Abstract
OBJECTIVE
The fuel sensor AMP-activated protein kinase (AMPK) in the hypothalamus regulates energy homeostasis by sensing nutritional and hormonal signals. However, the role of hypothalamic AMPK in glucose production regulation remains to be elucidated. We hypothesize that bidirectional changes in hypothalamic AMPK activity alter glucose production.
RESEARCH DESIGN AND METHODS
To introduce bidirectional changes in hypothalamic AMPK activity in vivo, we first knocked down hypothalamic AMPK activity in male Sprague-Dawley rats by either injecting an adenovirus expressing the dominant-negative form of AMPK (Ad-DN AMPKα2 [D157A]) or infusing AMPK inhibitor compound C directly into the mediobasal hypothalamus. Next, we independently activated hypothalamic AMPK by delivering either an adenovirus expressing the constitutive active form of AMPK (Ad-CA AMPKα1312 [T172D]) or the AMPK activator AICAR. The pancreatic (basal insulin)-euglycemic clamp technique in combination with the tracer-dilution methodology was used to assess the impact of alternations in hypothalamic AMPK activity on changes in glucose kinetics in vivo.
RESULTS
Injection of Ad-DN AMPK into the hypothalamus knocked down hypothalamic AMPK activity and led to a significant suppression of glucose production with no changes in peripheral glucose uptake during the clamps. In parallel, hypothalamic infusion of AMPK inhibitor compound C lowered glucose production as well. Conversely, molecular and pharmacological activation of hypothalamic AMPK negated the ability of hypothalamic nutrients to lower glucose production.
CONCLUSIONS
These data indicate that changes in hypothalamic AMPK activity are sufficient and necessary for hypothalamic nutrient-sensing mechanisms to alter glucose production in vivo.
AMP-activated protein kinase (AMPK) is an evolutionarily conserved cellular energy sensor that regulates cellular metabolism (1). Consisting of a catalytic α subunit and two regulatory β and γ subunits, AMPK responds to an increase in intracellular AMP-to-ATP ratio and phosphorylates intracellular targets involved in cellular metabolism to promote ATP-generating processes and inhibit energy-consuming pathways. AMPK is expressed in a variety of tissues including the liver, skeletal muscles, adipose tissue, and the hypothalamus (1). AMPK phosphorylates and inhibits acetyl-CoA carboxylase (ACC) (1), which prevents the conversion of acetyl-CoA to malonyl-CoA. A decrease in malonyl-CoA relieves the inhibition of carnitine palmitoyltransferase-1 (2) and favors the transfer of long-chain fatty acyl-CoA (LCFA-CoA) into the mitochondria for β-oxidation. Conversely, direct inhibition of AMPK increases malonyl-CoA and LCFA-CoA levels (3).
Studies have emerged implicating that AMPK in the hypothalamus integrates nutritional and hormonal signals to regulate food intake (4–8). In particular, direct inhibition of hypothalamic AMPK lowers food intake (8), whereas selective activation of hypothalamic AMPK negates the ability of leptin to activate hypothalamic ACC, increase hypothalamic malonyl-CoA levels, and lower food intake (9). In light of the fact that the hypothalamus integrates nutritional and hormonal signals to not only regulate energy (10–12) but also glucose (13–17) homeostasis, and that accumulation of hypothalamic malonyl-CoA and LCFA-CoA levels lowers food intake as well as hepatic glucose production (18–20), a possibility arises that direct inhibition of hypothalamic AMPK activity could alter hepatic glucose production (Fig. 1A). This working hypothesis was first tested in the current study.
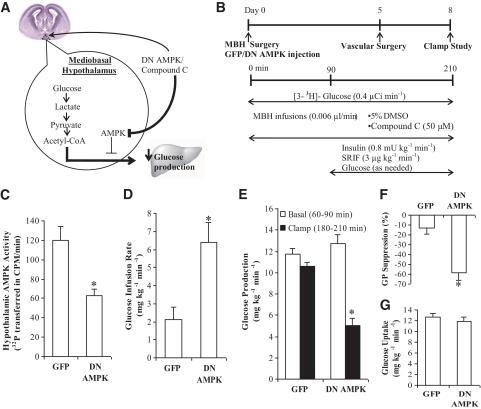
FIG. 1.
