Abstract
OBJECTIVE
Glucose-stimulated islet insulin or C-peptide secretion experiments are a fundamental tool for studying and assessing islet function. The goal of this work was to develop Ab-based fluorescent homogenous sensors that would allow rapid, inexpensive, near-instantaneous determinations of insulin and C-peptide levels in biological samples.
RESEARCH DESIGN AND METHODS
Our approach was to use molecular pincer design (Heyduk et al., Anal Chem 2008;80:5152–5159) in which a pair of antibodies recognizing nonoverlapping epitopes of the target are modified with short fluorochrome-labeled complementary oligonucleotides and are used to generate a fluorescence energy transfer (FRET) signal in the presence of insulin or C-peptide.
RESULTS
The sensors were capable of detecting insulin and C-peptide with high specificity and with picomolar concentration detection limits in times as short as 20 min. Insulin and C-peptide levels determined with the FRET sensors showed outstanding correlation with determinations performed under the same conditions with enzyme-linked immunosorbent assay. Most importantly, the sensors were capable of rapid and accurate determinations of insulin and C-peptide secreted from human or rodent islets, verifying their applicability for rapid assessment of islet function.
CONCLUSIONS
The homogeneous, rapid, and uncomplicated nature of insulin and C-peptide FRET sensors allows rapid assessment of β-cell function and could enable point-of-care determinations of insulin and C-peptide.
Diabetes comprises a heterogeneous group of hyperglycemic disorders. There are two major forms of diabetes: 1) type 1 diabetes, which is associated with an autoimmune-mediated attack and destruction of pancreatic β-cells resulting in insulin deficiency, and 2) type 2 diabetes, which is characterized by insufficient insulin production or impaired insulin action. For type 1 diabetic subjects, insulin injections are used to regulate plasma glucose levels, while type 2 diabetic subjects are usually treated by diet, oral agents, and insulin therapy. If uncontrolled, elevated plasma levels of glucose increase the risk for the development of diabetic complications such as cardiovascular disease, kidney disease, neurological disorders, and blindness. Intensive insulin therapy reduces the risk of such complications, although there is an increased risk of hypoglycemic episodes with this therapy, which, if severe, can result in coma or seizures. Insulin is synthesized as a precursor protein, proinsulin, that is processed by specific proteases found in insulin granules to the active hormone (containing an A and B chain connected by two disulfide linkages). During the processing of insulin, the connecting sequence (C-peptide) is also produced, and C-peptide is released into the bloodstream with insulin at times of insulin demand.
Glucose-stimulated insulin or C-peptide secretion by the islets is a fundamental tool for studying and assessing islet function. Current methodologies to determine β-cell function rely on the use of radioimmunoassay or enzyme-linked immunosorbent assay (ELISA)-based assays to detect either insulin or C-peptide produced by β-cells. These methods are time-consuming and, in many cases, require the use of radioactivity. Development of a specific methodology for detecting insulin and/or C-peptide that reduces the time required to determine β-cell function would provide a tremendous advantage over methods currently being used. Such a methodology could also have clinical applications, for example, as a rapid method to characterize β-cell function in the transplantation of human islets, a potential therapy for type 1 diabetic patients. Transplantation of human islets, isolated from cadaver donors, has been used for a number of years in an effort to gain insulin independence in type 1 diabetic subjects; however, this procedure has been only marginally successful (1,2). Recently, the Edmonton group has described a new protocol for the transplantation of human islets that was successful in attaining short-term insulin independence in seven of seven patients (3), and similar results have been reproduced by others (4–6). One key feature of the Edmonton protocol is the immediate transplantation of islets after isolation. Previous protocols cultured islets for extended periods of time in part to determine islet viability and function prior to transplantation (7). A methodology that would allow rapid assessment of glucose-stimulated insulin secretion potentially could be used to enhance characterization of islets before transplantation.
