Abstract
OBJECTIVE
We examined the effect of β-adrenergic receptor (βAR) activation and cAMP-elevating agents on respiration and mitochondrial uncoupling in human adipocytes and probed the underlying molecular mechanisms.
RESEARCH DESIGN AND METHODS
Oxygen consumption rate (OCR, aerobic respiration) and extracellular acidification rate (ECAR, anaerobic respiration) were examined in response to isoproterenol (ISO), forskolin (FSK), and dibutyryl-cAMP (DB), coupled with measurements of mitochondrial depolarization, lipolysis, kinase activities, and gene targeting or knock-down approaches.
RESULTS
ISO, FSK, or DB rapidly increased oxidative and glycolytic respiration together with mitochondrial depolarization in human and mouse white adipocytes. The increase in OCR was oligomycin-insensitive and contingent on cAMP-dependent protein kinase A (PKA)-induced lipolysis. This increased respiration and the uncoupling were blocked by inhibiting the mitochondrial permeability transition pore (PTP) and its regulator, BAX. Interestingly, compared with lean individuals, adipocytes from obese subjects exhibited reduced OCR and uncoupling capacity in response to ISO.
CONCLUSIONS
Lipolysis stimulated by βAR activation or other maneuvers that increase cAMP levels in white adipocytes acutely induces mitochondrial uncoupling and cellular energetics, which are amplified in the absence of scavenging BSA. The increase in OCR is dependent on PKA-induced lipolysis and is mediated by the PTP and BAX. Because this effect is reduced with obesity, further exploration of this uncoupling mechanism will be needed to determine its cause and consequences.
Adipose tissue is a key component in the management of whole-body energy balance and metabolic homeostasis. In mammals, adipose tissue is composed of white and brown adipose tissue (WAT, BAT). Both tissues are similar in that they are highly responsive to insulin to store energy as triglyceride, and both respond to catecholamines to catabolize these energy reserves into their constituent fatty acids (FAs) and glycerol. However, the fate of released FAs from BAT and WAT is different. Brown adipocytes possess a rich complement of mitochondria and are the only cell type to express uncoupling protein (UCP1). After catecholamine stimulation of the β-adrenergic receptors (βARs), UCP1 activation (by the released FAs) increases the proton conductance of the inner mitochondrial membrane (IMM) and dissipates the electrochemical proton gradient that is the driving force for ATP synthesis in a process termed “mitochondrial uncoupling” (rev. in 1,2). Catecholamine stimulation also increases Ucp1 gene expression and mitochondrial mass, altogether resulting in robust oxidation of FAs for heat production and energy expenditure. White adipocytes have fewer mitochondria and negligible amounts of UCP1. Upon βAR stimulation of WAT, FAs liberated by lipolysis are mostly released into the circulation. Although an important source of energy for other tissues, chronically elevated circulating FAs in obesity are associated with insulin resistance and progression to type 2 diabetes (3).
Because of recent evidence for the existence of BAT in adult humans (rev. in 4), (5–9) there is renewed interest in the idea that mitochondrial uncoupling could contribute to FA oxidation and weight reduction. However, it is not yet clear whether there are sufficient numbers of brown adipocytes to have a significant impact on body weight and energy expenditure, and most of the adipose tissue in adult humans consists of white adipocytes. More recently, white adipocytes are appreciated to have a greater complement of mitochondria than previously thought (10), and there are recent reports showing that FAs in adipocytes can be oxidized in situ (11–13). Earlier suggestions in the literature also noted that rodent white adipocytes can exhibit mitochondrial uncoupling after catecholamine stimulation (14,15). Also of note, previous experiments in mice with ectopic expression of UCP1 in WAT from the adipocyte FA–binding protein (aP2) promoter documented the potential of mitochondrial uncoupling in vivo and resistance to dietary obesity (16). The uncoupling role of FAs released during white adipocyte lipolysis and its molecular basis remain unclear, especially in less commonly studied human adipocytes. Therefore, a better understanding of the potential role for white adipocytes to engage in metabolic fuel oxidation and uncoupling is warranted.
Using an approach combining measures of oxygen consumption rate (OCR, aerobic respiration), extracellular acidification rate (ECAR, anaerobic respiration or glycolysis), mitochondrial inner membrane potential, and biochemical measurements, we present evidence that human white adipocytes can acutely increase aerobic and anaerobic respiration in response to βAR and protein kinase A (PKA)-dependent stimulation of lipolysis. Under conditions where the released FAs are not scavenged by BSA in the medium, we show that the increase in respiration results, in part, from mitochondrial uncoupling. Moreover, we present evidence that the molecular mechanism mediating this uncoupling involves the mitochondrial permeability transition pore (PTP) and its regulator protein BAX. Interestingly, this βAR-stimulated respiration is reduced with obesity. Such compromised capacity could contribute to increased adipocyte size, elevated plasma FA levels and oxidative stress—all of which exist in obesity and its metabolic complications.
RESEARCH DESIGN AND METHODS
Materials included forskolin (FSK), isoproterenol (ISO), dibutyryl-cAMP (DB), N-[2-(p-bromocinnamylamino)-ethyl]-5-isoquinoline-sulfonamide (H89), GlutaMAX, gelatin, carbonylcyanide-p-trifluoromethoxyphenyl-hydrazone (FCCP), cyclosporin-A (CSA), rotenone, and FA-free BSA (Sigma-Aldrich, St. Louis, MO), oligomycin (Oligo) (Calbiochem, San Diego, CA), the cAMP antagonist Rp-cAMPS (Enzo Life Sciences, Farmingdale, NY), tetramethylrhodamine methyl ester (TMRM), and MitoTracker Green (MTG) (Molecular Probes; Invitrogen, San Diego, CA).
Cell cultures.
