Abstract
WNIN/Ob, a mutant rat strain, developed at the National Center for Laboratory Animal Sciences (NCLAS) facility of National Institute of Nutrition (NIN), is a new animal model to study the metabolic syndrome. These animals have 47% fat in their body and isolation of islets from these animals were compounded due to the formation of amorphous viscous and jelly like material which reduced the islet yield. However, islets isolated from WNIN adult (≥12 months) control rats gave a good islet recovery, under standard isolation procedures using collagenase digestion. In the present study we optimized culture conditions in WNIN/Ob rats to isolate islets with higher yield, and also established primary islet cell cultures from these mutant rats, retaining cellular integrity and functionality.
Keywords: Obese rats, Collagenase, Islet isolation, Islet cell culture, Viability and islet function
Introduction
Animal models like ob/ob, db/db, Zucker, and Koletsky rats are the pioneer models in the field of obesity and diabetes and all the other existing models have been developed from these strains (Giridharan 1998). We are maintaining one of the oldest rat strain in the world, Wistar, in an inbred condition since 1920. From this WNIN rat colony we isolated and established a mutant obese rat strain, WNIN/Ob, in 1996, which are unique in its own way and share certain common traits with other mutant models of obesity like Zucker and Koletsky rats (Giridharan et al. 1996; Giridharan 1998). WNIN/Ob animals have “kinky” tails, due to fusion of tail vertebrae at two or three places of the tail and the mode of inheritance of obesity is by incomplete dominance, facilitating the development of three phenotypes, such as lean (+/+), carrier (+/−) and obese (−/−). Though, like other obese rats they are infertile, infertility can be easily reversed by diet restriction. Apart from being obese, WNIN/Ob mutants also develop tumors (60%), cataract and retinal degeneration (Reddy et al. 2009) and nearly all of them have kidney abnormalities. In addition, they also age faster with the average life span being 1.5 years compared to 2.5–3 years seen in the parental control (Giridharan 1998). In view of the importance of this model in biomedical research, development of an efficient and consistent method for obtaining large number of viable and functional islets from pancreas of these WNIN/Ob mutant rats have become mandatory.
Collagenase digestion of pancreatic tissue appears to be a promising method for islet isolation as reported in animal model systems (Gotoh et al. 1985; Hara et al. 1989; Lui and Shapiro1995) and in humans (Gray et al. 1984; Kneteman et al. 1987). Addition of Soy bean trypsin inhibitor and BSA to the collagenase digest, was found to further augment the yield of islets as demonstrated in mice pancreatic tissue (Shewade et al. 1999). However, when we adopted the same methodology (Shewade et al. 1999) for WNIN/Ob mutant rats, the islet yield was found to be very low due to the formation of the amorphous viscous and jelly like material. Paradoxically, under the same conditions we could isolate islets from WNIN rats (parental control) with good yield retaining the islet cell integrity. The present study, reports, our attempts, to optimize conditions of islet isolation and successfully establish primary islet cell cultures with cellular and functional integrity from WNIN/Ob mutant rats.
Materials and methods
Animals
All the animal experimental procedures described were approved by the institutional animal ethical committee (IAEC). Rats were obtained from NCLAS Hyderabad, India. Six male WNIN/Ob mutant rats aged (≥12 months) weighing 900–950 g were used for the study. They were housed in standard polypropylene cages and maintained at 22 ± 1 °C with 12 h dark/light cycles, and were fed standard laboratory rat chow prepared at our animal facility with free access to water as well. The rats were fasted overnight prior to any experimental procedures to maintain uniformity in their metabolic status.