In a previous study, we described a new Ab-based sensor technology that allowed simple fluorescence-based homogenous detection of target proteins (7). The goal of this work was to determine whether this sensor design could be adapted for rapid detection of insulin and C-peptide and used for very rapid determination of islet functional activity (insulin secretion). We show that our sensors allow near-instantaneous determination of the insulin or C-peptide produced by isolated human or rodent islets, validating their applicability for rapid assessment of islet quality. We believe that the uncomplicated and rapid characteristics of our assay will allow for the deployment of methods for insulin and C-peptide determinations in a point-of-care setting.
RESEARCH DESIGN AND METHODS
Sensor design.
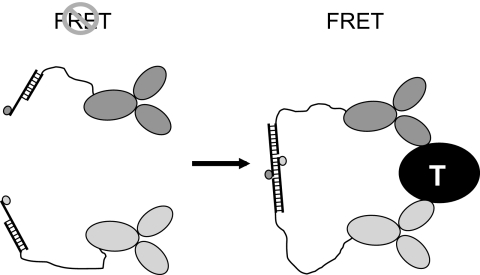
Figure 1 illustrates general design of homogenous sensors for insulin or C-peptide that is based on previously described molecular pincer assay (7). A pair of antibodies recognizing nonoverlapping epitopes of insulin (or C-peptide) are functionalized by attaching short complementary oligonucleotides via long nanometer-scale flexible linkers. Oligonucleotides are modified with a pair of fluorophores (fluorescein and Cy5) that could function as a donor and an acceptor in fluorescence resonance energy transfer (FRET) (8). In the presence of the target analyte, both antibodies will bind to the target molecule, resulting in a large increase of the local concentration of signaling oligonucleotides. This in turn will lead to annealing of the oligonucleotides bringing the fluorophores to close proximity, resulting in efficient FRET that can be used as a signal for target analyte detection.
FIG. 1.
Design of homogenous sensors for detecting insulin and C-peptide. “T” denotes a target (insulin or C-peptide).
Materials.
NHS-PEO8-maleimide and Traut's reagent were from Pierce (Rockland, IL). Oligonucleotides were obtained either from Keck Oligonucleotide Synthesis Facility at Yale University or from IDT (Coralville, IA). The following oligonucleotides were used (names in parentheses):
(A1) 5′-C6-amino-TAGGTGCTCGACGCTGAC
(A2) 5′-C6-amino-TAGGAGAGAGAGAGAGGA
(A3) 5′-fluorescein-GCTCATTGTCAGCGTCGAGCACCTA
(A4) 5′-Cy5-ATGAGCTTCCTCTCTCTCTCTCCAT
A3 and A4 oligonucleotides contain short sequences (italicized) that are used in target-induced annealing to generate FRET signal. Fluorescein and Cy5-labeled oligonucleotides were purified by reverse-phase high-performance liquid chromatography (9). Concentrations of oligonucleotides were calculated from UV absorbance at 260 nm, after correction for fluorophore absorbance at 260 nm.
Monoclonal anti-human insulin antibodies (clones E6E5, D4B8, 8E2, and 3A6) were purchased from Fitzgerald Industries International, Inc. (Concord, MA). Monoclonal anti-human C-peptide antibodies (clones 9101 and 9103) were from BiosPacific (Emeryville, CA). Insulin and C-peptide ELISA kits (EZH-14C and EZHCP-20C) were purchased from Millipore (Billerica, MA) and were used according to the manufacturer's instruction with the exception of using our assay buffer (20 mmol/l Tris-HCl [pH 8.0], 100 mmol/l NaCl, and 10 mmol/l EDTA containing 0.2 mg/ml BSA) and standards prepared by us in place of those provided in the ELISA kit.
Human, bovine, and porcine insulin, glucagon, insulin-like growth factor II, human C-peptide, angiotensin I and II, and ACTH were purchased from Sigma (St. Louis, MO). Canine and porcine C-peptide were obtained from Anaspec (Fremont, CA), and human proinsulin was from R&D Systems (Minneapolis, MN).