Human preadipocytes (Zen-Bio, Research Triangle Park, NC) were isolated from human subcutaneous adipose tissue using conventional techniques including enzymatic dissociation, differential centrifugation, and plating. Cells were seeded into 0.2% gelatin-covered 24-well XF24 plates (#100777-004; 13,000 cells/well) (Seahorse Bioscience, North Billerica, MA) for OCR and ECAR experiments, 60-mm dishes (8 × 105 cells) for confocal microscopy, and 6-well plates (4 × 105 cells/well) for Western blotting. Growth for 24–48 h in preadipocyte media (PM-1; Zen-Bio) contained Dulbecco's modified Eagle's medium F12 (DMEM/F12 [1:1, v/v]), HEPES (pH 7.4), 10% FBS, and antibiotics. Cells were differentiated for 7 days in media containing DMEM/F12 (1:1; v/v), HEPES (pH 7.4), 10% FBS, biotin, pantothenate, insulin, dexamethasone, isobutylmethylxanthine (IBMX), and a nonthiazolidinedione peroxisome proliferator–activated receptor (PPAR)-γ agonist (DM-2; Zen-Bio), followed by an additional week in adipocyte maintenance media (DM-2 without IBMX and PPARγ agonist [AM-1, Zen-Bio]).
Mouse adipocytes, 3T3-L1 and 3T3-F442A cells, were grown and differentiated as described (17) for up to 10 days. Twenty-four h before XF24 analysis, cells were trypsinized and seeded at 25,000 cells/well in the plates.
Animals.
Age-matched male Ucp2+/+ and Ucp2−/− mice (10–16 weeks old) on a C57BL/6J background were created in our lab as described previously (18,19). Animals were housed and genotyped as previously described (19) and fed standard rodent chow. All protocols for animal use were approved by the Institutional Animal Care and Use Committee of The Hamner Institutes in accordance with National Institutes of Health guidelines.
Cellular metabolic rate.
Human or mouse adipocytes were washed with 1 ml XF-DMEM (#D5030, Sigma-Aldrich) containing sodium pyruvate (1 mmol/l), GlutaMAX-1 (2 mmol/l), glucose (17.5 mmol/l), NaCl (1.85 g/l), phenol red (15 mg/l, pH 7.4), and 500 μl were added per well. Cellular OCR and ECAR were measured (model XF24, Seahorse Bioscience) as described previously (20). Per specific experiments, drugs were delivered to final concentrations of ISO (1 μmol/l), FSK (10 μmol/l), Oligo (1 μg/ml), FCCP (0.6 μmol/l), and rotenone (3 μmol/l). For some experiments, cells were pretreated with H89 (10 μmol/l), DB (1 mmol/l), Rp-cAMPS (0.5 mmol/l), 5% FA-free BSA or CSA (5 μg/ml). Optimal drug concentrations were determined in preliminary experiments.
Tissues.
Freshly isolated mouse gonadal WAT was rinsed with XF-DMEM containing 25 mmol/l HEPES, cleaned of nonadipose material and cut into pieces (∼10 mg). After extensive washing, one piece of tissue was placed in each well of a XF24-well plate (#101122-100, Seahorse Bioscience) and covered with a customized screen that allows free perfusion while minimizing tissue movement. XF-DMEM (500 μl) was added per well, and samples were analyzed in the XF24.
Mitochondrial membrane potential and mitochondrial mass by confocal microscopy.
Differentiated human or mouse 3T3-L1 adipocytes were trypsinized and seeded onto 0.2% gelatin coated glass-bottom 10-mm culture dishes (MatTak Corporation, Ashland, MA). After overnight incubation at 37°C, 5% CO2, cells were either treated with ISO (1 μmol/l, 30 min) or FSK (10 μmol/l, 60 min) and subsequently stained with 20 nmol/l TMRM or with 15 nmol/l MTG (45 min at 37°C, in the dark). Cells were washed twice with 1 ml phenol red-free DMEM (#31053; GIBCO) and visualized by laser scanning confocal microscopy (Zeiss, Thornwood, NY). FCCP (40 μmol/l, 60 min) was used as a control for mitochondrial uncoupling and depolarization. Fluorescence intensities were calculated with ImagePro Plus software (Media Cybernetics, Bethesda, MD).
Lipolysis and PKA activity.
Glycerol release and PKA activity in differentiated human adipocytes were measured as described (21) after ISO treatment (1 μmol/l, 2 h for lipolysis; 15 min for PKA activity).
Small interfering RNA.
Differentiated human adipocytes were transfected with small interfering RNAs (siRNAs) using INTERFERin reagent (Polyplus-transfection; Genesee Scientific, San Diego, CA) per the manufacturer for 24 or 48 h as detailed in Fig. 8 and in supplementary Fig. 6 in the online appendix available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0245/DC1. Total cellular RNA was extracted for real-time PCR (RT-PCR), or respiration measurements were performed in the XF24. Optimal transfection conditions were determined in preliminary experiments for each gene target, which also included siRNA positive control (Hs_MAPK1, Qiagen).
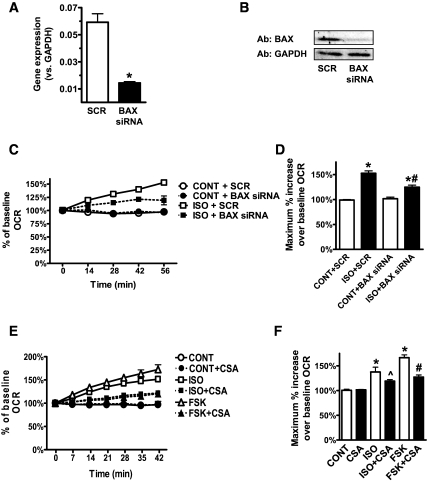
FIG. 8.