Islet isolation and purification
After CO2 asphyxiation, the skin from the abdomen was opened and the pancreatic duct was cannulated. The pancreas were then removed aseptically in cold RPMI-1640 (GIBCO) pH 7.2 containing 100 Units/mL penicillin, 0.2 mg/mL streptomycin and 2.5 mg/L amphotericin. The tissue was minced finely to obtain approximately 1 mm pieces, and were digested with filter sterilized collagenase solution (1:10) prepared in RPMI-1640 medium. During the digestion process, the pancreatic tissue obtained from WNIN/Ob rats were divided into three groups based on the collagenase concentration. This includes: Group-I (0.1 mg/mL), Group-II (0.5 mg/mL), Group-III (1 mg/mL) and Group-IV represents the parental control (WNIN) and the collagenase concentration used was 1 mg/mL similar to the procedure described by Shewade et al. (1999). In addition, the digestion mixture contained 2 mg/mL Soy bean trypsin inhibitor (Sigma), and 2% BSA fraction V (Sigma) and the digestion temperature was varied between 37 ± 1.5 °C. We have also worked by maintaining the pH in the range optima of 7.7–7.9 during the course of digestion (Amoli et al. 2005). The samples were processed in fractions (serial sampling) to assess the recovery of islets. The digestion was carried out under the same conditions for all the four groups (Group-I, Group-II, Group-III and Group-IV) i.e., for a period of 20–25 min at 37 °C maintaining 2–3 cycles/min. At the end of digestion (turbidity appearance), the reaction was stopped by addition of chilled RPMI-1640 medium containing10%FCS in the ratio of 1:3. The resultant homogenate, thus contained islets, acinar and RBCs. Repeated centrifugation (800 rpm × 2), and washings (3–4 times) of the supernatant facilitated the removal of the acinar cells, RBCs and other disrupted cells, resulting in a pellet which contained predominantly the islet enriched fraction which was >90%. Recovery of the islets were calculated and quantitated using ACT-2U/1.7 version soft ware attached to Nikon inverted microscope (TE-2000s).
Primary islet cell cultures
The islet enriched fraction from all the four groups (Group-I, Group-II, Group-III and Group-IV) were seeded in culture grade flasks (Corning, USA) and maintained in DMEM medium containing 10% FCS+ 100 Units/mL penicillin, 0.2 mg/mL streptomycin and 2.5 mg/L amphotericin. The primary islet cell cultures were maintained for a period of 48 h and parameters such as viability, integrity and functional response were measured following established protocol.
Viability and integrity
Islet cell viability was assessed by Trypan Blue Dye Exclusion (TBE), the islet integrity was measured using Dithiazone (DTZ) (Sigma St. Louis, MO, USA) staining which helps one to differentiate islets from acinar cells (Banerjee and Bhonde 2003; Vijayalakshmi et al. 2004). The number of islets in each group was determined by counting islets in triplicates (10% of the islet suspension) and multiplying the mean number of islets in these aliquots by 10. Bright field images of the TBE and DTZ staining were obtained with a Nikon inverted microscope TE-2000S (Nikon, Tokyo, Japan) equipped with a digital CCD camera and using ACT-2U software version 1.7.
Insulin secretion assay
The functional response measurements were carried out in the islets by insulin secretion assay as per our recently published method (Kiran et al. 2011). Briefly, 250 islets in triplicates from each group were placed in 6 well plate (Corning), containing 1 mL of Krebs–Ringer bicarbonate HEPES (KRBH) buffer (pH 7·4), 10 mmol/L HEPES, 1 mg/mL BSA with 5.5 mM glucose (basal level), 16.5 mM glucose (high glucose challenge) followed by 1 h incubation at 37 °C. The supernatant was collected and stored at −80 °C till assay. Insulin was determined at both basal and high glucose challenge using Mercodia High Range Rat Insulin ELISA kit (Uppsala, Sweden) and was expressed as μIU/mg protein/h.
Statistical analysis
All statistical analyse were performed using SPSS software version 12. The results were expressed as mean ± SE. Comparisons of data were carried out by one way ANOVA followed by Post Hoc test. Differences of P < 0.05 were considered significant.
Results and discussion
The purpose of the present study was to optimize the condition of getting good yield of primary islet cultures from WNIN/Ob rats maintained at our institute. This was necessary in order to obtain good islet cell yields with cellular and functional integrity on account of poor yield of islets which we had encountered using standard procedures described in the literature. We standardized islet isolation especially collagenase digestion and could obtain substantial increase in islet yield, which showed integrity and responded functionally well in cell culture conditions maintained for 48 h. A maximum islet yield of 4021 ± 157 per pancreas with a range of 200–350 microns in diameter was obtained from Group-II (0.5 mg/mL), almost similar to that of the WNIN control (4,500 ± 125/200–325 microns). As indicated in Table 1: Group-I gave an islet yield of 706 ± 64 per pancreas with a range of 400–600 microns and Group-III gave an islet yield of 1,160 ± 65.9 islets per pancreas with a range of 100–200 microns. This shows that lowering the concentration of collagenase to 0.5 mg/mL and maintaining a pH of 7.6 throughout the dissociation/digestion process rendered better isolation of islets and also retained the viability of the cells. The islets obtained with Group-II using 0.5 mg/mL collagenase were comparable with Group-IV (parental control) using 1 mg/mL collagenase.