Antibody modification.
Antibodies were labeled with signaling oligonucleotides using a previously described procedure (7) (method “c” in Fig. 1B from ref 7). A1 and A2 oligonucleotides were first attached to the antibodies via long linkers followed by annealing of A3 and A4 oligonucleotides to produce Ab-A1/A3 and Ab-A2/A4 conjugates, respectively. The first step of the procedure involves preparation of a thiol-reactive oligonucleotide that is subsequently used to react with thiolated antibody. Two hundred microliters of 5′-amine containing oligonucleotides (A1 or A2) at ∼250 μmol/l in 20 mmol/l NaH2PO4 (pH 7.4), 150 mmol/l NaCl, and 2.5 mmol/l EDTA buffer (conjugation buffer) were mixed with 5 μl of ∼250 mmol/l of NHS-PEO8-maleimide dissolved in dimethylformamide. The reaction mixtures were incubated for 1–1.5 h at room temperature. Oligonucleotide was purified from the excess of the cross-linker by ethanol precipitation in the presence of 1 mg/ml glycogen. Precipitated oligonucleotides were dried in Speed-Vac and were stored at −20°C until they were used for antibody modification. Antibody solutions (50–75 μl) containing 0.3–0.4 mg of the protein were run on a spin column (Zeba, Pierce, Rockford, IL) equilibrated with the conjugation buffer. Antibodies were thiolated for 1.5 h at room temperature with 40 molar excess of Traut's reagent added as ∼14 mmol/l stock solution in dimethylformamide. The excess of Traut's reagent was removed on a Zeba spin column equilibrated in the conjugation buffer. The thiolated antibody was then reacted with a 15–20 molar excess of linker-conjugated oligonucleotide (calculated assuming that ∼50% of the oligonucleotides were conjugated with the cross-linker). Reaction mixtures were incubated for 4 h at room temperature followed by an overnight incubation at 4°C.
Modified antibodies were purified from the excess of the oligonucleotides by size-exclusion fast-protein liquid chromatography using a 10/30GL Superdex 200 column (Pharmacia) equilibrated with 10-fold–diluted 20 mmol/l Tris (pH 8.0), 100 mmol/l NaCl, and 10 μmol/l EDTA buffer. Fractions containing modified antibodies were pooled and concentrated 10-fold in the Speed-Vac. The protein concentration was estimated using Bradford assay. Labeling of the antibodies with oligonucleotides was confirmed (and the extent of the labeling estimated) by analyzing the UV spectra of the purified final product. Observed spectra were fitted by a linear combination of the spectra of free antibody and free oligonucleotide to determine relative amounts of the protein and oligonucleotide in the sample.
Islet isolation.
Human islets were isolated from cadaver donors using protocols approved by the IRB at the University of Alabama in Birmingham. Rodent islets were isolated from male Sprague-Dawley rats (250–300 g) by collagenase digestion as previously described (10).
Islets were cultured overnight in CMRL-1066 (containing 2 mmol/l l-glutamine, 10% heat-inactivated FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin) at 37°C under an atmosphere of 95% air and 5% CO2 prior to experimentation.
Glucose-stimulated insulin secretion.
The islets were washed with Krebs-Ringer buffer (KRB) (25 mmol/l HEPES, 115 mmol/l NaCl, 24 mmol/l NaHCO3, 5 mmol/l KCl, 1 mmol/l MgCl2, 2.5 mmol/l CaCl2, and 0.1% BSA, pH 7.4) containing 3.3 mmol/l glucose followed by preincubation for 30-min at 37°C in KRB containing 3.3 mmol/l glucose. The islets (∼10 and ∼15 in the case of human or rodent, respectively) were aliquoted to vials containing either KRB with 3.3 mmol/l glucose or KRB with 16.7 mmol/l glucose and were incubated for 60 min (human islets) (KRB with 20 mmol/l glucose and 30-min incubation in the case of rodent islets) at an atmosphere of 95% air and 5% CO2 at 37°C. After the incubation, the supernatant was removed and the samples were stored frozen at −70°C until analyzed. In experiments where FRET sensors were compared with ELISA, the samples were thawed and analyzed in parallel by sensors and ELISA to eliminate any variability due to differences in sample treatment.
