Abstract
There are ongoing events where aircraft engine lubricant containing tricresyl phosphates (TCPs) contaminates aircraft cabins. Some individuals have experienced tremors or other neurological symptoms that may last for many months following exposures. Mass spectrometric (MS) protocols are being developed to determine the percentage of “biomarker proteins” that are modified by such exposures, specifically on active site serines. Both plasma butyrylcholinesterase (BChE) and red cell acylpeptide hydrolase (APH) are readily inhibited by 2-(o-cresyl)-4H-1:3:2:benzodioxaphosphoran-2-one (CBDP) or phenyl saligenin cyclic phosphate (PSP) and have the potential to provide information about the level of exposure of an individual. We have developed immunomagnetic bead-based single-step purification protocols for both BChE and APH and have characterized the active site serine adducts of BChE by MS.
Keywords: Biomarkers, Tricresyl phosphate, CBDP, Butyrylcholinesterase, Acylpeptide hydrolase, Aerotoxic syndrome
1. Introduction
Tricresyl phosphates (TCP) are organophosphate (OP) compounds used as anti-wear additives in jet engine lubricants. Their fire resistant properties are also beneficial (Solbu et al., 2007). Commercial TCPs are mixtures of triaryl phosphate (TAP) isomers (De Nola et al., 2008). The tri-ortho isomer (ToCP) was thought to be responsible for the 10–50,000 cases of paralysis resulting from the consumption of extracts of TCP adulterated ethanolic ginger during Prohibition in the US (Smith et al., 1931). Henschler demonstrated that the mono-ortho isomer of TCP was approximately 10-times more toxic than the ToCP isomer, with the di-ortho isomer exhibiting intermediate toxicity (Henschler, 1958).
Over the past 25 years, there have been numerous reports worldwide of aircrew and passengers reporting short or long-term symptoms such as dizziness, cognitive problems, disorientation and uncontrolled tremors following exposure to oil-contaminated cabin air. These events have been described in a number of publications (Hale and Al-Seffar, 2009; Montgomery et al., 1977; Murawski and Supplee, 2008; Ross, 2008; van Netten and Leung, 2001; Winder, 2006; Winder and Michaelis, 2005; Winder et al., 2002a, 2002b).. The ill health effects have been referred to as aerotoxic syndrome (Balouet and Winder, 1999).
A major aim of our research is to develop protocols for determining whether an individual has been exposed to TCPs. Aldridge reported that bioactivation by liver was required to convert TCP to highly toxic metabolites (Aldridge, 1954). In 1961, Eto and Casida (Casida et al., 1961) reported the structure of the bioactivated metabolite of tri-orthocresyl phosphate as 2- (o-cresyl)-4H-1:3:2:benzodioxaphosphoran-2-one, also known as cyclic saligenin cresyl phosphate (CBDP). Bioactivated TAPs inhibit many different serine active site enzymes including carboxylesterases (CESs), lipases [e.g., neuropathy target esterase (NTE)], and cholinesterases (Casida and Quistad, 2004; Glynn et al., 1994). The serine at the active site of these enzymes is covalently modified by OPs, and these modifications can be identified by mass spectrometry (MS). The use of OP-labeled enzymes as biomarkers of exposure is useful because unlike the parent OPs, they can remain in circulation for weeks (Kim et al., 2010).
Human butyrylcholinesterase (BChE, accession # P06276) is an 85 kDa tetrameric glycoprotein that is synthesized by the liver and has a 11-day half-life in plasma (Ostergaard et al., 1988). BChE can play an important role in the detoxification of ingested or inhaled OPs by stoichiometrically binding OP molecules (Masson and Lockridge, 2010). Inhibition or modification of BChE is commonly used as a biomarker of OP exposure (Schopfer et al., 2010; Sun and Lynn, 2009; Wieseler et al., 2006).