Molecular knockdown of hypothalamic AMPK by the dominant-negative form of AMPK (DN AMPK) is sufficient to lower glucose production. A: Schematic representation of the working hypothesis: Inhibition of hypothalamic AMPK activity by DN AMPK or compound C leads to the lowering of hepatic glucose production. B: Experimental procedure and clamp protocol. A bilateral MBH catheter was implanted on day 0. Adenovirus tagged with GFP (Ad-GFP) or adenovirus-expressing DN AMPK (Ad-DN AMPK) was injected into the MBH of a group of rats immediately after MBH catheter implantation. Venous and arterial cannulations were done on day 5, and the pancreatic clamp protocol was performed on day 8. In the Ad-GFP and Ad-DN AMPK–injected rats, no MBH infusions were given during the clamp experiments. In rats with no adenovirus injection, 5% DMSO control or compound C was infused into the MBH during the clamps. C: Hypothalamic AMPK activity was significantly diminished in animals injected with Ad-DN AMPK, compared with control animals with injection of Ad-GFP (*P < 0.001). Hypothalamic injection of Ad-DN AMPK led to an increase in glucose infusion rate (D) (*P < 0.01) and a decrease in glucose production (E) (*P < 0.001) compared with the GFP control. F: Suppression of glucose production during the clamp period (180–210 min) expressed as percentage reduction from basal steady state (60–90 min) (*P < 0.01 vs. GFP control). G: Glucose uptake was not significantly different from that of GFP control. Values are shown as means ± SEM. (A high-quality color representation of this figure is available in the online issue.)
Second, hypothalamus glucose metabolism to lactate, and the subsequent conversion of lactate to pyruvate and acetyl-CoA, have been reported to lower hepatic glucose production (21). However, the downstream biochemical pathways that mediate the ability of hypothalamic glucose/lactate sensing to lower glucose production remain unclear, although it was hypothesized that the formation of malonyl-CoA via the enhanced flux of acetyl-CoA could be a necessary step (3,15). Given the well-established regulatory role of AMPK on the formation of malonyl-CoA from acetyl-CoA and that hypothalamic malonyl-CoA regulates glucose production (18), we next tested the possibility that direct activation of hypothalamic AMPK negates the ability of central nervous system glucose/lactate sensing to regulate glucose production.
In summary, we tested the hypothesis that molecular and pharmacological changes in hypothalamic AMPK activity are sufficient and necessary for hypothalamic nutrient-sensing mechanisms to regulate glucose production in vivo.
RESEARCH DESIGN AND METHODS
The animal experimental protocols were reviewed and approved by the institutional animal care and use committee of the University Health Network. Adult 8-week-old male Sprague-Dawley rats were obtained from Charles River Laboratories (Montreal, Quebec, Canada) and maintained on a 12-h/12-h day/night cycle with access to rat diet and water ad libitum. The rats underwent stereotaxic surgeries for insertion of bilateral catheters into the mediobasal hypothalamus (MBH), as previously described (22) (Figs. 1B and 3B). The coordinates used for MBH cannulation are 3.1 mm posterior of bregma, 0.4 mm lateral of midline, and 9.6 mm below skull surface. Immediately poststereotaxic surgery, a group of rats received MBH administration of 3 μl of adenovirus expressing the dominant-negative form of AMPK (Ad-DN AMPKα2 [D157A], 1.1 × 1013 plague-forming units/ml) (23), the constitutive active form of AMPK (Ad-CA AMPKα1312 [T172D], 3.83 × 1010 plague-forming units/ml) (23), or green fluorescent protein (Ad-GFP, 1.4 × 109 plague-forming units/ml) (23) on each side of the MBH catheter. Adenoviral MBH injections were performed as previously described (18), indicating that using this MBH adenoviral animal injection protocol, GFP is localized in the MBH and not other regions of the brain. Five days later, catheters were placed in the internal jugular vein and the carotid artery for infusion and sampling during the clamps. Recovery from surgery was monitored by measuring daily food intake and weight gain.
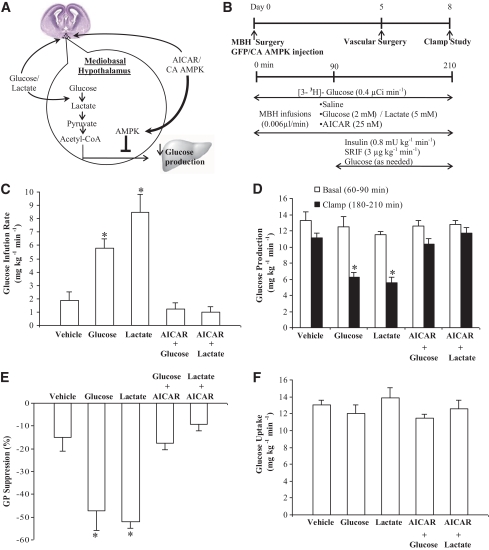
FIG. 3.