Table 1.
Islet yield, purity, size and viability of the islets
| Groups (collagenase concentrations) |
Islets number | Purity of islets (%) | Size of islet (micron) | Viability of islets (%) |
|---|---|---|---|---|
| Group-I (0.1 mg/mL) |
706 ± 64* | 65 | 400–600 | >90 |
| Group-II (0.5 mg/mL) |
4,021 ± 157 | 80 | 200–350 | >90 |
| Group-III (1 mg/mL) |
1,160 ± 65.9* | 40 | 100–200 | >90 |
| Group-IV Control (1 mg/mL) |
4,500 ± 125 | 85 | 200–325 | >90 |
Islets isolated from WNIN/Ob mutant rats with different collagenase concentrations: Group-I (0.1 mg/mL), Group-II (0.5 mg/mL)and Group-III (1 mg/mL) and WNIN control Group-IV (1 mg/mL. The data showed a comparison in islet yield, purity and size of the islets between Group-II and Group-IV as compared to Group-I and Group-III. However, viability was same in all four groups. The data represent an average of 6 values obtained from each group. The asterisk indicates statistical significance as compared to controls (Group-IV) at P < 0.05
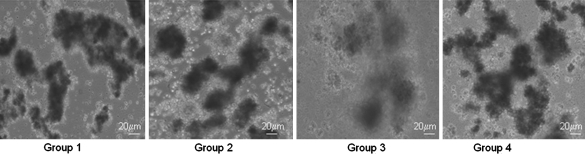
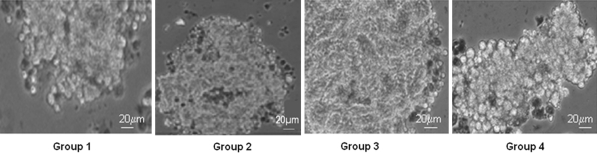
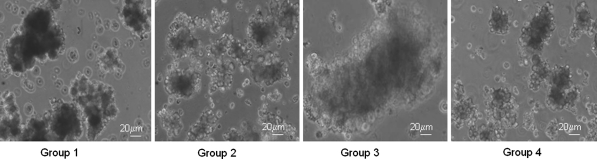
As demonstrated in Fig. 1, at a 1 mg/mL collagenase concentration (Group-III) the pancreatic digestion of WNIN/Ob mutant rats resulted in the formation of a gelatinous homogenate with entrapped islets. This was a major limiting and critical factor for the isolation of islets at this level. At the lowest concentration of 0.1 mg/mL (Group-I) the tissue was less digested and thus interfered with the isolation process resulting in poor islet cell yield (Table 2). However, at a 0.5 mg/mL concentration of the collagenase (Group-II) the interferences such as viscous and gel like formation were not observed suggesting that low concentration of collagenase appears to be optimal towards islet cell recovery. Further, during the incubation of the collagenase digest frequent mixing also permeates effective digestion and this might have also contributed to an increase in the islet yield. Hence, the feasibility of using a 0.5 mg/mL collagenase concentration appears promising for the isolation of islets from WNIN/Ob mutant rats for obtaining a good islet cell yield. Interestingly WNIN controls which served as the experimental control gave a good recovery of islets at 1 mg/mL collagenase concentration unlike WNIN/Ob mutant rats. The viability of the islets (Fig. 2) which was assessed by TBE showed >90% viability for all four groups maintained in primary islet cell cultures (48 h). On similar lines, we also measured islet cell integrity, based on DTZ staining, which also showed (Fig. 3) a comparison among the four groups in primary islet cell cultures.
Fig. 1.
Primary islet cell culture—bright field images. Islets digested with different collagenase concentrations from WNIN/Ob mutant rats: Group-1 (0.1 mg/mL), Group-2 (0.5 mg/mL), Group-3 (1 mg/mL) and parental control Group-4 (1 mg/mL). Group-3 islets showed gelatinous formation interfering with islet yield. The data represent an average of 6 animals from each group
Table 2.