Acylpeptide hydrolase (APH, accession # P13798) is a serine peptidase that cleaves N-acetylated peptides. The enzyme possesses both acylpeptide and esterase activities and is present in many tissues. APH is localized in red blood cells (RBCs), and has a lifespan of around 120 days. APH was also proposed as a sensitive biomarker of OP exposure (Quistad et al., 2005). OP-modified APH can possibly be detected several weeks following exposure since the half-life of RBCs is ≈33 days (Umlas et al., 1991).
This study is focused on the rapid purification from blood samples of TCP metabolite-modified BChE and APH from in vitro exposed samples.
2. Materials and Methods
2.1 Sample collection
The samples used for this study came from an institutional review board-approved project investigating organophosphate exposure. Blood samples were collected in lithium heparin tubes. RBCs were separated from plasma by centrifugation at 1285×g for 10 min at 4°C. Plasma samples were stored at −80°C until use. The RBCs were washed 3 times with PBS and then frozen at −80°C. The experiments reported here made use of blood fractions obtained from non-exposed control subjects. The samples were exposed in vitro to the chemically synthesized metabolic intermediates.
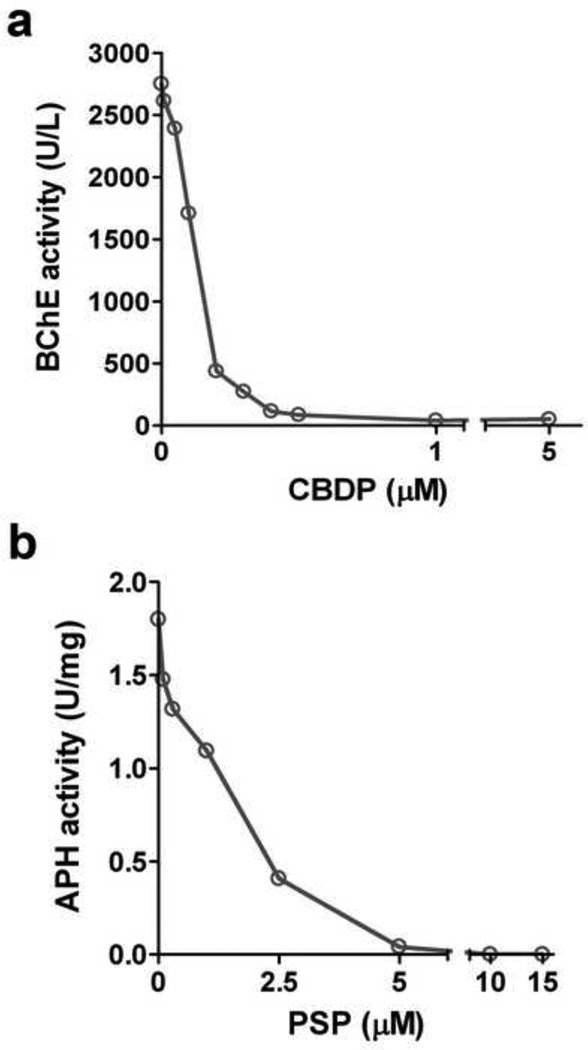
Plasma samples were incubated with 5 µM CBDP for 1h at room temperature. We performed a concentration dependence inhibition of BChE using plasma and different concentrations of CBDP, with a 10 min incubation (Fig. 1a). For the MS characterization of adducts, we used 5 µM CBDP and a 1h incubation to insure complete inhibition of the BChE, with activity determined before and after incubation with CBDP.
Figure 1.
Concentration dependence of a. CBDP inhibition of human plasma BChE, and b. PSP inhibition of human pure APH; following a 10 min incubation at ambient temperature. Results are represented as U/L or U/mg (U = µmol/min).
An analogue of CBDP, phenyl saligenin phosphate (PSP), was used to inhibit RBC APH, as described previously (Kim et al., 2010). The concentration dependence of PSP inhibition of APH is shown in Fig. 1b. For the MS characterization of adducts, 25 µM PSP was used with a 1h incubation to insure complete inhibition of the APH, with activity determined before and after the incubation.