Insulin and C-peptide determinations.
All insulin and C-peptide measurements were performed in 20 mmol/l Tris-HCl (pH 8.0), 100 mmol/l NaCl, and 10 μmol/l EDTA containing 0.2 mg/ml BSA (assay buffer). Standard solutions of insulin and C-peptide were prepared from stock solutions obtained by dissolving human insulin and C-peptide (from Sigma) in assay buffer. Concentrations of stock solutions were assigned based on information provided by the supplier. The standards were not calibrated to World Health Organization reference material. Stock solutions were stored frozen in small aliquots at −70°C. No significant differences were observed between the results obtained with different samples of stock insulin or C-peptide solutions. When comparing FRET sensors with ELISA, to eliminate variability of the results due to potential instability of calibrators included in commercial ELISA kits, FRET sensors and ELISA experiments were done in parallel using the same solutions of insulin or C-peptide standards for preparation of FRET sensor or ELISA calibration curves. Thus, ELISA was not performed exactly according to the manufacturer's instructions. While this may have affected somewhat the performance of ELISA, it was important for the purpose of validating sensor assay to perform sensor assays and ELISA under the same conditions. Typically, for a standard curve, eight samples of insulin (or C-peptide) at concentrations in the 0–15 nmol/l range were prepared by diluting stock solutions in assay buffer. Standard curves for quantitative determinations performed by ELISA were obtained by assaying 2.5–5 μl of standard solutions diluted to 60 μl with the assay buffer. Standard curves using the fluorescent sensor methodology were obtained by assaying 5 μl of standard solutions diluted into 20 μl of assay mix. Concentrations of insulin and C-peptide in unknown samples were determined from the previously described calibration curves using absorbance values (ELISA) obtained with 2.5–5 μl of the samples diluted to 60 μl with the assay buffer or using fluorescence values (sensors) obtained with 5 μl of the samples diluted into 20 μl of assay mix. Because the samples from islet secretion experiments were in modified Krebs-Ringer bicarbonate buffer, 5 μl of the buffer was included in the 20 μl assay mix used to measure calibration standards in this case. Absorbance at 450 nm of ELISA samples was measured in 96-well plates using SpectrofluorPLus plate reader (Tecan). Fluorescences of sensor mixes were measured in 20 μl in 384-well low-volume black microplates (Corning cat. no. 3676) at 25°C. Typically, sensor assay mixtures contained (final concentrations in 20 μl) 20 and 25 nmol/l or 10 and 12.5 nmol/l of fluorescein-labeled antibody and Cy5-labeled antibody, respectively. Prior to fluorescence measurements, the A1- and A2-labeled antibodies were annealed with fluorescent A3 and A4 oligonucleotides by incubating 100 nmol/l antibodies with equimolar amounts of A3 or A4 for 30 min at room temperature. These 100 nmol/l stocks of labeled antibodies were then used to prepare 2× sensor mix solutions in the assay buffer. Appropriate volumes of samples and assay buffer were then added to 10 μl of 2× assay solutions to obtain final assay volume of 20 μl that was used for fluorescence measurements.
The donor (fluorescein; excitation at 485 nm, emission at 535 nm) and sensitized acceptor emission (Cy5; excitation at 485 nm, emission at 665 nm) signals were read with an Analyst AD plate reader (LJL Biosystems, Sunnyvale, CA). Reaction mixtures were incubated for at least 20 min before fluorescence measurements were made (with the exception of kinetic experiments, where incubation time varied).