Insulin secretion in the primary islet cell cultures
| Groups (Collagenase concentrations) |
Basal (5.5 mM glucose) |
Stimulated (16.5 mM glucose) |
|---|---|---|
| Group-I (0.1 mg/mL) |
45.56 ± 1.52* | 53.85 ± 1.97* |
| Group-II (0.5 mg/mL) |
38.78 ± 5.82* | 44.23 ± 3.84* |
| Group-III (1 mg/mL) |
Not detectable | Not detectable |
| Group-IV Control (1 mg/mL) |
14.55 ± 1.88 | 28 ± 1.92 |
Insulin secretion was measured in 250 islets at both basal (5.5 mM glucose) and stimulated (16.5 mM glucose) from WNIN/Ob mutant rats at different collagenase concentrations: Group-I (0.1 mg/mL), Group-II (0.5 mg/mL) and Group-III (1 mg/mL) and WNIN control Group-IV (1 mg/mL). Group-III islets resulted in viscous and gelly like formation with collagenase digestion and insulin secretion could not be measured. The asterisk indicates statistical significance as compared to control (Group-IV) (basal and stimulated) at p < 0.05. The insulin values are expressed as μIU/mg protein/h
Fig. 2.
Islet cell viability-TBE. The figure shows TBE of the isolated islets digested with different concentrations of collagenase from WNIN/Ob mutant rats: Group-1 (0.1 mg/mL), Group-II (0.5 mg/mL), Group-III (1 mg/mL) and parental control Group-IV (1 mg/mL). The TBE/viability was comparable among the four groups
Fig. 3.
Islet cell integrity-DTZ. DTZ staining of isolated islets digested with different concentrations of collagenase from WNIN/Ob mutant rats: Group-1 (0.1 mg/mL), Group-II (0.5 mg/mL), Group-III (1 mg/mL) and parental control Group-IV (1 mg/mL). All four Groups demonstrated staining with DTZ (crimson red) indicating islet cell integrity. Group-III islets had a viscous and gelly like appearance
We next examined the functional responses of islets in terms of their insulin secretion at basal and upon high glucose stimulation. As expected, the WNIN control rats were most responsive (Table 2) to high glucose challenge as compared to WNIN/Ob mutant rats which demonstrated hyperglycemia and were less responsive to stimulation. Further the magnitude of insulin secretion was only marginal in WNIN/Ob mutant rats as compared to their controls. The functional response from Group-III was poor because of the interference from the jelly like material. We included in our isolation protocol, both Soy bean trypsin inhibitor and BSA for increasing the yield of the islets as it was shown earlier to protect the pancreas against warm ischemic injury (Perdrizet et al. 1995). Culturing islets for 48 h were equally effective as this permitted disintegration of the acinar cells and improved overall the islet cell yield (Figs. 1, 2, 3).
Conclusion
The present method describes a simple, viable and economical approach for obtaining a large number of islets from the pancreatic tissue of WNIN/Ob mutant rats having a high percent of fat in the body. Earlier we had problems in getting good yields from these animals by conventional procedures which was easily overcome by keeping the collagenase concentration at 0.5 mg/mL and culturing the cells for 48 h and also adding Soy bean trypsin inhibitor and BSA.
The modifications we have made thus in the existing procedure were able to overcome the difficulties while trying to isolate islets from obese rats like WNIN/Ob, having high percentage of fat. This model is now increasingly being used (Reddy et al. 2009; Bandaru et al. 2011; Madhira et al. 2011; Sakamuri et al. 2011) and in the long run the establishment of a primary cell line from this model will be useful for studies related to obesity and diabetes.
Acknowledgments
We thank Director of National Institution of Nutrition for providing us the infrastructure facility as well as intramural grant support to carry out this work. The manuscript corrections and support extended by Ms K. Sowmya is acknowledged.