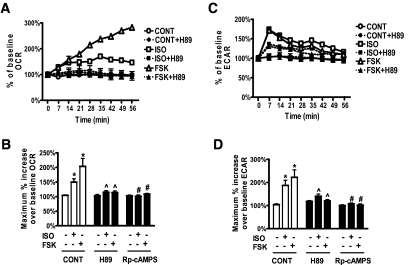
cAMP-induced mitochondrial uncoupling is mediated by BAX and the PTP. A and B: Human adipocytes were transfected with scrambled (SCR) or BAX siRNAs (30 nmol/l, 48 h). A: BAX gene expression was measured by RT-PCR and normalized to GAPDH. B: Representative Western blots (one of four replicates) of BAX protein level compared with GAPDH. C and E: Representative measurements of the percent increase in OCR relative to baseline rates, in response to ISO or FSK. Human adipocytes were transfected with SCR or BAX siRNAs (C) or pretreated or not with CSA (5 μg/ml, 72 h) (E). At 0 min, ISO (1 μmol/l), FSK (10 μmol/l), or DMEM (CONT) was injected into cells. Each data point is the mean of four wells, and error bars not visible are contained within symbols. D and F: Histograms summarizing the maximum percent increase of OCR over the baseline rates in response to DMEM (CONT), ISO, or FSK. In D, results are the average of six subjects measured in three independent experiments. *P < 0.001, compared with respective control; #P < 0.001, compared with ISO + SCR. In F, results are the average of a total of 12 subjects measured in five independent experiments. *P < 0.001, compared with CONT; ∧P < 0.05, compared with respective ISO without CSA; #P < 0.001, compared with respective FSK without CSA.
RNA extraction and RT-PCR.
Total RNA was isolated using TRIzol in combination with PureLink Micro-to-Midi Total RNA Purification System (Invitrogen). Reverse transcription was performed using High-Capacity cDNA Kit with random primers on a Veriti 96-well Thermal Cycler (Applied Biosystems). RT-PCR was done using TaqMan or SYBR Green (Applied Biosystems) in an ABI PRISM 7900HT Sequence Detection System. RT-PCR primers are described in supplementary Fig. 6. All data were normalized to g lyceraldehyde - 3-phosphate dehydrogenas (GAPDH) content.
Western blotting.
Cells were lysed and protein concentrations measured by BCA (Pierce); 2.5 μg of each sample was fractionated by electrophoresis for Western blotting, all exactly as described previously (21). Primary and secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology (Santa Cruz, CA).
Data analysis.
Statistical analysis was performed by one-way repeated-measures ANOVA with Newman-Keuls post hoc test. For comparison of two groups, significance was analyzed by Student t test. Results are represented as means ± SE, unless otherwise noted in the figure legends.
RESULTS
Elevated cAMP levels acutely induce respiration in white adipocytes.
Continuous measurements of OCR and ECAR were collected from human adipocytes over time. The addition of ISO, FSK, or DB led to a rapid increase in OCR, which gradually peaked after 20–30 min (ISO) or 60 min (FSK, DB) and then slowly declined (Fig. 1A). Figure 1B presents the average maximal OCR changes from several experiments, with maximal rates of respiration measured by the mitochondrial uncoupler FCCP. Similar increases in OCR in response to elevations in cAMP were also observed in mouse WAT (supplementary Fig. 1A) and cell lines (supplementary Figs. 1B and 2A). Note from a technical standpoint that the same responses and kinetics are observed for freshly isolated adipose tissue, adipocytes, or cell lines, although the response in the cell lines was weaker.
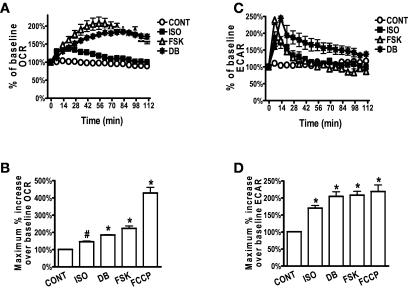
FIG. 1.
Elevated cAMP levels acutely induce respiration in white adipocytes. Representative measurements of the percent increase in OCR (A) or ECAR (C), respectively, relative to baseline rates in response to ISO, FSK, or DB in human adipocytes. As indicated, at 0 min ISO (1 μmol/l), FSK (10 μmol/l), DB (1 mmol/l), or DMEM (CONT) were injected. OCR (A) or ECAR (C) measurements before drug injection were set as 100%. Adipocytes were pooled from five different human subjects, and each data point is a mean of 4–6 wells. Histograms summarizing the average maximal percent increase of OCR (B) or ECAR (D), respectively, over their baseline rates in response to ISO, FSK, DB, and FCCP (0.6 μmol/l). Data are collected from 8 to 10 experiments, using a total of 20 subjects (BMI 21.7–35.5 kg/m2). All experiments included wells that received ISO, FSK, or DB and wells that received FCCP. *P < 0.001; #P < 0.01 compared with CONT.
The increase in ECAR was more rapid than for OCR, reaching a peak after 10 min and then declining (Fig. 1C). This rise in ECAR was fully inhibited by the lactate dehydrogenase inhibitor, oxamate (5 mmol/l, not shown), indicating an acute increase in glycolysis (20). The maximum percent change in ECAR is summarized in Fig. 1D. ECAR was also increased in 3T3-L1 and 3T3-F44A adipocytes in response to ISO or FSK (supplementary Figs. 1C and 2B).
cAMP-induced mitochondrial uncoupling.
