INTRODUCTION
Prostate cancer is the second leading cause of death from cancer for men in the United States, claiming over 32,000 lives each year.1 Patients who present with bone metastasis have a 5-year survival rate of 3%.2 Metastatic prostate cancer is initially treated with either hormonal therapy or orchiectomy to deprive prostate cancer cells of testosterone, which binds to the androgen receptor and induces cellular proliferation. Although these treatments may initially limit tumor growth, the cancer cells will ultimately proliferate in spite of decreased levels of testosterone, a state known as castration resistance,3 increasing the potential for metastasis. Conventional treatment for metastatic castration-resistant prostate cancer (mCRPC) employs additional agents, such as androgen receptor antagonists (ARAs) and cytotoxic chemotherapy (i.e., docetaxel and cabazitaxel), which has been shown to prolong survival and palliate symptoms in patients with mCRPC.4
The recent approval by the U.S. Food and Drug Administration (FDA) of sipuleucel-T for mCRPC and ipilimumab for metastatic melanoma have generated significant momentum in the field of cancer immunotherapy. PSA-TRICOM (PROSTVAC®) is an investigational, off-the-shelf, vector-based therapeutic cancer vaccine genetically engineered with transgenes for prostate-specific antigen (PSA) and 3 human T-cell costimulatory molecules.5 A phase II randomized, placebo-controlled trial in 125 patients with mCRPC demonstrated a survival benefit of 8.5 months for patients treated with PSA-TRICOM compared to patients treated with placebo (25.1 vs. 16.6 months; P = 0.0061).6 In a separate single-arm study in 32 patients with mCRPC, the median overall survival was 26.6 months, and enhanced PSA-specific T-cell responses were associated with improved survival.7 The most common adverse event in these trials was a mild, local injection-site reaction, with only a subset of patients experiencing systemic flu-like symptoms. One patient had grade 3 injection-site cellulitis.6
Here we present a case report of a patient who, despite multiple negative prognostic factors, demonstrated prolonged survival with minimal toxicity after receiving treatment with PSA-TRICOM. Furthermore, the patient had a prolonged stable disease course while on PSA-TRICOM in spite of fluctuations in his PSA.
CASE PRESENTATION
A 49-year-old male presented to a primary care physician with diffuse bone pain. A radiographic study showed multiple osteoblastic lesions,. PSA value at the time of presentation was 468 mcg/L. A prostate biopsy showed moderately differentiated adenocarcinoma (Gleason score 3+4 = 7). A staging workup revealed extensive osseous metastases causing debilitating pain that required high doses of analgesics. He underwent surgical castration with bilateral orchiectomy. One month after this surgery, his PSA was 237 mcg/L. However, within another month his PSA began to rise, suggesting that the prostate cancer had become castration-resistant. The patient was started on bicalutamide, an FDA-approved ARA. After a short course of therapy, the PSA continued to rise and bicalutamide was withdrawn. Five months later, the PSA had risen to 291 mcg/L.
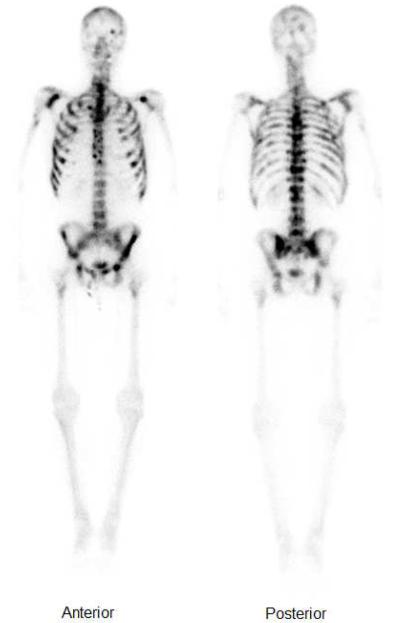
The patient was referred to the National Cancer Institute and evaluated for a phase II trial of PSA-TRICOM vaccine in chemotherapy-naïve patients with mCRPC. At presentation, he had significant pain in his shoulders, anterior bilateral ribs, right hip, and lower back, which was managed with high doses of multiple analgesics, including methadone, fentanyl transdermal patch, topical lidocaine, etodolac, hydrocodone/acetaminophen, carisoprodol, amitriptyline, and monthly zoledronic acid. He previously worked as a graphic designer but had retired on disability due to significant pain from metastatic disease. His Eastern Cooperative Oncology Group performance status at presentation was 1. Physical examination was otherwise unremarkable. Labs revealed PSA 259 mcg/L, lactate dehydrogenase 187 units/L, alkaline phosphatase 78 units/L, and hemoglobin 12.1 mg/dL. A baseline bone scintigraphy demonstrated extensive involvement of the axial skeleton and portions of the appendicular skeleton, nearly all ribs, and the calvarium (Figure 1). Computed tomography of the chest, abdomen, and pelvis revealed no evidence of lymphadenopathy or metastatic visceral disease.