Hypothalamic administration of AICAR, the pharmacological activator of AMPK, negates the ability of hypothalamic glucose/lactate-sensing mechanisms to decrease glucose production. A: Schematic representation of the working hypothesis: Activation of hypothalamic AMPK by AICAR or the constitutively active form of AMPK (CA AMPK) prevents the ability of hypothalamic glucose/lactate to decrease glucose production. B: Experimental procedure and clamp protocol. A bilateral MBH catheter was implanted on day 0. Adenovirus tagged with GFP (Ad-GFP) or adenovirus expressing CA AMPK (Ad-CA AMPK) was injected into the MBH of a group of rats immediately after MBH catheter implantation. Venous and arterial cannulations were done on day 5, and the pancreatic clamp protocol was performed on day 8. In rats with no adenovirus injection, AICAR, saline, glucose, lactate, AICAR+glucose, or AICAR+lactate was infused into the MBH during the clamp experiments. In rats injected with Ad-GFP or Ad-CA AMPK, saline, glucose, or lactate was infused into the MBH during the clamp studies. C: Direct MBH infusion of glucose or lactate during the clamps increased glucose infusion rate (*P < 0.001) and lowered glucose production (D) (*P < 0.001) compared with those of MBH vehicle (AICAR/saline) treatments. MBH glucose or lactate coinfused with AICAR failed to increase glucose infusion rate (C) and lower glucose production (D) compared with those of vehicle treatments. E: Suppression of glucose production during the clamp period (180–210 min) expressed as the percentage reduction from the basal steady state (60–90 min) (*P < 0.05 vs. other groups). F: Glucose uptake was comparable in all groups. Values are shown as means ± SE. (A high-quality color representation of this figure is available in the online issue.)
Clamp procedure.
Infusion studies lasted a total of 210 min (Figs. 1B and 2B), and all rats were restricted to ∼60 kcal of food the night before the infusion studies to ensure the same nutritional status. MBH infusions were initiated at 0 min and maintained throughout the experiments at a rate of 0.006 μl/min. Treatments included 5% DMSO, 50 μmol/l AMPK inhibitor compound C (dissolved in 5% DMSO; Calbiochem), 2 mmol/l glucose or 5 mmol/l lactate alone, 25 mmol/l AMPK activator AICAR (dissolved in saline; Sigma) plus 2 mmol/l glucose, 25 mmol/l AICAR plus 5 mmol/l lactate, and vehicle (either saline or 25 mmol/l AICAR). A primed-continuous intravenous infusion of 3-3H-glucose (40 μCi bolus, 0.4 μCi/min; Perkin Elmer) was also initiated at 0 min and maintained throughout the study to assess glucose kinetics. At 90 min, the pancreatic clamp was initiated to assess the effect of MBH treatments on glucose metabolism independent of differences in glucoregulatory hormones. This was done by the continuous intravenous infusion of exogenous insulin (0.8 mU/kg/min) and somatostatin (3 μg/kg/min). A total of 25% glucose was infused intravenously and adjusted periodically to maintain plasma glucose levels comparable among groups. Plasma samples for determination of [3H]-glucose–specific activity and plasma glucose levels were obtained at every 10-min interval to assess the glucose kinetics (glucose infusion rate needed to maintain euglycemia, glucose production, and glucose uptake) under basal (60–90 min) and clamped (180–210) conditions. Of note, since the clamp studies were performed in the presence of basal insulin replacement, any observable changes in glucose kinetics due to hypothalamic treatments are not under insulin-stimulated conditions. At the end of all studies, rats were anesthetized and 3 μl of diluted bromophenol blue was injected through each side of the MBH catheter to ensure the correct placement of the catheter. The MBH wedges were obtained and stored at −80°C for subsequent AMPK activity assay.
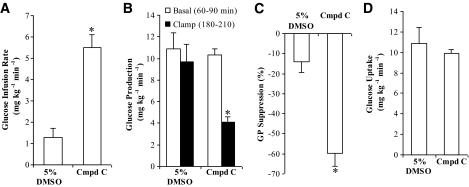
FIG. 2.
Hypothalamic administration of compound C, the pharmacological inhibitor of AMPK, lowers glucose production. Direct infusion of compound C (Cmpd C), the pharmacological inhibitor of AMPK, into the MBH significantly increased the glucose infusion rate (A) (*P < 0.001) and decreased the glucose production (B) (*P < 0.05) during the clamps compared with the 5% DMSO control group. C: Suppression of glucose production during the clamp period (180–210 min) expressed as the percentage reduction from the basal steady state (60–90 min) (*P < 0.001). D: Glucose uptake in the compound C–treated group did not differ significantly from that of the 5% DMSO treated–control group. Values are shown as means ± SE.
AMPK activity assay.
AMPK activity was determined essentially as previously described (24). In brief, MBH wedge samples were lyzed in 200–500 μl ice-cold lysis buffer (in mmol/l: 50 Tris HCl [pH 7.4, 4°C], 250 sucrose, 50 NaF, 1 Na pyrophosphate, EDTA, 1 EGTA, 1 dithiothreitol, 0.1 benzamidine, and 0.1 phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride, 5 μg/ml soybean trypsin inhibitor, and 1% [vol/vol] Triton X-100), and cell debris was removed by centrifugation at 13,200 rpm for 5 min at 4°C. Protein concentration was determined using a bicinconinic acid–based protein assay kit (Pierce, Rockford, IL). Total extract (10 μg protein) was used for activity measurement by phosphotransfer, with synthetic “SAMS” peptide (HMRSAMSGLHLVKRR) as substrate (24). Results were analyzed by linear regression using GraphPad software and were expressed in counts per minute. Background (in the presence of lysis buffer only, non–AMPK dependent) incorporation of radioactivity was subtracted from all values. Assays were performed in triplicate.