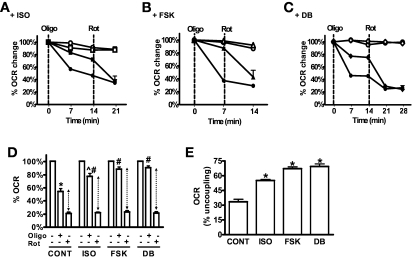
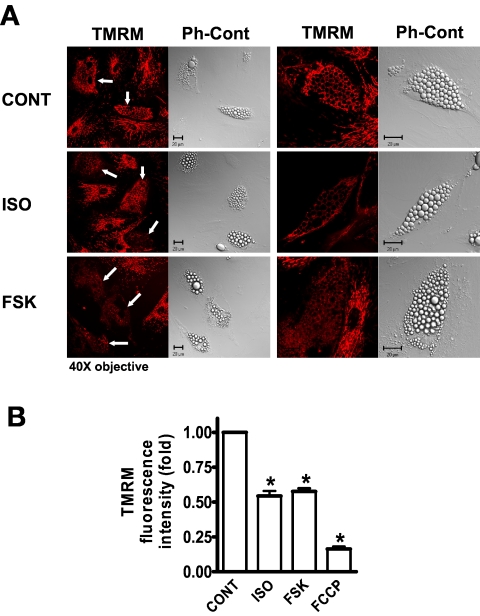
We next determined whether mitochondrial uncoupling was a contributor to the increased OCR in response to cAMP-elevating agents by using the ATP synthase inhibitor Oligo and by examining cells stained with the fluorescent dye TMRM, which accumulates specifically in the mitochondrial matrix as a function of IMM potential. As shown in Fig. 2A–D, under basal conditions Oligo inhibited OCR by 50–60%. However, in ISO-, FSK-, or DB-treated cells, OCR was less sensitive to Oligo, inhibited by less than 25%. This finding suggests that some of the cAMP-induced OCR is uncoupled respiration. To further assess percent of uncoupling, cells were treated with rotenone, which inhibits respiratory chain activity at Complex I, with the remaining OCR from nonmitochondrial sources. Figure 2D shows that about 20% of total OCR remains in the presence of rotenone and is similar for all groups. After accounting for this nonmitochondrial OCR (shown by the dotted arrows in Fig. 2D and illustrated as percent uncoupling in Fig. 2E), mitochondrial uncoupling accounts for 30% of OCR in untreated control cells, ∼55% of ISO-induced respiration and 65–70% of FSK- or DB-stimulated respiration. We next analyzed TMRM fluorescence intensity by confocal microscopy. Figure 3 shows that TMRM intensity is significantly reduced in either ISO- or FSK-treated human adipocytes relative to untreated control cells. Note that the changes in TMRM intensity occur specifically in the differentiated adipocytes, recognized by their lipid accumulation (arrows in Fig. 3A) and occur in the presence of serum-containing media. The changes in fluorescence intensity are summarized in Fig. 3B. Both ISO and FSK decreased IMM potential by about 50%. Similar results were obtained in mouse 3T3-L1 adipocytes (supplementary Fig. 3). All together, these results indicate that βAR stimulation or cAMP-elevating agents can acutely increase mitochondrial uncoupling in white adipocytes.
FIG. 2.
Elevated cAMP levels induce Oligo-insensitive OCR. Representative measurements of the percent change in OCR in human adipocytes after injections of DMEM (open symbols) or the inhibitors (closed symbols) ATP synthase inhibitor oligomycin (Oligo (1 μg/ml) or the complex 1 inhibitor rotenone (3 μmol/l), as indicated by the dashed vertical lines. Oligo was injected 30–40 min after ISO (1 μmol/l) (A), FSK (10 μmol/l) (B), DB (1 mmol/l) (C), or DMEM (CONT). OCR before Oligo injection was set as 100%. The results are from adipocytes pooled from five subjects, and each data point is a mean of 4–5 wells. D: Histogram summarizing the percent decrease in OCR in response to Oligo or rotenone injections. Data are relative to OCR levels before Oligo injection (and after ISO, FSK, or DB injections). The data are an average of 3–5 experiments using a total of 15 subjects (BMI 21.7–35.5 kg/m2). *P < 0.001, compared with untreated CONT; ∧P < 0.01, compared with untreated ISO; #P < 0.001, compared with CONT + Oligo. E: The relative changes in OCR between Oligo and rotenone are expressed as % uncoupling (calculated from the results presented in D, dotted arrows). Rot, rotenone.
FIG. 3.
Elevated cAMP levels induce mitochondrial depolarization. A: Microscopy images of human adipocytes stained with TMRM and visualized under confocal microscopy or their phase-contrast (Ph-Cont) mode. Cells were treated with ISO (1 μmol/l, 30 min) or FSK (10 μmol/l, 60 min) in DMEM + 10% FBS, stained with TMRM and immediately analyzed, as described in research design and methods. The scale bar in the images represents 20 microns. B: Histogram summarizing TMRM fluorescence intensity of untreated (CONT) and ISO-, FSK-, or FCCP- (40 μmol/l, 60 min) treated cells of 50 images collected from three independent experiments. TMRM intensity in the untreated cells (CONT) was set as 1. *P < 0.001, compared with CONT. (A high-quality digital representation of this figure is available in the online issue.)
cAMP-induced OCR is FA-dependent.