CBDP (99.5% pure, CAS 1222-87-3) was custom synthesized by Starks Associates, Buffalo, NY, USA. PSP was a generous gift of Dr. Marion Ehrich.
2.2 BChE and APH purification
We have developed single-step immunomagnetic bead-based protocols (IMB) for purifying both plasma BChE and RBC APH to high levels of purity starting with just 200 µL of plasma or packed RBCs. For both protocols, we used magnetic beads (Invitrogen, Carlsbad, CA, USA), coupled to either mouse monoclonal anti-human plasma BChE antibodies (Thermo Fisher Scientific, Waltham, MA, USA) or in-house biotinylated, affinity purified polyclonal anti-human RBC APH antibodies (generated in rabbits by Cocalico Biologicals Inc., Reamstown, PA, USA—this company maintains a current USDA research license and a current Animal Welfare Assurance from the NIH’s Office of Laboratory Animal Welfare). We used 2.5% v/v (0.4 M) acetic acid to elute the antibody bound proteins from the IMBs.
We tested the efficiency of the IMB protein capture by activity assays of the samples before and after incubation with the beads. Enzyme activity was reduced by approximately 85–90%. All procedures were performed in Protein LoBind Tubes (Eppendorf, Hamburg, Germany).
The purified proteins were analyzed by gel electrophoresis using SDS-PAGE gels (NuPAGE® 4–12% BisTris Gel, Invitrogen, Carlsbad, CA, USA), stained with the Pierce® Silver Stain kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) or with the Imperial Protein Stain solution (Thermo Fisher Scientific Inc.).
2.3 Determination of BChE and APH activity
Plasma BChE activity was measured as described by Ellman (Ellman et al., 1961), using 1 mM butyrylthiocholine in 0.1 M phosphate buffer pH 8.0 at 25°C. The reaction was started by the addition of 100 µL of diluted BChE to 100 µL of substrate solution and was monitored continuously at 405 nm over 3 min at 25°C.
Since APH has esterase activity, we adapted a protocol from Mastropaolo (Mastropaolo and Yourno, 1981) to assay the RBC APH activity. A 0.73 M stock solution of alpha-naphthyl butyrate (ANB) in methanol was diluted to 0.2 mM with 20 mM Tris pH 7.0. The reaction was initiated by the addition of 200 µL of substrate solution to the sample, and was monitored continuously at 235 nm over 4 min at 25°C. We have also used the APH specific substrate N-acetyl-L-alanyl-p-nitroanilide (AcAlaPNA) to measure APH activity as described (Quistad et al., 2005).
The assays were carried out in a SPECTRAmax® PLUS Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA). The AcAlaPNA was obtained from Bachem (Torrance, CA, USA). The other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.4 Mass spectrometry protocols
Samples were analyzed by high resolution MS with the aim of detecting and quantifying active site serine modifications.
Samples obtained from IMB protocol were digested with 1 µg of chymotrypsin (Promega, Madison, WI, USA) for 2 h at 37°C and loaded onto an LC-MS/MS system nanoAcquity (Waters Corporation, Milford, MA, USA)/LTQ-FT (Thermo Fisher Scientific, Waltham, MA, USA). The system was equipped with a 30 cm column of 75 µm I.D. fused silica capillary pulled to a 5 µm I.D. tip using a Sutter Instruments P-2000 CO2 laser puller. The analytical column was packed with C-12 reversed phase chromatography material (Phenomenex, Jupiter 4µ, Proteo 90 Å) using an in-house constructed pressure bomb and compressed helium gas. Peptides were eluted using a 60 min water:acetonitrile gradient. SEQUEST was used for the databank search using the following dynamic modifications: S (Serine): 170.0138 (cresyl phosphoserine) and 79.9668 (phosphoserine) for BChE.