Results of fluorescence measurements were expressed as normalized FRET signal change (ΔFRET):
where FSA, FD, FSA0, and FD0 are sensitized acceptor and donor emission intensities in the presence and absence of insulin (or C-peptide), respectively. Buffer background was subtracted from the measured fluorescence intensities before FRET values were calculated. Using the ratio of sensitized acceptor and donor emission intensities for calculating FRET according to Eq. 1 reduces the variability of FRET measurements due to dilution and instrumentation errors. Radioimmunoassay (RIA) determinations of insulin in rat islet secretion experiments were performed by Washington University DRTC (St. Louis, MO).
RESULTS
We have previously demonstrated feasibility of the sensor design illustrated in Fig. 1 using thrombin, cardiac troponin, and C-reactive proteins as models and using antibodies (7) or aptamers (11) as the binders. The assay depicted in Fig. 1 requires only adding the sample to the sensor mix followed by a simple fluorescence intensity measurement. We thus hypothesized that, if the sensors with adequate sensitivity and specificity could be developed for insulin or C-peptide, they could be an extremely useful tool in rapid assessment of islet function.
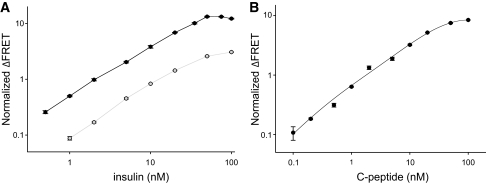
We first prepared an insulin sensor using anti-insulin monoclonal antibody clones E6E5 and D4B8 (Fitzgerald Industries International). When titrated with increasing concentrations of insulin, a robust and highly reproducible insulin concentration–dependent FRET signal was observed (Fig. 2A, gray symbols). While robust, the signal was lower when compared with what we have previously observed in the case of troponin or C-reactive protein (7). We wondered if the antibody pair we selected for the sensors was indeed the most optimal. We tested another monoclonal antibody pair (clones 8E2 and 3A6 from Fitzgerald Industries International, Inc.) that, according to the manufacturer's specifications, had higher insulin-binding affinity. The sensors prepared from this antibody pair exhibited a much better (∼5-fold higher) FRET signal in the presence of insulin (Fig. 2A, black symbols). Limit of detection was also improved substantially (from ∼1 nmol/l [gray symbols] to ∼100 pmol/l [black symbols]). The upper limit of detection is a function of sensor concentration used and, thus, can be tailored to a desired value by changing the sensor concentration (data not shown). Analytical sensitivity (defined as the ratio of the mean of the measured concentrations to the actual concentrations) was 1.13 ± 0.13. Comparison of these two datasets obtained with two pairs of monoclonal antibodies shows that the binding affinity of the antibodies that are used for preparing the sensors is a key determinant of the performance of the sensors.
FIG. 2.
A: Response of the insulin sensor to indicated amounts of human insulin. Experiments were performed with a sensor mixture containing 20 nmol/l fluorescein-labeled antibody and 25 nmol/l of Cy5-labeled antibody. Black and gray symbols correspond to experiments performed with sensors using 8E2/3A6 and E6E5/D4B8 antibody pairs, respectively. The average and SD of three measurements are shown. B: Response of the human C-peptide sensor to indicated amounts of C-peptide. Experiments were performed with a sensor mixture containing 20 nmol/l fluorescein-labeled antibody and 25 nmol/l of Cy5-labeled antibody. The average and SD of three measurements are shown. FRET signals were read after a minimum of 20 min of incubation after adding sample to the sensor mix.
In the next set of experiments, a sensor of similar quality to that obtained for insulin was developed for C-peptide. Figure 2B shows that, as in the case of insulin, a C-peptide sensor exhibited a robust and highly reproducible C-peptide concentration–dependent response. The estimated limit of detection (∼50 pmol/l) was slightly better than the limits for the insulin sensor. Analytical sensitivity was 0.99 ± 0.06.
