POMC mRNA was nonsignificantly increased.
The molecular mechanisms as to how Rb1 acts on cells are not clear. Recent reports suggest that Rb1 might act as a phytoestrogen and exert estrogen-like activity through estrogen receptors (ERs) (16,48). Rb1 activated both ERα and ERβ dose-dependently, and the activation was inhibited by ICI 182,780, an estrogen receptor antagonist, indicating that these effects were mediated through the ER. Treatment with Rb1 increased ER expression in MCF-7 cells and of AP-1–driven luciferase genes in COS cells (16), but these actions are likely independent of a direct ER association because Rb1 did not displace specific binding of [3H]17β-estradiol from estrogen receptors in MCF-7 whole-cell ligand binding assays (16). These observations suggest that Rb1 is functionally similar to 17β-estradiol. Because estrogen signaling plays an important role in the control of energy homeostasis, whether ER mediates the catabolic action of Rb1 will require further study.
In summary, we have demonstrated that intraperitoneal Rb1 significantly reduces food intake without causing malaise in ad libitum–fed or fasted rats. Moreover, chronic intraperitoneal treatment with Rb1 significantly reduced food intake and body weight gain and increased energy expenditure in HFD-induced obese rats. Rb1 also significantly decreased fasting blood glucose and improved glucose tolerance to a greater extent than pair-feeding in the HFD-induced obese rats, raising the possibility that Rb1 has important effects on glucose homeostasis. These results identify a novel role for Rb1 as an antiobesity and antihyperglycemic agent.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants DK70992, DK 017844, and DK63907.
No potential conflicts of interest relevant to this article were reported.
Ye X. researched data and wrote the manuscript. L.S. researched data. K.J.L. researched data. P.T. edited the manuscript. Yu. X. reviewed the manuscript. G.W. reviewed the manuscript. S.C.W. reviewed/edited the manuscript. M.L. wrote and edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Blumenthal M, Ferrier GKL. Total sales of herbal supplements in United States show steady growth. Herbal Gram 2006;71:64–66 [Google Scholar]
- 2. Court WE. Ginseng: The Genus Panax. Amsterdam, the Netherlands, Harwood Academic Publishers; 2006;71:64–66 [Google Scholar]
- 3. Wang XY, Zhang JT. Effect of ginsenoside Rb1 on mouse sexual function and its mechanism. Acta Pharm Sin 2002;35:492–495 [Google Scholar]
- 4. Kim DY, Chang JC. Radioprotective effect of ginseng components on antioxidant enzymes, glutathione and lipid peroxidation of liver in irradiated mice. Korean J Ginseng Sci 1998;22:1–10 [Google Scholar]
- 5. Lee FC. Facts about Ginseng, the Elixir of Life. Elizabeth, NJ, Hollyn International, 1992 [Google Scholar]
- 6. Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 1997;54:1–8 [DOI] [PubMed] [Google Scholar]
- 7. Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci 2005;97:124–131 [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Kang SA, Han SM, Shim I. Comparison of the antiobesity effects of the protopanaxadiol- and protopanaxatriol-type saponins of red ginseng. Phytother Res 2009;23:78–85 [DOI] [PubMed] [Google Scholar]
- 9. Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002;51:1851–1858 [DOI] [PubMed] [Google Scholar]
- 10. Chung SH, Choi CG, Park SH. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res 2001;24:214–218 [DOI] [PubMed] [Google Scholar]
- 11. Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995;18:1373–1375 [DOI] [PubMed] [Google Scholar]
- 12. Yun SN, Moon SJ, Ko SK, Im BO, Chung SH. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch Pharm Res 2004;27:790–796 [DOI] [PubMed] [Google Scholar]
- 13. Vuksan V, Stavro MP, Sievenpiper JL, Koo VY, Wong E, Beljan-Zdravkovic U, Francis T, Jenkins AL, Leiter LA, Josse RG, Xu Z. American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J Am Coll Nutr 2000;19:738–744 [DOI] [PubMed] [Google Scholar]
- 14. Washida D, Kitanaka S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2003;51:1314–1317 [DOI] [PubMed] [Google Scholar]
- 15. Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. [Chemical studies of crude drugs (1). Constituents of Ginseng radix rubra]. Yakugaku Zasshi 1983;103:612–622 [PubMed] [Google Scholar]
- 16. Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab 2004;89:3510–3515 [DOI] [PubMed] [Google Scholar]