Glossary
- WNIN
Inbred Wistar rat strain established at NIN
- WNIN/Ob
Wistar rats raised in the National Institute of Nutrition, with obesity trait
- NCLAS
National Center for Laboratory Animal Sciences
- NIN
National Institute of Nutrition
- DTZ
Dithiazone
- KRBH
Krebs–Ringer bicarbonate HEPES
References
- Amoli MM, Moosavizadeh R, Larijani B. Optimizing conditions for rat pancreatic islets isolation. Cytotechnology. 2005;48:75–78. doi: 10.1007/s10616-005-3586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru P, Rajkumar H, Nappanveettil G. Altered or impaired immune response upon vaccination in WNIN/Ob rats. Vaccine. 2011;16:3038–3042. doi: 10.1016/j.vaccine.2011.01.107. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Bhonde RR. Islet generation from intra islet precursor cells of diabetic pancreas: in vitro studies depicting in vivo differentiation. JOP. 2003;4:137–145. [PubMed] [Google Scholar]
- Giridharan NV. Animal models of obesity and their usefulness in molecular approach to obesity. Indian J Med Res. 1998;108:225–242. [PubMed] [Google Scholar]
- Giridharan NV, Harishankar N, Satyavani M. A new rat model for the study of obesity. Scand J Anim Sci. 1996;23:131–137. [Google Scholar]
- Gotoh M, Maki T, Kiyozumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Gray DWR, Mc Shane P, Grant A, Morris PJ. A method for isolation of islets of langerhans from the human pancreas. Diabetes. 1984;33:1055–1061. doi: 10.2337/diabetes.33.11.1055. [DOI] [PubMed] [Google Scholar]
- Hara Y, Taniguchi H, Ishihara K, et al. Simple and easy method for harvesting of a large number of isolated islets and their function. Transplant Proc. 1989;21:2632–2634. [PubMed] [Google Scholar]
- Kiran SG, Dorisetty RK, Umrani MR, Boindala S, Bhonde RR, Chalsani M, Singh H, Venkatesan V. Pyridoxal 5′ phosphate protects islets against streptozotocin-induced beta-cell dysfunction–in vitro and in vivo. Exp Biol Med. 2011;236:456–465. doi: 10.1258/ebm.2011.010361. [DOI] [PubMed] [Google Scholar]
- Kneteman N, Alderson D, Scharp DW. The isolation and purification of human pancreatic islets. Transplant Proc. 1987;19:3469–3470. [PubMed] [Google Scholar]
- Lui M, Shapiro ME. A new method for isolation of murine islets with markedly improved yields. Transplant Proc. 1995;27:3208–3210. [PubMed] [Google Scholar]
- Madhira SL, NappanVeettil G, Kodavalla V, Venkatesan V. Comparison of adipocyte–specific gene expression from WNIN/Ob mutant obese rats, lean control and parental control. Mol Cell Biochem. 2011;357:217–225. doi: 10.1007/s11010-011-0892-4. [DOI] [PubMed] [Google Scholar]
- Perdrizet GA, Rewinski MJ, Bartus SA, Hull D, Schweizer RT, Scharp DW. Albumin improves islet isolation: specific versus nonspecific effects. Transplant Proc. 1995;27:3400–3402. [PubMed] [Google Scholar]
- Reddy GB, Vasireddy V, Mandal MN, Tiruvalluru M, Wang XF, Jablonski MM, Nappanveettil G, Ayyagari R (2009) A novel rat model with obesity-associated retinal degeneration. Invest Ophthalmol Vis Sci 50:3456–3463. doi:10.1167/iovs.08-2498 [DOI] [PMC free article] [PubMed]
- Sakamuri VP, Ananthathmakula P, Veettil GN, Ayyalasomayajula V (2011) Vitamin A decreases pre-receptor amplification of glucocorticoids in obesity—study on the effect of Vitamin A on 11 beta—hydroxysteroid dehydrogenase type 1 activity in liver and visceral fat of WNIN/Ob obese rats. Nutr J 10:70. doi:10.1186/1475-2891-10-70 [DOI] [PMC free article] [PubMed]
- Shewade Y, Umrani M, Bhonde RR. Large-scale isolation of islets by tissue culture of adult mouse pancreas. Transplant Proc. 1999;31:1721–1723. doi: 10.1016/S0041-1345(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi V, Naseem B, Khan AA, Capoor AK, Habibullah CM (2004) Comparison of biochemical and cytotoxic functions of hepatocytes from goat, pig and human fetuses. J Gastroenterol Hepatol 19:1029–1035. doi:10.1111/j.1440-1746.2004.03402.x [DOI] [PubMed]