Immunohistochemistry.
Male Sprague-Dawley rats with Ad-GFP injections in the MBH were anesthetized 8 days after and perfused transcardially with saline (40 ml) and 4% parafomaldehyde (35 ml) for tissue fixation. Brains were removed and 4-mm-thick coronal sections containing the mediobasal hypothalamus were embedded and frozen in optimal cutting temperature compound (Tissue-Tek) and stored at −80°C. Ten-micrometer-thick coronal tissue sections mounted on glass slides were obtained via cryostat sectioning of the frozen brain sample. To costain GFP with AgRP or proopiomelanocortin (POMC), tissues were first blocked for 1 h with 10% normal goat serum and 0.2% Triton X-100 and then incubated overnight at 4°C with a combination of either chicken anti-GFP (1:1,800; Abcam) plus rabbit anti-POMC (1:1,500; Phoenix Pharmaceuticals) antibodies or chicken anti-GFP (1:1,800) plus rabbit anti-AgRP (1:200; Phoenix Pharmaceuticals) antibodies. On the following day, tissues were washed and incubated with goat anti-chicken IgG (1:1,000; Alexa-Fluor 488) and goat anti-rabbit IgG (1:1,000 for POMC and 1:700 for AgRP; Alexa-Fluor 546) secondary antibodies. The slides were viewed under the fluorescence microscope.
Biochemical analysis.
Plasma glucose concentrations were measured using the glucose oxidase method (Glucose analyzer GM9; Analox Instruments, Lunenbertg, MA). Plasma insulin and glucagon concentration was measured using a radioimmunoassay (Linco Research, St. Charles, MO).
Statistical analysis.
Data are presented as means + SE. Statistical analysis was done by two-way ANOVA followed by Tukey post hoc test. Statistical analysis was accepted as significant with P < 0.05.
RESULTS
Molecular inhibition of hypothalamic AMPK lowers glucose production.
We first examined whether inhibition of hypothalamic AMPK activity is sufficient to alter glucose production in vivo. Adenovirus expressing the dominant-negative form of AMPK (Ad-DN AMPK) was injected directly into MBH of rats immediately after the stereotaxic surgery (Fig. 1B). The infusion clamp studies were carried out 8 days after brain surgery and adenoviral injections and 3 days after vascular surgery (Fig. 1B). On the morning of the infusion clamp studies (day 8), there was an observable trend (although not statistically significant) in the Ad-DN AMPK–injected rats to weigh less than Ad-GFP–injected control rats (P = 0.07) (Table 1). In addition, we detected a 40.7 ± 10.5% decrease in overnight food intake of Ad-DN AMPK–injected rats versus Ad-GFP–injected control rats only on day 8 (P < 0.05).
TABLE 1.
Body weights and plasma insulin, glucagon, and glucose concentrations of rats treated with Ad-GFP or Ad-DN AMPK in the mediobasal hypothalamus
| Body weight (kg) | Insulin (ng/ml) | Glucagon (pg/ml) | Glucose (mg/dl) | |
|---|---|---|---|---|
| Ad-GFP (n = 6) | ||||
| Basal | 0.282 ± 0.004 | 0.8 ± 0.2 | 60 ± 2 | 146 ± 4 |
| Clamp | 0.8 ± 0.1 | 53 ± 4 | 140 ± 6 | |
| Ad-DN AMPK (n = 14) | ||||
| Basal | 0.254 ± 0.012 | 0.8 ± 0.1 | 82 ± 9* | 153 ± 8 |
| Clamp | 0.8 ± 0.1 | 54 ± 5 | 128 ± 7 |
Data are means ± SE. Basal (t = 0); clamp (t = 180–210).
*P < 0.05 vs. Ad-GFP at basal.
Direct Ad-DN AMPK hypothalamic injection reduced hypothalamic AMPK activity compared with Ad-GFP injection immediately following the clamp studies (Fig. 1C). In addition, we performed immunohistochemistry against GFP in our rat hypothalamic slices injected with Ad-GFP. GFP staining was discovered in the mediobasal hypothalamic regions, and ∼40% of the GFP was colocalized with AgRP-positive neurons and another ∼40% was colocalized with POMC-positive neurons (supplementary Fig. 1 in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0221/DC1). Using the tracer dilution methodology in combination with the pancreatic (basal insulin)-euglycemic clamp technique, we assessed the effects of Ad-DN AMPK on glucose kinetics (Fig. 1B). During the clamps, the exogenous glucose infusion rate required to prevent hypoglycemia and maintain euglycemia was approximately threefold higher in comparison with the Ad-GFP–injected rats (Fig. 1D). The increase in the glucose infusion rate was fully accounted for by an inhibition in the rate of glucose production (Fig. 1E and F) and was independent of differences among groups in peripheral circulating insulin and glucagon levels (Table 1) as well as the rate of peripheral glucose uptake (Fig. 1G). Our results indicate, for the first time, that molecular inhibition of hypothalamic AMPK activity is sufficient to suppress glucose production in vivo.