Figure 1.
Baseline whole body bone scan. Note the extensive involvement of the axial skeleton and portions of the appendicular skeleton, nearly all ribs, and the calvarium.
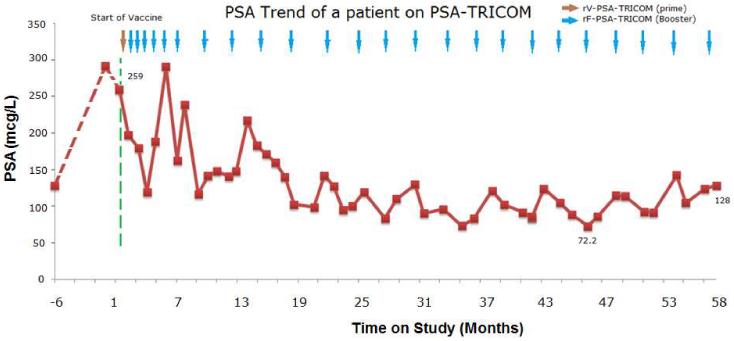
The patient was enrolled on trial and received PSA-TRICOM monthly for 6 months and once every 3 months thereafter. Other than a grade 2 injection-site reaction and flu-like symptoms, which were manageable with an antipyretic, he had no other toxicities attributable to the vaccine. After the first 3 monthly injections of vaccine, his PSA declined > 50% from baseline (Figure 2). Over this same period of time he mounted greater than six-fold increase in PSA-specific T cells from baseline. Bone scans continued to show extensive osseous metastases, but did not reveal any new lesions. The patient’s pain was adequately controlled with narcotics. Given his PSA response and clinical and radiographic stability, he was continued on monthly booster vaccinations for 3 more months, then once every 3 months thereafter. Interestingly, he had several periods of apparent "PSA progression." However, apart from rising PSA values, other clinical parameters,(i.e., pain, weight, ECOG Performance Status and other laboratory findings) and radiographic studies suggested stable disease. Furthermore, his subsequent PSA’s declined. Despite these fluctuations, shown in Figure 2, his PSA levels trended downward, reaching a nadir at 72.2 mcg/L, a 72% decline from baseline. He remained on trial for 59 months and received a total of 25 doses of PSA-TRICOM. He was eventually taken off study after 5 years of vaccination based on the rationale that he had likely received maximal benefit from this therapy. At the time he was taken off study, his PSA was 128 mcg/L, about 50% below his on-study PSA, and he did not have any newly defined areas of disease or clinical progression. His PSA doubling time was 20.6 month during his last 12 months on study versus 5.5 months before vaccination. Two years later, his disease remains stable and has not required further treatment.
Figure 2.
Plot of PSA trend from 6 month prior to the start of the vaccine until the time off the study. Note the overall downward trend of PSA with interval fluctuations.