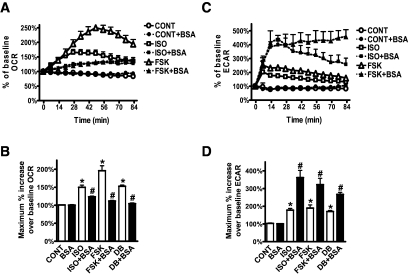
In adipocytes, βAR agonists stimulate lipolysis to generate FFAs. This response depends largely on the activation of PKA (17). We examined the involvement of PKA as well as the liberated FAs on cAMP-induced changes in adipocyte respiration. We used two mechanistically different inhibitors of PKA: the catalytic inhibitor H89 (22) and the competitive cAMP antagonist Rp-cAMPS (23). Figure 4A and B shows that both H89 and Rp-cAMPS inhibited ISO- or FSK-induced OCR. Both inhibitors also effectively blocked the increase in ECAR (Fig. 4C; average of several experiments presented in Fig. 4D). To examine the role of the liberated FAs on respiration, cells were provided with 5% BSA (FA-free) prior to ISO or FSK injections (15). A sample dataset of OCR is shown in Fig. 5A, and results from several experiments are summarized in Fig. 5B. The increase in OCR induced by either ISO or FSK was significantly reduced due to scavenging by BSA of the FAs released from lipolysis. Similar results were obtained in mouse 3T3-F442A adipocytes (supplementary Fig. 2A). Together, the data suggest that the FAs released by PKA-dependent lipolysis contribute to the cAMP-dependent mitochondrial oxidative respiration of white adipocytes, consistent with other studies (15,24). Although these data do not clearly distinguish between FAs as a substrate, uncoupler, or both, two additional observations suggest that FAs contribute to both. First, in contrast to OCR, ECAR levels in the presence of BSA were even higher after ISO or FSK (Fig. 5C and D; supplementary Fig. 2B). This suggests that the reduction of available FAs from lipolysis for oxidation resulted in increased glycolysis. The cells respire well on other substrates (e.g., pyruvate) because they increase respiration in response to FCCP and in the absence of cAMP elevation. Second, a much earlier study in primary rat adipocytes reported that mitochondrial depolarization observed after ISO treatment was largely prevented by pretreatment with FA-free BSA (15). Also, the reduction in mitochondrial membrane potential measured by confocal microscopy was performed in the presence of complete serum-containing media.
FIG. 4.
cAMP-induced respiration is PKA-dependent. Representative measurements of the percent increase in OCR (A) and ECAR (C), relative to baseline rates, in response to ISO or FSK in human adipocytes pretreated or not with H89. At 0 min, ISO (1 μmol/l), FSK (10 μmol/l), or DMEM (CONT) was injected to cells that were pretreated (closed symbols) or not (open symbols) with H89 (10 μmol/l, 1 h). Each data point is the mean of four wells, and error bars not visible are contained within the symbols. Histograms summarizing the maximum percent increase of OCR (B) or ECAR (D), over their baseline rates in response to DMEM, ISO, or FSK. Cells were pretreated with or without H89 or Rp-cAMPS (0.5 mmol/l, 1 h). Results are the average of a total of 11 subjects (BMI 21.7–35.5 kg/m2), measured in three independent experiments. *P < 0.05, compared with untreated respective control; ∧P < 0.05, compared with respective ISO or FSK without H89; #P < 0.05, compared with respective ISO or FSK without Rp-cAMPS.
FIG. 5.
cAMP-induced OCR is FA-dependent. Representative measurements of the percent increase in OCR (A) and ECAR (C), relative to baseline rates, in response to ISO or FSK in adipocytes in the presence or absence of FA-free BSA. At 0 min, ISO (1 μmol/l), FSK (10 μmol/l), or DMEM (CONT) was injected to cells that were pretreated (closed symbols) or not (open symbols) with BSA (5%, 1 h). Each data point is the mean of four wells, and error bars not visible are contained within the symbols. Histograms summarizing the maximum percent increase of OCR (B) or ECAR (D) in response to DMEM (CONT), ISO, FSK or DB (1 mmol/l) over their baseline rates. Data are an average of 11 subjects (BMI 21.7–35.5 kg/m2), measured in three independent experiments. *P < 0.05, compared with CONT; #P < 0.05, compared with respective samples without BSA.
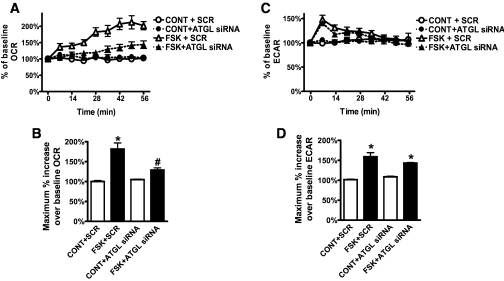
To further evaluate the importance of lipolysis in this cAMP-induced respiration, we used a siRNA approach to suppress the expression of the important lipase, adipose triglyceride lipase (ATGL) (25). Knock-down of ATGL (supplementary Fig. 5B) resulted in a dramatic inhibition of FSK-induced OCR (Fig. 6A and B). The portion of OCR that was not inhibited may be due to residual ATGL. FSK-induced ECAR was not changed with ATGL knock-down (Fig. 6C and D). These data support the role of lipolysis in the cAMP-induced oxidative respiration in white adipocytes.
FIG. 6.
cAMP-induced OCR is dependent upon ATGL. Human adipocytes were transfected with siRNA targeted to ATGL or scrambled sequence (SCR), as described in research design [scap]and [scap]m[scap]ethods, which resulted in 50% suppression (supplementary Fig. 5B). Representative measurements of the percent increase in OCR (A) and ECAR (C) relative to baseline rates in response to FSK (10 μmol/l) in human adipocytes treated with SCR (open symbols) or ATGL siRNA (closed symbols). As indicated, at 0 min FSK or DMEM (CONT) was injected. OCR measurements before FSK injection were set as 100%. Adipocytes were pooled from five different human subjects, and each data point is a mean of six wells. Histograms summarizing the maximum percent increase of OCR (B) or ECAR (D), respectively, relative to respective baseline rates, collected from three experiments using a total of 20 subjects (BMI 21.7–35.5 kg/m2). *P < 0.01, compared with respective control samples; #P < 0.05, compared with FSK + SCR.
cAMP-induced OCR is reduced with obesity.