Pharmacological inhibition of hypothalamic AMPK lowers glucose production.
To evaluate whether direct inhibition of hypothalamic AMPK lowers glucose production independent of changes in food intake and body weight, AMPK inhibitor compound C was directly infused at 50 μmol/l (dissolved in 5% DMSO) into the hypothalamus of rats that had similar food intake (all rats were restricted to ∼60 kcal of food the night before the infusion experiments to ensure the same nutritional status) and body weight (Fig. 1B) (Table 2). During the pancreatic (basal insulin) clamps, hypothalamic compound C potently increased the glucose infusion rate required to maintain euglycemia compared with 5% DMSO (Fig. 2A). This elevation of the glucose infusion rate was due to a suppression of glucose production (Fig. 2B and C) and was independent of differences among groups in peripheral circulating insulin and glucagon levels (Table 2) as well as the rate of peripheral glucose uptake (Fig. 2D). Thus, pharmacological inhibition of hypothalamic AMPK lowers glucose production independent of changes in food intake and body weight.
TABLE 2.
Body weights and plasma insulin, glucagon, and glucose concentrations of rats treated with 5% DMSO or compound C in the mediobasal hypothalamus
| Body weight (kg) | Insulin (ng/ml) | Glucagon (pg/ml) | Glucose (mg/dl) | |
|---|---|---|---|---|
| 5% DMSO (n = 6) | ||||
| Basal | 0.298 ± 0.014 | 0.9 ± 0.1 | 58 ± 3 | 144 ± 10 |
| Clamp | 0.7 ± 0.1 | 51 ± 3 | 124 ± 16 | |
| Compound C (n = 6) | ||||
| Basal | 0.306 ± 0.004 | 0.9 ± 0.1 | 56 ± 2 | 132 ± 6 |
| Clamp | 0.7 ± 0.1 | 47 ± 1 | 110 ± 7 |
Data are means ± SE. Basal (t = 0); clamp (t = 180–210).
Pharmacological activation of hypothalamic AMPK negates the ability of hypothalamic glucose/lactate to lower glucose production.
Since inhibition of hypothalamic AMPK mimics the ability of hypothalamic metabolism of glucose to lactate and pyruvate to lower glucose production (21) (Fig. 3A), we next addressed whether activation of hypothalamic AMPK via the pharmacological activator AICAR would negate the ability of central glucose/lactate to lower glucose production (Fig. 3A and B). First, consistent with previous findings (21), MBH glucose/lactate increased glucose infusion rate (Fig. 3C) and lowered glucose production during the clamps (Fig. 3D and E) in the presence of comparable levels of plasma insulin, glucagon, glucose, and body weights (Table 3). In contrast, when the AMPK activator AICAR (25 mmol/l) was coadministered with glucose or lactate into the MBH (Fig. 3A and B), MBH infusion of glucose/lactate completely failed to increase the glucose infusion rate (Fig. 3C) and to lower glucose production (Fig. 3D and E) in the presence of comparable levels of plasma insulin, glucagon, glucose, and body weight (Table 3). Of note, MBH AICAR infused alone at 25 mmol/l (n = 3) had minimal effects on basal glucose production (13.9 ± 1.0 mg/kg/min), clamp glucose production (10.8 + 0.3), and glucose uptake (13.2 ± 0.9) compared with the MBH saline–infused group (n = 3) (12.4 ± 0.7, 11.5 ± 1.3, and 12.7 ± 1.0, respectively). Thus, MBH AICAR and saline infusion clamp experiments were pooled together into a single vehicle group as reported in Table 3 and Fig. 3C–F. Glucose uptake was comparable among groups (Fig. 3F). These data indicate that pharmacological activation of hypothalamic AMPK blocked the ability of hypothalamic glucose/lactate to lower glucose production.
TABLE 3.
Body weights and plasma insulin, glucagon, and glucose concentrations of rats treated with vehicle, glucose, lactate, AICAR plus glucose, or AICAR plus lactate in the mediobasal hypothalamus
| Body weight (kg) | Insulin (ng/ml) | Glucagon (pg/ml) | Glucose (mg/dl) | |
|---|---|---|---|---|
| Vehicle (n = 6)* | ||||
| Basal | 0.291 ± 0.010 | 1.0 ± 0.1 | 63 ± 5 | 144 ± 2 |
| Clamp | 0.9 ± 0.1 | 56 ± 5 | 146 ± 6 | |
| Glucose (n = 5) | ||||
| Basal | 0.294 ± 0.007 | 1.0 ± 0.1 | 60 ± 7 | 145 ± 9 |
| Clamp | 0.9 ± 0.1 | 49 ± 4 | 149 ± 6 | |
| Lactate (n = 5) | ||||
| Basal | 0.295 ± 0.014 | 0.9 ± 0.1 | 58 ± 3 | 143 ± 3 |
| Clamp | 0.9 ± 0.1 | 45 ± 3 | 140 ± 9 | |
| AICAR + glucose (n = 5) | ||||
| Basal | 0.306 ± 0.002 | 0.9 ± 0.1 | 56 ± 7 | 150 ± 5 |
| Clamp | 0.8 ± 0.2 | 42 ± 7 | 152 ± 7 | |
| AICAR + lactate (n = 5) | ||||
| Basal | 0.314 ± 0.003 | 0.8 ± 0.1 | 61 ± 9 | 142 ± 5 |
| Clamp | 0.8 ± 0.1 | 49 ± 3 | 140 ± 8 |
Data are means ± SE. Basal (t = 0); clamp (t = 180–210).