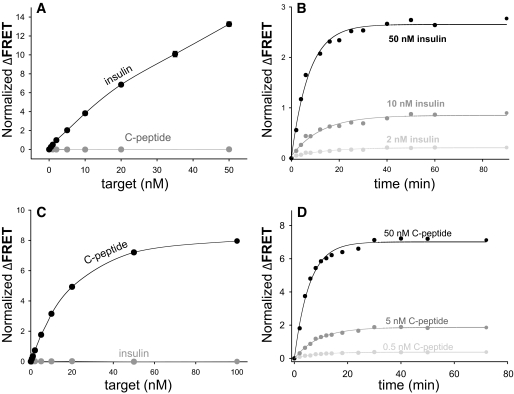
The data shown in Fig. 3A demonstrate specificity of the insulin sensor. While a robust response in the presence of insulin is observed, no FRET signal was detected in the presence of the negative control (C-peptide). More extensive data regarding insulin sensor specificity is summarized in supplementary Table S9 (available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0088/DC1). No significant cross-reactivity was observed for any of the C-peptides or various unrelated polypeptides tested. The sensor could detect bovine, porcine, and rat insulin as well (in some cases better) as human insulin. Partial cross-reactivity with human proinsulin was observed. Specificity of the sensors reflects specificity of the antibodies that were used to prepare them so it could be altered by using a different set of antibodies for sensor preparation, if available. We expected that our sensors, because of their homogenous nature, would allow quick, uncomplicated measurement of insulin in a sample. Figure 3B shows the time dependence of insulin sensor response (FRET signal) upon addition of indicated amounts of insulin to the sensor mix. Full response was obtained within ∼20 min, with ∼50% of the maximum signal observed 5 min after addition of the test sample. Data in Fig. 3B demonstrate a key feature of our sensors, the ability to detect insulin in a very rapid manner using a simple homogenous assay format that does not require specialized instrumentation (all the data were collected using a standard fluorescence plate reader). The C-peptide sensor was also highly specific, as the FRET signal was not observed in the presence of human insulin (Fig. 3C). More extensive data regarding C-peptide sensor specificity are summarized in supplementary Table S10. No significant cross-reactivity was observed for any of the C-peptides from other species, any insulins, or various unrelated polypeptides tested. Partial cross-reactivity with human proinsulin was observed. The time course of the response of the C-peptide sensor (Fig. 3D) was very similar to the insulin sensor requiring ∼20 min for the maximal FRET signal and exhibiting >50% of maximal signal change after 5 min of incubation.
FIG. 3.
A: Specificity of insulin sensor. Response of insulin sensor to human insulin and human C-peptide is compared. Sensor mixture containing 20 nmol/l fluorescein-labeled 8E2 antibody and 25 nmol/l of Cy5-labeled 3A6 antibody was used. FRET signals were read after a minimum of 20 min of incubation after adding sample to the sensor mix. B: Kinetics of insulin sensor response at indicated human insulin concentrations. Sensor mixture containing 20 nmol/l fluorescein-labeled E6E5 antibody and 25 nmol/l of Cy5-labeled D4B8 antibody was used. C: Specificity of C-peptide sensor. Response of C-peptide sensor to human C-peptide and human insulin is compared. FRET signals were read after a minimum of 20 min of incubation after adding sample to the sensor mix. D: Kinetics of C-peptide sensor response at indicated C-peptide concentrations. Sensor mixtures containing 20 nmol/l fluorescein-labeled antibody and 25 nmol/l of Cy5-labeled antibody were used.