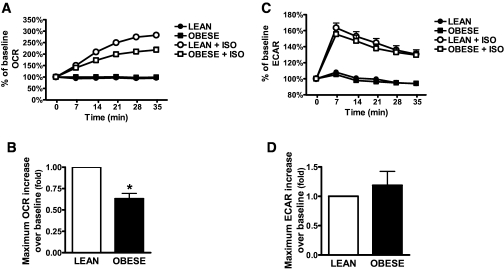
Catecholamine responsiveness of adipose tissue to increase lipolysis has been reported to become impaired with increasing BMI (rev. in 26,27). Therefore, an interesting question is whether adipocytes of obese individuals have an equivalent capacity for this βAR-activated increase in cAMP-dependent respiration. We compared ISO-induced OCR and ECAR in adipocytes from lean (BMI 21.7–24.6 kg/m2) or obese (BMI 30–35.5 kg/m2) donors. As seen in Fig. 7, the increase in OCR by ISO was impaired in adipocytes from obese subjects (Fig. 7A and B). ECAR was not changed (Fig. 7C and D). This deficit in OCR response from obese individuals was not due to impaired differentiation of the adipocytes, as measured by Nile Red staining and the expression of such differentiation markers as aP2, CCAAT/enhancer-binding protein (C/EBP)-α, PPARγ coactivator (PGC)-1α, PPARγ2, and hormone-sensitive lipase (not shown). There were also no discernible differences in mitochondrial mass, as measured by MTG (supplementary Fig. 4A) or by gene expression of mitochondrial proteins (cytochrome C, ATP synthase, and cytochrome C oxidase subunits [not shown]). Moreover, other parameters that were not different between lean and obese included ISO-stimulated lipolysis (supplementary Fig. 4B) or PKA activity (supplementary Fig. 4C), basal OCR (supplementary Fig. 4D), and βAR subtype expression (not shown). Interestingly, maximal OCR evoked by FCCP (supplementary Fig. 4E) was also significantly reduced in adipocytes from obese subjects, while basal respiration was less sensitive to Oligo (supplementary Fig. 4F).
FIG. 7.
ISO-induced OCR is reduced with obesity. Representative measurements of the percent increase in OCR (A) and ECAR (C), in response to ISO of adipocytes taken from lean or obese humans. Cells were pooled from five lean subjects (BMI 21.7–24.6 kg/m2) or five obese subjects (BMI 30–35.5 kg/m2). As indicated, at 0 min ISO (1 μmol/l, open symbols) or DMEM (closed symbols) was injected. Each data point is a mean of 6–8 wells, and error bars not visible are contained within the symbols. Histograms summarizing the maximum percent increase of OCR (B) or ECAR (D) in response to ISO and relative to baseline rates. Results are presented as fold vs. lean. Data are the mean of 12 lean and 9 obese samples; each was measured in three different experiments, containing six to eight replicates. *P < 0.001, compared with lean.
cAMP-induced mitochondrial uncoupling is mediated by BAX and the PTP.
A number of mitochondrial proteins have been associated with uncoupling induced by FAs (rev. in 2,28). These include members of the mitochondrial carrier family such as UCP1, the adenine nucleotide translocases (ANTs), phosphate carrier (PiC), aspartate/glutamate carrier (AGC), and the PTP complex. The subunit composition of the PTP is not completely clear (28,29). It is thought to consist of the integral membrane proteins ANT and voltage-dependent anion channel 1 (VDAC1) and regulatory proteins of the BCL-2 family, which control the open-closed states of the pore (29,30).
We assessed the involvement, if any, of major proteins in these groups in WAT that could be candidates for mediating this cAMP-triggered respiration in white adipocytes. First, we measured their expression levels in human white adipocytes by RT-PCR. Expression of ANT3, PiC, UCP2, VDAC1, BAX, and to a lesser extent ANT2/1 is relatively abundant in white adipocytes, whereas that of UCP1, UCP5, ANT4, and AGC is very low (supplementary Fig. 5A). Therefore, we focused on the former group, measuring the OCR response to ISO after targeted reduction of these candidates. The involvement of UCP2 was assessed using WAT from Ucp2+/+ and Ucp2−/− mice (18,19), and the increase in OCR was identical between the two genotypes (supplementary Fig. 5C). Expression of ANT3, ANT2, PiC, VDAC1, or BAX in human adipocytes was suppressed by siRNAs. The efficiency of targeted knock-down was 75–85% versus the scrambled siRNA (supplementary Fig. 5B). The OCR response was unchanged in cells receiving the siRNAs for PiC, ANT3, ANT2, ANT2+ANT3, or VDAC1 (supplementary Fig. 5C). However, knock-down of BAX, as shown in Fig. 8A and B, resulted in a significant decrease in the OCR response to ISO (Fig. 8C and D). Because BAX can increase mitochondrial uncoupling by opening the PTP (30), we also treated cells with the specific PTP inhibitor CSA (29) prior to measuring cAMP-induced OCR. Figure 8E and F shows that CSA similarly inhibited the OCR response to either ISO or FSK. Together these results suggest an important role for BAX to regulate PTP opening for this cAMP and FA-induced mitochondrial uncoupling in white adipocytes.
DISCUSSION
The ability of adipocytes to oxidize FAs and uncouple mitochondrial respiration in response to βAR activation is best understood in brown adipocytes, where the high density of mitochondria and the presence of the unique protein UCP1 allow for robust OCR and energy expenditure. Obviously, white adipocytes have less oxidative capacity than brown adipocytes, and their ability to oxidize FA is much lower (24,31). Nevertheless, a case can be made for assessing the relevance of WAT as a target for increasing energy expenditure based on the sheer amount of the tissue as a percentage of body mass. In fact, evidence exists in rodent adipocytes for a moderate increase in FA oxidation in response to βAR activation (12,24), and various futile cycles have been examined in adipocytes (32).
Several years ago, a collection of unrelated reports suggested that epinephrine could increase OCR in rat epididymal fat pads ([33] and references therein) or primary cultures of rat adipocytes (14). βAR-stimulated lipolysis in these adipocytes was also associated with both FA-dependent IMM depolarization (15,34) and a decrease in ATP levels (14,15,34,35). A more direct link between lipolysis and ATP levels was shown in 3T3-L1 cells in which the generic lipase inhibitor orlistat suppressed FSK-stimulated lipolysis as well as the drop in ATP content (36). Because a high OCR together with mitochondrial depolarization and ATP reduction are common characteristics of mitochondrial uncoupling, all together these studies suggest there is mitochondrial uncoupling in response to βAR activation. However, no single study has presented cohesive evidence for βAR-induced uncoupling in white adipocytes, especially in human adipocytes, and a molecular mechanism was not identified.