*Vehicle includes saline or AICAR infusion alone.
Molecular activation of hypothalamic AMPK negates the ability of hypothalamic glucose/lactate to lower glucose production.
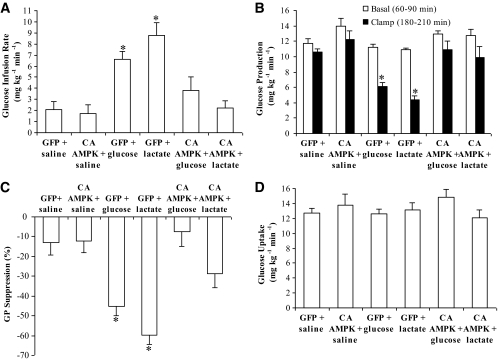
To alternatively evaluate whether activation of hypothalamic AMPK negates nutrient-sensing mechanisms to regulate glucose production, hypothalamic AMPK was activated through the injection of adenovirus expressing the constitutively active form of AMPK (Ad-CA AMPK) (Fig. 3A and B). First, infusion of MBH glucose or lactate injected with Ad-GFP significantly increased the glucose infusion rate needed to maintain euglycemia (Fig. 4A) during the clamp studies in the presence of similar levels of plasma insulin and glucagon as well as body weight (Table 4). The increase in the glucose infusion rate was in association with a reduction in the rate of glucose production (Fig. 4B and C) and not a change in glucose uptake (Fig. 4D). In rats injected with Ad-CA AMPK, however, MBH infusion of glucose or lactate during the clamps failed to increase the glucose infusion rate and lower glucose production (Fig. 4A–C) in the presence of similar levels of plasma insulin and glucagon as well as body weight (Table 4). Ad-GFP or Ad-CA AMPK injected alone into the MBH did not alter glucose kinetics in our experimental settings (Fig. 4A–D). Together with the pharmacological gain-of-function data, these molecular gain-of-function experiments indicate that selective activation of hypothalamic AMPK negates the ability of central nervous system glucose/lactate sensing to regulate glucose production.
FIG. 4.
Hypothalamic administration of the constitutively active form of AMPK (CA AMPK) negates the ability of hypothalamic glucose/lactate-sensing mechanisms to decrease glucose production. Direct MBH administration of glucose or lactate to the GFP treatment groups increased glucose infusion rate (A) (*P < 0.01) and lowered glucose production (B) (*P < 0.001) compared to those of GFP/saline and CA AMPK/saline groups during the clamps. Direct MBH administration of glucose or lactate during the clamps to the CA AMPK treatment groups failed to increase glucose infusion rate (A) and lower glucose production (B). C: Suppression of glucose production during the clamp period (180–210 min) expressed as the percentage reduction from the basal steady state (60–90 min) (*P < 0.01 vs. other groups). D: Glucose uptake was comparable in all groups. Values are shown as means ± SE.
TABLE 4.
Body weights and plasma insulin, glucagon, and glucose concentrations of rats treated with Ad-GFP plus saline, Ad-CA AMPK plus saline, Ad-GFP plus glucose, Ad-GFP plus lactate, Ad-CA AMPK plus glucose, or Ad-CA AMPK plus lactate in the mediobasal hypothalamus
| Body weight (kg) | Insulin (ng/ml) | Glucagon (pg/ml) | Glucose (mg/dl) | |
|---|---|---|---|---|
| Ad- GFP + saline (n = 5) | ||||
| Basal | 0.270 ± 0.014 | 0.8 ± 0.2 | 60 ± 2 | 146 ± 4 |
| Clamp | 0.9 ± 0.1 | 54 ± 5 | 141 ± 5 | |
| Ad-CA AMPK + saline (n = 6) | ||||
| Basal | 0.273 ± 0.017 | 0.7 ± 0.2 | 54 ± 4 | 127 ± 14 |
| Clamp | 0.7 ± 0.1 | 46 ± 2 | 121 ± 9 | |
| Ad-GFP + glucose (n = 6) | ||||
| Basal | 0.283 ± 0.016 | 0.8 ± 0.2 | 54 ± 4 | 152 ± 10 |
| Clamp | 0.7 ± 0.1 | 52 ± 3 | 144 ± 11 | |
| Ad-GFP + lactate (n = 6) | ||||
| Basal | 0.284 ± 0.013 | 0.8 ± 0.1 | 58 ± 5 | 142 ± 4 |
| Clamp | 0.9 ± 0.1 | 46 ± 2 | 136 ± 7 | |
| Ad-CA AMPK + glucose (n = 6) | ||||
| Basal | 0.262 ± 0.014 | 0.5 ± 0.1 | 59 ± 5 | 144 ± 8 |
| Clamp | 0.5 ± 0.1 | 46 ± 2 | 125 ± 4 | |
| Ad-CA AMPK + lactate (n = 7) | ||||
| Basal | 0.278 ± 0.024 | 0.8 ± 0.2 | 56 ± 4 | 156 ± 13 |
| Clamp | 0.7 ± 0.1 | 54 ± 4 | 136 ± 10 |
Data are means ± SE. Basal (t = 0); clamp (t = 180–210).