3. Results
3.1 Target proteins isolation
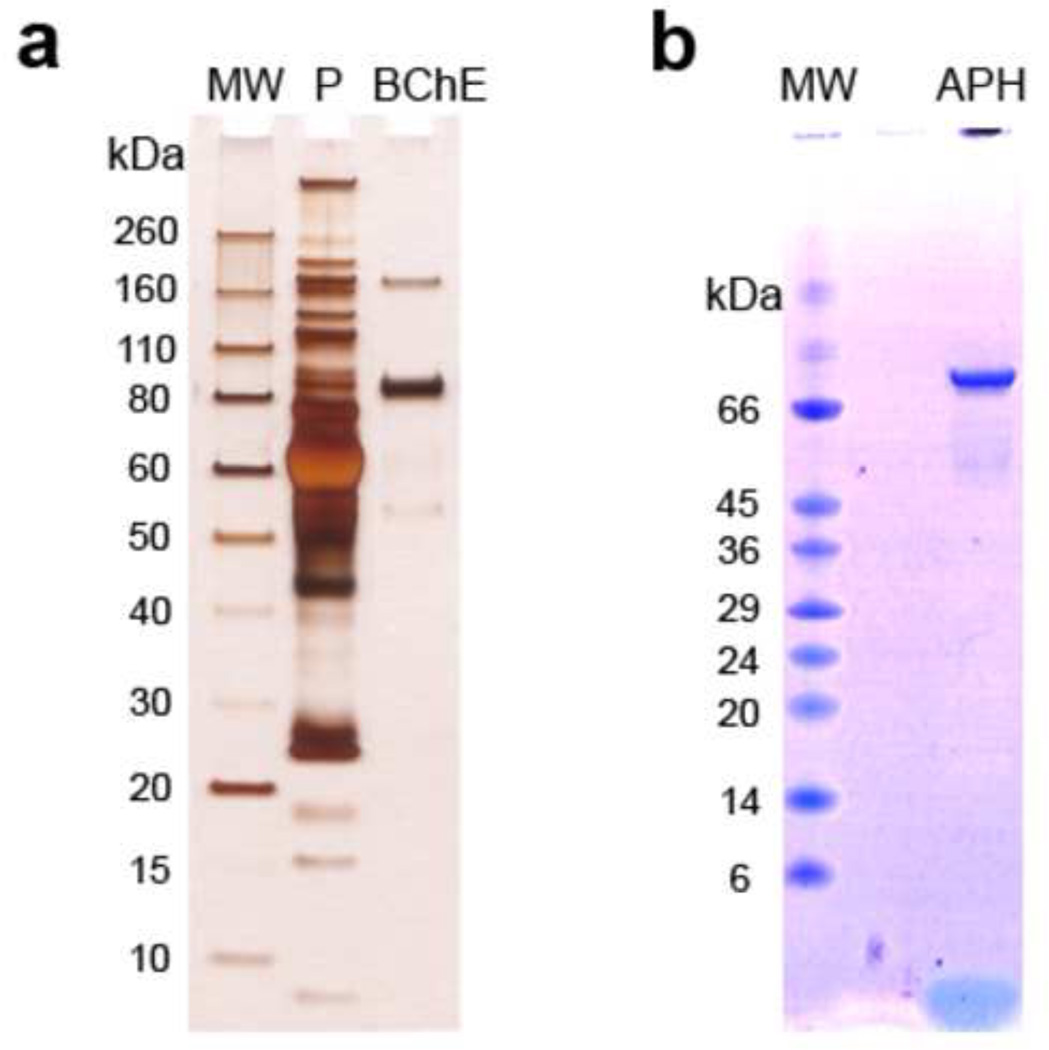
One of our aims was to develop protocols for fast and accurate purification of the biomarker proteins BChE and APH. We were able to obtain a high level of purity of BChE and APH in single steps (Fig. 2). The samples obtained from the IMB protocol, when digested and analyzed by MS, provided modified target peptides that were easily identified.
Figure 2.
SDS-PAGE analysis of the IMB-purified target proteins; a. Silver stained gel of IMB-purified plasma BChE, and b. Coomassie blue stained gel of IMB-purified RBC APH. Abbreviations: MW = molecular weight markers; P = plasma.