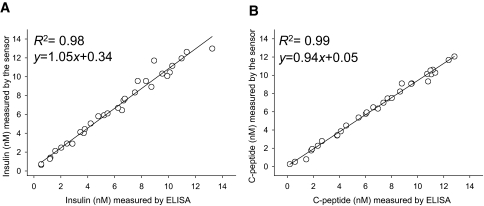
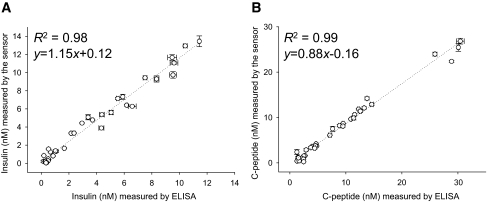
The data presented in Figs. 2 and 3 established the feasibility of homogenous FRET sensors for insulin and C-peptide. To examine the performance of these sensors for quantitative determination of insulin and C-peptide, we compared our sensors with standard and established methodologies used in commercially available ELISA kits (Fig. 4). Thirty-two samples containing randomly chosen concentrations of insulin or C-peptide (in the 0–14 nmol/l range) were prepared in the assay buffer. The levels of insulin (Fig. 4A) and C-peptide (Fig. 4B) in these samples were quantified in parallel using our sensors and ELISA. There was outstanding correlation between the two methods (R2 ≥ 0.98; Fig. 4) demonstrating the utility of FRET sensors for quantitative determination of insulin and C-peptide.
FIG. 4.
Correlation between measurements using the sensors and ELISA in 32 samples containing randomly selected concentrations of human insulin (A) and human C-peptide (B) dissolved in a buffer.
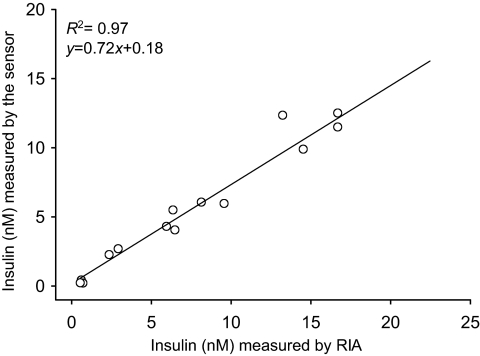
To confirm the functionality and to examine the practical utility of our sensors, the levels of insulin and C-peptide were determined in randomly chosen samples from glucose-stimulated secretion experiments performed with human islets. Thirty-two samples from secretion experiments at low and high glucose levels were analyzed in parallel by FRET sensors and ELISA (Fig. 5). In every sample tested, it was possible to determine the level of insulin (or C-peptide) using FRET sensors, showing that the sensors could be used for measuring insulin and C-peptide secreted at low and high glucose levels. There was a cluster of data points in Fig. 5 at low insulin (or C-peptide) corresponding to low glucose samples and a more diffuse cluster at higher insulin (or C-peptide) corresponding to high glucose samples. For both insulin and C-peptide, the correlation between the results obtained with the sensors and ELISA was outstanding (R2 = 0.98 and 0.99, respectively), demonstrating the utility of the sensors for rapid assessment of islet function. Similarly, in randomly chosen samples from rodent islet secretion experiments, insulin levels were determined using our sensors and RIA assay (Fig. 6), and excellent correlation between the two techniques was observed.
FIG. 5.
Correlation between measurements of human insulin (A) and human C-peptide (B) using the sensors and ELISA in human islet secretion experiments. The average and SD of three measurements of 32 samples are shown.
FIG. 6.
Correlation between measurements of rat insulin using the sensors and RIA in 15 samples from rodent islet secretion experiments.
Results of experiments used to obtain basic performance characteristics of FRET sensor assays are presented in the supplementary data. Inter- and intra-assay precision (supplementary Tables S1–S4), spike and recovery (supplementary Tables S5 and S6), assay linearity (supplementary Tables S7 and S8), and assay cross-reactivity (supplementary Tables S9 and S10) were determined and were found to be similar to what is typically observed for ELISA.
DISCUSSION
Insulin and C-peptide sensors that we have developed allow rapid determination of insulin and C-peptide with high specificity and sensitivity by a procedure that requires only the addition of the sample to the sensor mix. The sensors exhibit sensitivity that is sufficient for rapid assessment of islet functionality by near-instantaneous measurement of insulin or C-peptide secreted by the islets. The use of the sensors does not require any complicated specialized instrumentation; any standard fluorescence plate reader will be sufficient.