DISCUSSION
In this case, treatment with a therapeutic cancer vaccine was associated with minimal toxicity and prolonged stable disease in a patient who might otherwise have been treated with docetaxel-based systemic therapy, most likely incurring significant toxicities. Of particular interest is the fact that this patient had aggressive disease characteristics at baseline, including rapidly rising PSA and extensive osseous metastases causing pain requiring treatment with narcotics, all of which argued against such a prolonged survival. Interestingly, a retrospective analysis of 599 patients with mCRPC suggested an inverse association between degree of pain and risk of death. Patients with a higher degree of pain had a median survival of 10.2 months vs. 17.6 months for patients with less pain (P < 0.001).8 Based on his disease characteristics (PSA, performance status, lactate dehydrogenase, alkaline phosphatase, hemoglobin, Gleason score, and presence/absence of visceral disease), his predicted survival was approximately 17 months using the Halabi nomogram. This nomogram is derived from a retrospective analysis of 1,101 mCRPC patients from six Cancer and Leukemia Group B trials during the pre-docetaxel era.9
Although the reason for the patient’s prolonged survival with stable disease is not clear, evidence that he developed an immune response to the vaccine is noteworthy. The patient mounted an immune response after 3 monthly vaccinations, as demonstrated by the 6.6-fold increase in PSA-specific T cells from baseline. This magnitude of immune response was associated with a favorable survival outcome in a phase II trial.7 Correlation of these immunology assays with survival will be prospectively evaluated in a phase III clinical trial (NCT 01322490).10
The patient’s PSA trend is also worth noting, given his survival far beyond that predicted by the Halabi nomogram. Although his PSA nadir was 72% below baseline, his PSA clearly fluctuated over time in a range of less than 50% to 112% of the baseline PSA. Prostate Cancer Clinical Trials Working Group (PCWG) 2 emphasized that early changes in PSA may not reflect overall disease status and should not be the sole basis for a decision to discontinue potentially effective treatment without symptomatic or radiographic evidence of disease progression.11 As this case illustrates, early large fluctuations in PSA, including consecutive increases over 25% above the nadir, did not foreshadow clinical progression. A delayed maximal improvement in PSA kinetics may be particularly probable for prostate cancer patients treated with immunotherapy.12 Currently, there is no consensus on how to follow patients treated with immunotherapy, specifically with sipuleucel-T.13 However, it is becoming clear that increases in PSA in the absence of symptomatic or radiographic evidence of disease progression should not automatically lead to a change in treatment.11
Another interesting point is that the patient did not require further therapy for at least 2 years after he was taken off study. Data from the phase III trial of sipuleucel-T also showed that about half of patients treated with the vaccine did not receive subsequent docetaxel.14 While this patient’s outcome may suggest an indolent disease course in spite of poor prognostic indicators; a theory inconsistent with his pre-vaccine clinical course, it may also suggest the ability of therapeutic cancer vaccines to induce a persistent immunologic antitumor effect beyond the treatment period, an effect that decelerates tumor growth trajectories.15
CONCLUSION
This case reinforces the potential pitfalls of using PSA progression alone as the basis for discontinuing potentially effective immune-based therapy. It also suggests that there is little toxicity associated with long-term therapeutic vaccine administration. It is worth noting that this patient’s immune response to the vaccine was among the highest of all the patients on study. While further analysis is called for, this fact does suggest a possible association between antitumor immune response and clinical outcome. To that end, PSA-TRICOM will be prospectively evaluated in a global phase III, double-blind, placebo-controlled clinical trial in 1,200 mCRPC patients, with overall survival as the primary endpoint. An important secondary goal of this trial is further elucidation of the kinetics of clinical and immune responses following vaccine.
CLINICAL PRACTICE POINTS.
In the absence of symptomatic or radiographic evidence of disease progression, the decision to change treatment in patients with mCRPC should not be based solely on PSA changes. This is especially true for immune based therapies, such as vaccines, where short term changes in progression are often not seen.
Phase II data suggest that PSA-TRICOM, a therapeutic cancer vaccine, can improve long-term clinical outcomes with minimal toxicity in patients with metastatic castration- resistant prostate cancer (mCRPC). PSA-TRICOM will be prospectively evaluated in a phase III, double-blind, placebo-controlled clinical trial in 1,200 mCRPC patients, with overall survival as the primary endpoint.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.JEMAL A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Norgaard M, Jensen AO, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–7. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Zhu W, Zhu DS, Madan RA, et al. Treatment of castration-resistant prostate cancer: updates on therapeutics targeting the androgen receptor signaling pathway. Am J Ther. 2010;17:176–81. doi: 10.1097/MJT.0b013e3181c6c0b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JL Gulley, PM Arlen, RA Madan, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–9. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 9.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed: June 2011];A Trial of PROSTVAC +/− GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer (mCRPC) (Prospect) Available from: http://clinicaltrials.gov/ct2/show/NCT01322490.
- 11.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17:3884–91. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madan RA, Gulley JL. The current and emerging role of immunotherapy in prostate cancer. Clin Genitourin Cancer. 2010;8:10–6. doi: 10.3816/CGC.2010.n.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 15.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]