Here, we find an acute increase in the cellular energetics of human white adipocytes in response to βAR activation or cAMP-elevating agents that is dependent on the FAs liberated by lipolysis. In following cAMP-induced changes in cellular respiration over time, the increases in both aerobic (measured as OCR) and anaerobic (measured as ECAR) respiration were immediate and transient. The ECAR response was faster than OCR, implying that glucose oxidation preceded FA oxidation. Importantly, a major portion of cAMP-induced OCR was Oligo-insensitive, implying that some respiration was independent of ATP synthase activity. These results, together with mitochondrial depolarization, represent mitochondrial uncoupling.
The physiological significance of mitochondrial uncoupling in white fat is not fully understood and is debated (13,31,37) (rev. in 38). One consequence of mitochondrial uncoupling in WAT is an increase of the AMP/ATP ratio and activation of AMP kinase to 1) promote ATP-generating processes such as FA and glucose oxidation ([38,39] and references therein) and 2) inhibit ATP-consuming pathways such as lipogenesis, triglyceride synthesis and to some extent lipolysis (36,38,40,41). Another outcome of uncoupling in WAT could be protection against mitochondrial-derived reactive oxygen species and oxidative stress directly within the adipocyte, which can lead to cellular damage (12,36,42). Interestingly, we observed that adipocytes derived from obese donors had a significantly lower OCR response to βAR activation. In adipose tissue from obese individuals, there is evidence for an impaired lipolytic response in vivo and a reduced mitochondrial mass (10,43), with a variety of mechanisms proposed (rev. in 26,27). Although we did not observe a difference in lipolysis, our results suggest that there is a difference in the ability of the mitochondria from obese versus lean subjects to increase respiratory activity in response to βAR stimulation, mirroring notions that an impaired response of WAT to catecholamines at some level could be significant in the development or maintenance of obesity (27).
The ability of FAs to uncouple mitochondrial respiration has been extensively investigated, particularly in tissues such as BAT, muscle, and liver because of their high aerobic capacity. The molecular basis mediating FA-induced uncoupling has been vigorously discussed, and a number of mitochondrial carriers have been suggested (2,28,44,45). In BAT, the general consensus is that FAs allosterically activate the “carrier” or “channel” properties of UCP1 (46) (mechanistically still debated in some circles). By comparison, in WAT UCP1 levels are fairly negligible whereas the UCP1 homologue, UCP2, is abundant (47). UCP2 has periodically been proposed to mediate proton leak or mitochondrial uncoupling ([1] and references therein), but this point is not fully agreed upon (48,49). However, regarding UCP2 there was no difference in the OCR response to ISO in WAT of both wild-type and Ucp2-null mice. Moreover, the contribution of ANT2, ANT3, PiC, and VDAC1 was excluded. Instead, a significant portion of βAR and cAMP-induced OCR was inhibited by the specific PTP inhibitor, CSA, as well as by suppressing BAX expression—altogether suggesting that the opening of the mitochondrial PTP by the regulatory protein BAX is important for the observed mitochondrial uncoupling in WAT. Given that the amplitude of the OCR and uncoupling response in the obese subjects was reduced, we measured whether there was a change in expression of BAX and the PTP in obese WAT. In our sample set, no differences were detected in the expression levels of BAX (or VDAC1 or ANTs) between lean and obese WAT. However, proteins of the BCL-2 family are known to be post-translationally modified, which controls their activity and regulates PTP opening (30,50). Such modifications in WAT mitochondria could reasonably be a basis for the observed differences in mitochondrial function between lean and obese, but will require further study and must be extended to a larger sample set.
Because white adipocytes comprise the bulk of adipose tissue in the body, even a moderate, sustained ability of adipocytes to oxidize FAs could have several positive consequences. First, limiting the amount of FAs released into the circulation (13,24,37) would be protective against lipotoxicity in other tissues, and second, it might contribute to whole body energy expenditure. The concept of mitochondrial uncoupling as a strategy to reduce body weight has been clearly demonstrated with the past clinical use of such strong global uncouplers as 2,4-dinitrophenol, but that enterprise was not without major side effects, including fatalities (rev. in 48). Therefore, greater understanding of the mechanism(s) responsible for this cAMP-dependent uncoupling in WAT and the role of the PTP is warranted. The potential to identify novel agents and/or processes that could be harnessed for selectively stimulating moderate uncoupling are needed to combat the metabolic disease epidemic.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the North Carolina Biotechnology Center (CFG-8006).
No potential conflicts of interest relevant to this article were reported.