3.2 MS analysis of adducted target proteins
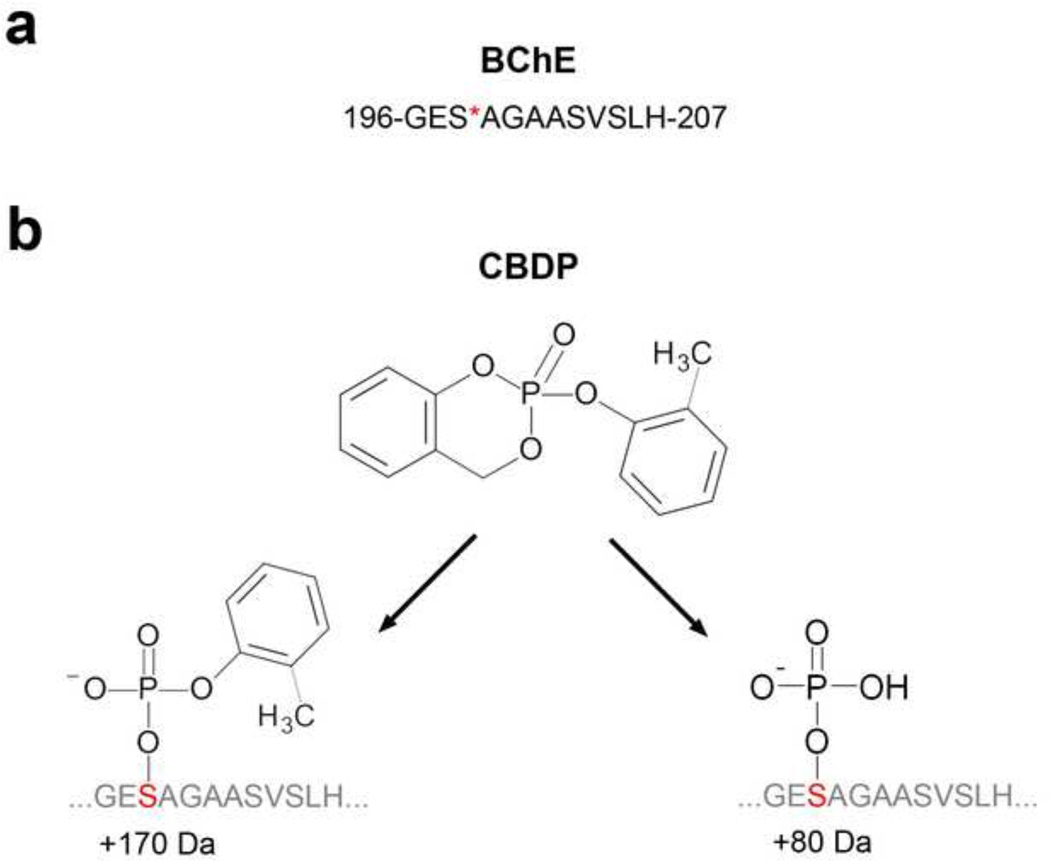
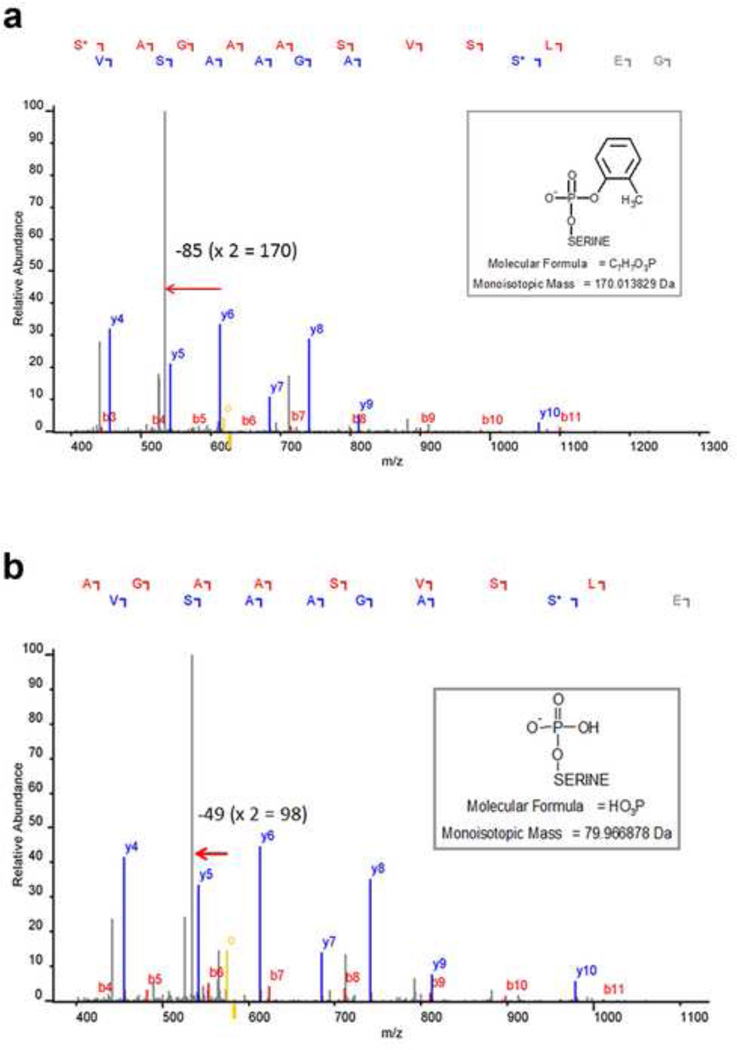
The chymotryptic sequence of the BChE active site peptide, GESAGAASVSLH, is shown in Fig. 3a. A peptide database search of the acquired data identified active site serine modifications, corresponding to +170 (C7H7PO3) and +80 (HPO3) for BChE (Fig. 3b). Fragmentation of parent ion 1255.5 for the cresyl-phosphate derivative in Fig.4a yielded a b-ion series (b3 to b11) that carried the +170 modification, and a y-ion series (y4–y10) where only the y10 ion carried the modification. Fragmentation of parent ion 1165.5 for the phosphate derivative in Fig. 4b yielded a b-ion series (b4 to b11) that carried the +80 modification and a y-ion series (y4–y10) where only the y10 ion carried the modification. Signals corresponding to the neutral loss of the modification were also observed in both spectra (denoted by the mass difference between parent ion, yellow mass labeled with “o”, and mass marked with the arrow) indicating partial dissociation of the label. Taken together, these data confirmed our identification of peptide modifications. The phenyl-phosphate adduct on the active site serine of APH corresponding to +156 (C6H5PO3) has been described previously following in vitro inhibition with PSP (Kim et al, 2010).
Figure 3.
a. BChE active site serine modified by CDBP, and b. structures of the active site serine adducts.
Figure 4.
Mass spectra showing the identification and characterization of the BChE modified active site serine of: a. the partially aged cresyl phosphoserine and b. the more extensively aged phosphoserine.
4. Discussion
OP-modified enzymes are more stable in the organism than intact OPs, due to the rapid elimination of free OPs. For this reason, new methods to identify and quantify the degree of modification of biomarker proteins need to be developed.
An ideal protein purification/enrichment strategy would be amenable to high-throughput scale-up. Affinity chromatography is currently the most commonly used technique for the isolation of peptides and proteins. The main disadvantages are the large amount of sample required and the necessity of more than one step to achieve a high level of purity. IMB isolation protocols solve these disadvantages. The process is simple, can be done in just a few steps, does not require expensive equipment or large volumes of sample, is very efficient, and is amenable to automation (Safarik and Safarikova, 2004). In this study we report a single-step purification procedure that is very efficient and only uses small amounts of sample.