- 17. Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res 2001;63:509–515 [DOI] [PubMed] [Google Scholar]
- 18. Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 1997;28:191–200 [DOI] [PubMed] [Google Scholar]
- 19. Liu M, Zhang J. Effects of ginsenoside Rb1 and Rg1 on synaptosomal free calcium level, ATPase and calmodulin in rat hippocampus. Chin Med J (Engl) 1995;108:544–547 [PubMed] [Google Scholar]
- 20. Jiang KY, Qian ZN. Effects of Panax notoginseng saponins on posthypoxic cell damage of neurons in vitro. Zhongguo Yao Li Xue Bao 1995;16:399–402 [PubMed] [Google Scholar]
- 21. Shang W, Yang Y, Zhou L, Jiang B, Jin H, Chen M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3–L1 adipocytes. J Endocrinol 2008;198:561–569 [DOI] [PubMed] [Google Scholar]
- 22. Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, Chen M. Ginsenoside Rb1 promotes adipogenesis in 3T3–L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci 2007;80:618–625 [DOI] [PubMed] [Google Scholar]
- 23. Park S, Ahn IS, Kwon DY, Ko BS, Jun WK. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3–L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci Biotechnol Biochem 2008;72:2815–2823 [DOI] [PubMed] [Google Scholar]
- 24. Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain apolipoprotein E: an important regulator of food intake in rats. Diabetes 2008;57:2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav 2003;78:149–155 [DOI] [PubMed] [Google Scholar]
- 26. Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D'Alessio D, Seeley RJ, Tso P, Woods SC. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol 2006;290:R202–R207 [DOI] [PubMed] [Google Scholar]
- 27. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed New York, Academic Press, Harcourt Brace, 1982, p. 20–34 [Google Scholar]
- 28. Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 2003;133:1081–1087 [DOI] [PubMed] [Google Scholar]
- 29. Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 2004;127:139–144 [DOI] [PubMed] [Google Scholar]
- 30. Shen L, Tso P, Woods SC, Sakai RR, Davidson WS, Liu M. Hypothalamic apolipoprotein A-IV is regulated by leptin. Endocrinology 2007;148:2681–2689 [DOI] [PubMed] [Google Scholar]
- 31. Huang KC. The Pharmacology of Chinese Herbs. CRC Press, Boca Raton, FL, 1999 [Google Scholar]
- 32. Wang JH. Chemical study on ginseng. In The Chemistry, Metabolism and Biological Activiries of Ginseng. Zhang JT, Chui DH. Eds. Chemical Industry Press, 2006, p. 3–17 [Google Scholar]
- 33. Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 2000;160:1009–1013 [DOI] [PubMed] [Google Scholar]
- 34. Xie JT, Wang CZ, Wang AB, Wu J, Basila D, Yuan CS. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharmacol Sin 2005;26:1104–1110 [DOI] [PubMed] [Google Scholar]
- 35. Xie JT, Mehendale SR, Li X, Quigg R, Wang X, Wang CZ, Wu JA, Aung HH, Rue A, Bell GI, Yuan CS. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys Acta 2005;1740:319–325 [DOI] [PubMed] [Google Scholar]
- 36. Xie JT, Zhou YP, Dey L, Attele AS, Wu JA, Gu M, Polonsky KS, Yuan CS. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine 2002;9:254–258 [DOI] [PubMed] [Google Scholar]
- 37. Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 1990;296:517–530 [DOI] [PubMed] [Google Scholar]
- 38. Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 2009;29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D'Alessio D, Seeley RJ, Tso P, Woods SC. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol 2006;290:R202–R207 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci 2006;361:1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 2008;149:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000;85:1–17 [DOI] [PubMed] [Google Scholar]
- 43. Etou H, Sakata T, Fujimoto K, Terada K, Yoshimatsu H, Ookuma K, Hayashi T, Arichi S. [Ginsenoside-Rb1 as a suppressor in central modulation of feeding in the rat]. Nippon Yakurigaku Zasshi 1988;91:9–15 [DOI] [PubMed] [Google Scholar]
- 44. Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem 1998;70:1781–1792 [DOI] [PubMed] [Google Scholar]
- 45. Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res 1995;12:1395–1406 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 47. Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 1998;280:1378–1383 [DOI] [PubMed] [Google Scholar]
- 48. Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol 2010;242:18–28 [DOI] [PubMed] [Google Scholar]