DISCUSSION
In the recent decade, the role of AMPK has expanded from being a simple gauge of cellular energy status to a key regulator of whole-body energy homeostasis. Specifically, in the hypothalamus, nutrients and anorexigenic signals decrease AMPK activity, whereas orexigenic signals stimulate AMPK to regulate energy homeostasis (4–8,19). Selective knockout of AMPK in POMC or AgRP neurons also leads to a disruption of energy balance (25), further highlighting the importance of hypothalamic AMPK as an integrator to control energy homeostasis. In addition to the hypothalamic control on food intake by AMPK, hypothalamic AMPK regulates glucocounterregulatory responses to acute hypoglycemia induced by hyperinsulinemia (26) as well as neuronal survival and cognitive ability (27). The metabolic regulatory role of hypothalamic AMPK in the presence of basal insulin levels is now extended by the current study to glucose production regulation. Specifically, molecular and pharmacological inhibition of hypothalamic AMPK lowered glucose production, whereas selective activation of hypothalamic AMPK negated hypothalamic glucose/lactate-sensing mechanisms to lower glucose production independent of changes in plasma insulin and glucagon levels compared with the appropriate controls.
Although molecular knockdown of hypothalamic AMPK in our experimental setting decreased glucose production in association with hypophagia, pharmacological inhibition of hypothalamic AMPK still potently lowered glucose production independent of changes in food intake and body weight. Furthermore, molecular and pharmacological activation of hypothalamic AMPK negated the hypothalamic nutrient-sensing mechanism to lower glucose production independent of changes in food intake and body weight as well. In addition, we performed infusion clamp studies to Ad-GFP–injected rats (n = 4) who had similar body weight and food intake (on day 8) as the Ad-DN AMPK–injected rats. We found that the glucose production during the clamps of these weight-matched Ad-GFP–injected rats was 10.4 ± 1.9 mg/kg/min, and this rate of glucose production was comparable to the Ad-GFP–injected rats reported in Fig. 1. This finding indicated that molecular knockdown of hypothalamic AMPK in our experimental settings lowered glucose production independent of changes in food intake and body weight. Thus, these data overall suggest that bidirectional changes in hypothalamic AMPK activity alter glucose production independent of changes in food intake and body weight.
Of note, in contrast to the ability of inhibition of hypothalamic AMPK, per se, to lower glucose production, both molecular and pharmacological activation of hypothalamic AMPK did not increase glucose production but were sufficient to negate the metabolic effects of hypothalamic nutrient-sensing mechanisms. Future studies are required to explore this discrepancy in details, but it is important to point out that a selective lowering of malonyl-CoA levels in the hypothalamus via overexpression of malonyl-CoA decarboxylase, per se, also did not increase glucose production but was sufficient to negate hypothalamic nutrient-sensing mechanisms to lower glucose production (18).
Within the mediobasal hypothalamus, AgRP and POMC neurons are implicated to sense hormonal and nutritional signals to regulate energy as well as glucose homeostasis (11,28). Our current study did not evaluate whether bidirectional changes of hypothalamic AMPK activity within the AgRP and/or POMC neurons regulate glucose production. However, we reported that Ad-GFPs that were injected via the current MBH adenoviral injection protocol were colocalized either with AgRP or POMC. Thus, the data raises the possibility that hypothalamic AMPK in the AgRP and/or POMC neurons may regulate glucose production in our experimental settings, but future studies are required to address this working hypothesis. In addition, the current study used the pancreatic (basal insulin)-euglycemic clamp technique to evaluate the regulatory impact of hypothalamic AMPK activity on glucose production regulation. Thus, the physiological relevance of hypothalamic control of glucose homeostasis by AMPK remains to be assessed.