E.Y.-S. performed experiments and wrote the manuscript. B.B. and J.P. performed experiments and contributed to the discussion. N.K. performed experiments. S.C. wrote and edited the manuscript.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The authors acknowledge Seahorse Bioscience for their advice and technical support of this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 2005;33:897–904 [DOI] [PubMed] [Google Scholar]
- 2. Rial E, Zardoya R. Oxidative stress, thermogenesis and evolution of uncoupling proteins. J Biol 2009;8:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabete Metab Res Rev 2002;18(Suppl. 2):S5–S9 [DOI] [PubMed] [Google Scholar]
- 4. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–E452 [DOI] [PubMed] [Google Scholar]
- 5. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 6. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 8. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. Faseb J 2009;23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 9. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 2004;114:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenson BM, Rydén M, Steffensen KR, Wåhlén K, Pettersson AT, Jocken JW, Arner P, Laurencikiene J. Activation of liver X receptor regulates substrate oxidation in white adipocytes. Endocrinology 2009;150:4104–4113 [DOI] [PubMed] [Google Scholar]
- 12. Cho SY, Park PJ, Shin ES, Lee JH, Chang HK, Lee TR. Proteomic analysis of mitochondrial proteins of basal and lipolytically (isoproterenol and TNF-alpha)-stimulated adipocytes. J Cell Biochem 2009;106:257–266 [DOI] [PubMed] [Google Scholar]
- 13. Maassen JA, Romijn JA, Heine RJ. Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 2007;50:2036–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hepp D, Challoner DR, Williams RH. Respiration in isolated fat cells and the effects of epinephrine. J Biol Chem 1968;243:2321–2327 [PubMed] [Google Scholar]
- 15. Davis RJ, Martin BR. The effect of beta-adrenergic agonists on the membrane potential of fat-cell mitochondria in situ. Biochem J 1982;206:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 1995;96:2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robidoux J, Kumar N, Daniel KW, Moukdar F, Cyr M, Medvedev AV, Collins S. Maximal beta3-adrenergic regulation of lipolysis involves Src and epidermal growth factor receptor-dependent ERK1/2 activation. J Biol Chem 2006;281:37794–37802 [DOI] [PubMed] [Google Scholar]
- 18. Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 2000;26:435–439 [DOI] [PubMed] [Google Scholar]
- 19. Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, Brownlee M, Corkey BE, Collins S. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology 2009;150:3040–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 2007;292:C125–C136 [DOI] [PubMed] [Google Scholar]
- 21. Kumar N, Robidoux J, Daniel KW, Guzman G, Floering LM, Collins S. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem 2007;282:9244–9250 [DOI] [PubMed] [Google Scholar]
- 22. Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 1990;265:5267–5272 [PubMed] [Google Scholar]
- 23. Botelho LH, Rothermel JD, Coombs RV, Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol 1988;159:159–172 [DOI] [PubMed] [Google Scholar]
- 24. Wang T, Zang Y, Ling W, Corkey BE, Guo W. Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res 2003;11:880–887 [DOI] [PubMed] [Google Scholar]
- 25. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009;50:3–21 [DOI] [PubMed] [Google Scholar]
- 26. Dodt C, Lönnroth P, Wellhöner JP, Fehm HL, Elam M. Sympathetic control of white adipose tissue in lean and obese humans. Acta Physiol Scand 2003;177:351–357 [DOI] [PubMed] [Google Scholar]
- 27. Jocken JW, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav 2008;94:219–230 [DOI] [PubMed] [Google Scholar]
- 28. Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim Biophys Acta 2006;1757:1330–1337 [DOI] [PubMed] [Google Scholar]
- 29. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 2009;46:821–831 [DOI] [PubMed] [Google Scholar]
- 30. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999;13:1899–1911 [DOI] [PubMed] [Google Scholar]
- 31. Frayn KN, Langin D, Karpe F. Fatty acid-induced mitochondrial uncoupling in adipocytes is not a promising target for treatment of insulin resistance unless adipocyte oxidative capacity is increased. Diabetologia 2008;51:394–397 [DOI] [PubMed] [Google Scholar]
- 32. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 2010;281:31894–31908 [DOI] [PubMed] [Google Scholar]
- 33. Ball EG, Jungas RL. On the action of hormones which accelerate the rate of oxygen consumption and fatty acid release in rat adipose tissue in vitro. Proc Natl Acad Sci U S A 1961;47:932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallano ML, Lee MY, Sonenberg M. Hormones modulate adipocyte membrane potential ATP and lipolysis via free fatty acids. Am J Physiol 1983;245:E266–E272 [DOI] [PubMed] [Google Scholar]
- 35. Angel A, Desai KS, Halperin ML. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res 1971;12:203–213 [PubMed] [Google Scholar]
- 36. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 2008;283:16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maassen JA, Romijn JA, Heine RJ. Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: do adipocytes consume sufficient amounts of oxygen to oxidise fatty acids? Diabetologia 2008;51:907–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kopecký J, Rossmeisl M, Flachs P, Bardová K, Brauner P. Mitochondrial uncoupling and lipid metabolism in adipocytes. Biochem Soc Trans 2001;29:791–797 [DOI] [PubMed] [Google Scholar]
- 39. Rognstad R, Katz J. The effect of 2,4-dinitrophenol on adipose-tissue metabolism. Biochem J 1969;111:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol 2006;574:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huber CT, Duckworth WC, Solomon SS. The reversible inhibition by carbonyl cyanide m-chlorophenyl hydrazone of epinephrine-stimulated lipolysis in perifused isolated fat cells. Biochim Biophys Acta 1981;666:462–467 [DOI] [PubMed] [Google Scholar]
- 42. Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab 2007;293:E159–E164 [DOI] [PubMed] [Google Scholar]
- 43. Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjölin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 2007;50:2526–2533 [DOI] [PubMed] [Google Scholar]
- 44. Andreyev AYu, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Tsofina LM, Volkov NI, Vygodina TV. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem 1989;182:585–592 [DOI] [PubMed] [Google Scholar]
- 45. Wojtczak L, Schönfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta 1993;1183:41–57 [DOI] [PubMed] [Google Scholar]
- 46. Garlid KD, Jabůrek M, Jezek P. The mechanism of proton transport mediated by mitochondrial uncoupling proteins. FEBS Lett 1998;438:10–14 [DOI] [PubMed] [Google Scholar]
- 47. Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 1997;15:269–272 [DOI] [PubMed] [Google Scholar]
- 48. Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2001;2:255–265 [DOI] [PubMed] [Google Scholar]
- 49. Nedergaard J, Ricquier D, Kozak LP. Uncoupling proteins: current status and therapeutic prospects. EMBO Rep 2005;6:917–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yehuda-Shnaidman E, Kalderon B, Azazmeh N, Bar-Tana J. Gating of the mitochondrial permeability transition pore by thyroid hormone. Faseb J 2010;24:93–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.