Limits of detection that we observe with these sensors are near the concentrations of insulin and C-peptide observed in serum (for example, normal insulin concentrations in plasma are 40–160 pmol/l). However, in its current formulation, the sensors are not yet suitable for serum measurements. Although they do work with serum samples (data not shown), the significant background fluorescence produced by serum requires significant sample dilution. In order to use these sensors for serum measurements, the sensors will need to be further improved to increase their sensitivity (∼5- to 10-fold) and to reduce the background of the serum (by employing fluorescence probes with excitation at higher wavelength or by using time-resolved fluorescence signal detection). We believe these improvements will be possible, and if successful, they will widen significantly the applicability of our sensors. With these improvements, we envision that the sensors could be adopted for use with a simple handheld fluorescence reader, allowing point-of-care insulin and C-peptide determination.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Juvenile Diabetes Research Foundation (JDRF) grant 5-2007-347 and the National Institutes of Health (DK-52194). Special thanks to Juan L. Contreras (University of Alabama at Birmingham [UAB]) and the Islet Cell Resources Consortium including the UAB Islet Program consisting of Stacie M.J. Bryant (UAB), Hank K. Fortinberry (UAB), Brett D. Yancey (UAB), Henry K. Fisher (UAB), and Brad R. Stephen (UAB) (supported by grant U42-RR-023246 from the National Center for Research Resources [NCRR]) for providing samples from human islet insulin secretion experiments. The Islet Research Lab at UAB is supported by the cooperative efforts of the NCRR and the National Institute of Diabetes and Digestive and Kidney Diseases, in conjunction with the generous contributions of the JDRF.
T.H. has received research grant subcontracts from Mediomics, a company that is commercializing the molecular pincer assay design that was developed in T.H.'s laboratory and that was used in insulin and C-peptide homogenous assays described in this work. No other potential conflicts of interest relevant to this article were reported.
E.H. researched data, contributed to the discussion, reviewed/edited the manuscript, and wrote the manuscript. M.M.M. contributed to the discussion and reviewed/edited the manuscript. A.S. researched data and reviewed/edited the manuscript. J.A.C. and T.H. contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Boker A, Rothenberg L, Hernandez C, Kenyon NS, Ricordi C, Alejandro R. Human islet transplantation: update. World J Surg 2001;25:481–486 [DOI] [PubMed] [Google Scholar]
- 2. Biancone L, Ricordi C. Pancreatic islet transplantation: an update. Cell Transplant 2002;11:309–311 [PubMed] [Google Scholar]
- 3. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 4. Ricordi C, Hering BJ, Shapiro AM. Beta-cell transplantation for diabetes therapy. Lancet 2008;372:27–28 [DOI] [PubMed] [Google Scholar]
- 5. Shapiro AM, Ricordi C, Hering B. Edmonton's islet success has indeed been replicated elsewhere. Lancet 2003;362:1242. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 7. Heyduk E, Dummit B, Chang YH, Heyduk T. Molecular pincers: antibody-based homogeneous protein sensors. Anal Chem 2008;80:5152–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selvin PR. Fluorescence resonance energy transfer. Methods Enzymol 1995;246:300–334 [DOI] [PubMed] [Google Scholar]
- 9. Heyduk E, Heyduk T. Thiol-reactive, luminescent Europium chelates: luminescence probes for resonance energy transfer distance measurements in biomolecules. Anal Biochem 1997;248:216–227 [DOI] [PubMed] [Google Scholar]
- 10. Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 2003;83:3–14 [DOI] [PubMed] [Google Scholar]
- 11. Heyduk E, Heyduk T. Nucleic acid-based fluorescence sensors for detecting proteins. Anal Chem 2005;77:1147–1156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.