MS analysis provides sequence information as well as the localization and mass change of the OP-modification on the biomarker proteins (Schopfer et al., 2010; Sun and Lynn, 2009; Wieseler et al., 2006). Here, we describe protocols for identifying OP-modified BChE peptide in samples inhibited by the chemically-synthesized TCP metabolite CBDP in vitro. As reported earlier with an affinity chromatographic protocol, both the partially aged cresyl phosphoserine and the more extensively aged phophoserine modifications were identified.
The methods described in the manuscript are compatible with the Thermo Scientific KingFisher Magnet Particle Processors, as well as other related systems for automation of sample analyses. Furthermore, this general approach should be useful for analyzing other OP exposures such as OP insecticides and nerve agents.
5. Conclusions
We report here a rapid and efficient method for purifying two protein biomarkers of OP exposure, BChE and APH, and MS protocols for identifying the sequence modifications. These protocols are readily adaptable for automated high throughput assessment of OP exposures.
Acknowledgments
Supported by U.S. Army Medical Research and Materiel Command (W81XWH-07-2-0034), the NIH (U01 NS058056, P30CA36727, R01ES09883, P42ES04696, and T32ES007032), and funding from pilot and flight attendant unions, the Royal Australian Air Force, the Norwegian Union of Energy Workers (SAFE), and NYCO S.A. JM was supported by a Beatriu de Pinós postdoctoral fellowship (2008 BP A 00166) from Comissionat per a Universitats i Recerca del Departament d’Innovació, Universitats i Empresa, Catalunya, Spain. SS was supported by an EP/T training grant from the NIH (ES 007032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest
References
- Aldridge WN. Tricresyl phosphates and cholinesterase. Biochem J. 1954;56:185–189. doi: 10.1042/bj0560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouet J-C, Winder C. Aerotoxic syndrome in air crew as a result of exposure to airborne contaminants in aircraft; Paper presented at the American Society of Testing and Materials Symposium on Air Quality and Comfort in Airliner Cabins; 27–28 October; New Orleans: 1999. [Google Scholar]
- Casida JE, Eto M, Baron RL. Biological activity of a trio-cresyl phosphate metabolite. Nature. 1961;191:1396–1397. doi: 10.1038/1911396a0. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- De Nola G, Kibby J, Mazurek W. Determination of ortho-cresyl phosphate isomers of tricresyl phosphate used in aircraft turbine engine oils by gas chromatography and mass spectrometry. J Chromatogr A. 2008;1200:211–216. doi: 10.1016/j.chroma.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Glynn P, Read DJ, Guo R, Wylie S, Johnson MK. Synthesis and characterization of a biotinylated organophosphorus ester for detection and affinity purification of a brain serine esterase: neuropathy target esterase. Biochem J. 1994;301(Pt 2):551–556. doi: 10.1042/bj3010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MA, Al-Seffar JA. Preliminary report on aerotoxic syndrome (AS) and the need for diagnostic neurophysiological tests. Am J Electroneurodiagnostic Technol. 2009;49:260–279. [PubMed] [Google Scholar]
- Henschler D. [Tricresylphosphate poisoning; experimental clarification of problems of etiology and pathogenesis] Klin Wochenschr. 1958;36:663–674. doi: 10.1007/BF01488746. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stevens RC, Maccoss MJ, Goodlett DR, Scherl A, Richter RJ, et al. Identification and characterization of biomarkers of organophosphorus exposures in humans. Adv Exp Med Biol. 2010;660:61–71. doi: 10.1007/978-1-60761-350-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P, Lockridge O. Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys. 2010;494:107–120. doi: 10.1016/j.abb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastropaolo W, Yourno J. An ultraviolet spectrophotometric assay for alpha-naphthyl acetate and alpha-naphthyl butyrate esterases. Anal Biochem. 1981;115:188–193. doi: 10.1016/0003-2697(81)90544-3. [DOI] [PubMed] [Google Scholar]