In summary, molecular and pharmacological modulation of hypothalamic AMPK regulates glucose production. These findings suggest that inhibition of AMPK in the hypothalamus could potentially lower glucose production and plasma glucose levels in diabetes and obesity. It should be noted, however, that contrary to hypothalamic AMPK, activation (and not inhibition) of AMPK in the peripheral tissues lowers glucose levels. For example, hepatic AMPK activation inhibits gluconeogenesis and reduces blood glucose levels (29,30). An increase in skeletal muscle AMPK activity also mediates some of the beneficial effects of exercise on glucose uptake (31). Due to these differential roles of peripheral and hypothalamic AMPK in glucose regulation, caution should be taken in developing drugs that target AMPK to lower glucose levels in diabetes and obesity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a research grant to T.K.T.L. from the Canadian Institute of Health Research (MOP-86554). C.K.L.L. was supported by the Canadian Institute of Health Research (CIHR) Graduate Scholarship. M.C. was supported by the Ontario Graduate Scholarship and a graduate studentship from the Banting and Best Diabetes Centre at the University of Toronto (BBDC). G.W.C.C. and A.K. are supported by the CIHR Graduate Scholarship as well as the BBDC Graduate Studentship. G.A.R. thanks the Wellcome Trust (program grant no. 081958/Z/07/Z), the Medical Research Council (G0401641), the National Institutes of Health (ROI DKO71962-01), and the European Union FP6 (SaveBeta). I.L. and G.A.R. thank Diabetes U.K. (BDA:RD04/0002895) and the Wellcome Trust (WT082366MA) for project grant support. T.K.T.L. holds the John Kitson McIvor Endowed Chair in Diabetes Research and Canada Research Chair in Obesity at the Toronto General Research Institute and University of Toronto.
No potential conflicts of interest relevant to this article were reported.
C.S.Y. conducted and designed in vivo and immunohistochemistry experiments, performed data analyses, and wrote the manuscript. C.K.L.L. conducted in vivo experiments and performed data analyses. M.C., G.W.C.C., and A.K. assisted with in vivo experiments. S.G., I.L., and G.A.R. supplied the adenovirus expressing the DN form of AMPK or GFP control and performed hypothalamic AMPK activity assay. T.K.T.L. supervised the project, designed experiments, and edited the manuscript.
The authors are extremely grateful to P. Wang from the Toronto General Research Institute for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 2. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 3. Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 2005;8:579–584 [DOI] [PubMed] [Google Scholar]
- 4. Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68 [DOI] [PubMed] [Google Scholar]
- 5. Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 2005;280:25196–25201 [DOI] [PubMed] [Google Scholar]
- 6. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 2004;10:727–733 [DOI] [PubMed] [Google Scholar]
- 7. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 8. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 9. Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A 2007;104:17358–17363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, Ebling FJ, Vickers SP, Cheetham S, Ghatei MA, Bloom SR, Dhillo WS. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes 2010;59:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 12. You J, Yu Y, Jiang L, Li W, Yu X, Gonzalez L, Yang G, Ke Z, Li W, Li C, Liu Y. Signaling through Tyr985 of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol Cell Biol 2010;30:1650–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang L, You J, Yu X, Gonzalez L, Yu Y, Wang Q, Yang G, Li W, Li C, Liu Y. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci U S A 2008;105:18619–18624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caspi L, Wang PY, Lam TK. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab 2007;6:99–104 [DOI] [PubMed] [Google Scholar]
- 16. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–275 [DOI] [PubMed] [Google Scholar]
- 17. Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med 2010;16:392–395 [DOI] [PubMed] [Google Scholar]
- 18. He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006;9:227–233 [DOI] [PubMed] [Google Scholar]
- 19. Hu Z, Dai Y, Prentki M, Chohnan S, Lane MD. A role for hypothalamic malonyl-CoA in the control of food intake. J Biol Chem 2005;280:39681–39683 [DOI] [PubMed] [Google Scholar]
- 20. Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 2003;9:756–761 [DOI] [PubMed] [Google Scholar]
- 21. Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–947 [DOI] [PubMed] [Google Scholar]
- 22. Ross R, Wang PY, Chari M, Lam CK, Caspi L, Ono H, Muse ED, Li X, Gutierrez-Juarez R, Light PE, Schwartz GJ, Rossetti L, Lam TK. Hypothalamic protein kinase C regulates glucose production. Diabetes 2008;57:2061–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 2003;371:761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci U S A 2000;97:4023–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 2007;117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008;57:444–450 [DOI] [PubMed] [Google Scholar]
- 27. Dagon Y, Avraham Y, Magen I, Gertler A, Ben Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem 2005;280:42142–42148 [DOI] [PubMed] [Google Scholar]
- 28. Lam CK, Chari M, Lam TK. CNS regulation of glucose homeostasis. Physiology (Bethesda) 2009;24:159–170 [DOI] [PubMed] [Google Scholar]
- 29. Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y, Kurihara H, Asano T. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 2008;283:33902–33910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vincent MF, Erion MD, Gruber HE, Van den BG. Hypoglycaemic effect of AICAriboside in mice. Diabetologia 1996;39:1148–1155 [DOI] [PubMed] [Google Scholar]
- 31. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 2001;7:1085–1094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.