- Montgomery MR, Wier GT, Zieve FJ, Anders MW. Human intoxication following inhalation exposure to synthetic jet lubricating oil. Clin Toxicol. 1977;11:423–426. doi: 10.3109/15563657708988205. [DOI] [PubMed] [Google Scholar]
- Murawski JTL, Supplee DS. An Attempt to Characterize the Frequency, Health Impact, and Operational Costs of Oil in the Cabin and Flight Deck Supply Air on U.S. Commercial Aircraft. J of ASTM Int. 2008;5:1–15. [Google Scholar]
- Ostergaard D, Viby-Mogensen J, Hanel HK, Skovgaard LT. Half-life of plasma cholinesterase. Acta Anaesthesiol Scand. 1988;32:266–269. doi: 10.1111/j.1399-6576.1988.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol Sci. 2005;86:291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Ross SM. Cognitive function following exposure to contaminated air on commercial aircraft: a case series of 27 pilots seen for clinical purposes. J Nutr Environ Med. 2008;17:111–126. [Google Scholar]
- Safarik I, Safarikova M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol. 2004;2:7. doi: 10.1186/1477-044X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer LM, Furlong CE, Lockridge O. Development of diagnostics in the search for an explanation of aerotoxic syndrome. Anal Biochem. 2010;404:64–74. doi: 10.1016/j.ab.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MI, Lillie RD, Surg PA. The histopathology of triorthocresyl phosphate poisoning - The etiology of so-called cancer paralysis (third report) Arch Neuro Psychiatr. 1931;26:976–992. [Google Scholar]
- Solbu K, Thorud S, Hersson M, Ovrebo S, Ellingsen DG, Lundanes E, et al. Determination of airborne trialkyl and triaryl organophosphates originating from hydraulic fluids by gas chromatography-mass spectrometry. Development of methodology for combined aerosol and vapor sampling. J Chromatogr A. 2007;1161:275–283. doi: 10.1016/j.chroma.2007.05.087. [DOI] [PubMed] [Google Scholar]
- Sun J, Lynn BC. Development of a LC/MS/MS method to analyze butyrylcholinesterase inhibition resulting from multiple pesticide exposure. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3681–3685. doi: 10.1016/j.jchromb.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Umlas J, Jacobson M, Kevy SV. Suitable survival and half-life of red cells after frozen storage in excess of 10 years. Transfusion. 1991;31:648–649. doi: 10.1046/j.1537-2995.1991.31791368344.x. [DOI] [PubMed] [Google Scholar]
- van Netten C, Leung V. Hydraulic fluids and jet engine oil: pyrolysis and aircraft air quality. Arch Environ Health. 2001;56:181–186. doi: 10.1080/00039890109604071. [DOI] [PubMed] [Google Scholar]
- Wieseler S, Schopfer L, Lockridge O. Markers of organophosphate exposure in human serum. J Mol Neurosci. 2006;30:93–94. doi: 10.1385/JMN:30:1:93. [DOI] [PubMed] [Google Scholar]
- Winder C. Hazardous chemicals on jet aircraft: case study- jet engine oils and aerotoxic syndrome. Curr Top Toxicol. 2006;3:65–88. [Google Scholar]
- Winder C, Balouet JC. Aerotoxic syndrome: adverse health effects following exposure to jet oil mist during commercial flights. In: Eddington I, editor. Towards a Safe and Civil Society Proceedings of International Congress on Occupational Health Conference. Brisbane: ICOH; 2000. pp. 196–199. [Google Scholar]
- Winder C, Balouet JC. The toxicity of commercial jet oils. Environ Res. 2002a;89:146–164. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- Winder C, Michaelis S. Aircraft air quality malfunction incidents-crew effects from toxic exposures on aircraft. Air quality in airplane cabins and similar enclosed spaces. In: Hocking M, editor. The handbook of environmental chemistry. Part H. Vol. 4. Heidelberg: Springer-Verlag; 2005. pp. 211–228. [Google Scholar]
- Winder C, Fonteyn P, Balouet JC. Aerotoxic syndrome: a descriptive epidemiological survey of aircrew exposed to in-cabin airborne contaminants. J Occup Health Safety-Aust.NZ. 2002b;18:321–338. [Google Scholar]