Abstract
Lacrimal gland acinar cells are an important cell type to study due to their role in production and release of tear proteins, a function essential for ocular surface integrity and normal visual acuity. However, mechanistic studies are often limited by problems with transfection using either plasmid DNA or siRNA. Although various gene delivery methods are available, many have been unproductive due to consistently low transfection efficiencies. We have developed a method using nucleofection that can result in 50% transfection efficiency and 60% knockdown efficiency for plasmid DNA and siRNA, respectively. These results are vastly improved relative to previous studies, demonstrating that nucleofection offers an efficient transfection technique for primary lacrimal gland acinar cells.
Keywords: Transfection, Nucleofection, Primary epithelial cells, Acinar cells, Lacrimal gland
Introduction
A major category of eye diseases, dry-eye disorders, collectively affect tens of millions of people in the United States alone and represent the most common cause of visits to eye care specialists. Dry-eye disorders are most commonly associated with inefficient or decreased secretion by the lacrimal gland acinar cells (LGAC) of the lacrimal gland. In a subset of these cases, these changes are accompanied by autoimmune inflammation of the lacrimal gland associated with Sjögren’s syndrome (SjS). LGAC are the principal cells responsible for the production and release of tear proteins to the ocular surface by the lacrimal gland, a process that is necessary for a healthy ocular surface and visual acuity. These cells are primary differentiated epithelial secretory cells that comprise approximately 80% of the mass of the lacrimal gland. Acinar cells within the gland are organized with their apical domains oriented to a central lumen into which proteins and fluid are released by exocytosis of secretory vesicles at the apical membrane. Although the functional acinar secretory unit is a highly specialized structure, individual acinar cells isolated from rabbit lacrimal gland can re-form into acinar-like structures in the presence of specific extracellular matrix additives (Gierow et al. 1995, 1996; Hamm-Alvarez et al. 1997). These reconstituted acini are poorly adherent and form loosely tethered clusters that can be anchored to the coverslip surface only in the presence of a significant amount of Matrigel™.
Many studies investigating the mechanisms associated with the development of dry eye disorders and the autoimmune disease, SjS, utilize rabbit LGAC cultures (Jerdeva et al. 2005; Marchelletta et al. 2008; Mircheff et al. 1998; Xie et al. 2002) while others use rat and mouse LGAC (Chen et al. 1998; da Costa et al. 2006; Hodges et al. 2004; Rios et al. 2005; Sanghi et al. 2000; Zoukhri and Kublin 2002). Primary cultured LGAC have previously exhibited very low transfection efficiency using non-viral, lipid-based methods, thus significantly limiting the types of experiments that may be conducted. To date, the only successful gene delivery methodology that is achievable with these cells has been the use of replication-defective Adenovirus Type 5 (Ad5). Although effective, this approach relies on the customized production of viral constructs, which is often time consuming and expensive, and is not conducive to easy delivery of siRNAs. Some previous work has shown that exposure of the LGAC to Ad5 capsid proteins may modify cellular function (Hamm-Alvarez et al. 2003). Finally, additional precautions to enhance safety and avoid contamination of other cells including duplicate culture facilities are often needed when working with Ad5. The difficulty with gene expression using typical transfection reagents in LGAC extends to siRNA. Previous attempts at siRNA delivery using a cationic lipid reagent resulted at best in a 30% knockdown of the target protein (Xie et al. 2006). It has been shown that gene delivery using non-viral vectors, while simple to use and inexpensive, is often dependent on a number of factors for effectiveness, many of which are difficult to control in primary culture and in terminally differentiated cells (Uchida et al. 2002).
Recently, a number of cell types that have previously shown to be difficult to transfect have been successfully transfected using nucleofection. Among these are human neural progenitor cells (Dieterlen et al. 2009), primary human keratinocytes (Distler et al. 2005) and endothelial cells (Thiel and Nix 2006), human embryonic stem cells (Lakshmipathy et al. 2007; Siemen et al. 2005, 2008), and mammalian neurons (Lakshmipathy et al. 2007; Zeitelhofer et al. 2007, 2009). Nucleofection is a newer transfection method based on the physical method of electroporation. It uses a combination of electrical parameters with cell-type specific reagents to enable transfer of nucleic acids (DNA, RNA, siRNA) directly into the nucleus. Because the method does not rely on cell division, it allows for transfection of non-dividing cells. Here we investigated and optimized transfection of LGAC using nucleofection technology for plasmid delivery using two genes encoding cytosolic and membrane-encapsulated proteins, GFP and Cathepsin S-GFP, respectively. We also demonstrated its improved efficacy relative to previous methods for delivery of siRNA directed against the Coxsackievirus-adenovirus receptor (CAR). This receptor is the common receptor for both group B coxsackieviruses and adenoviruses and is highly expressed in rabbit LGAC (Xie et al. 2006). Also, we compared the transfection efficiency of nucleofection with additional non-viral, lipid-based transfection methods. The results show that nucleofection may represent a simple and viable strategy for modulating selective acinar cell functions through which physiological and pathophysiological changes can be deduced through use of plasmid transfection or siRNA.
Materials and methods
Materials
The nucleofector device and the Basic Nucleofector Kit for Primary Epithelial Cells were purchased from Amaxa, now Lonza (Gaithersburg, MD). The GeneSilencer siRNA transfection reagent was purchased from Genlantis (San Diego, CA), while Lipofectamine™ 2000 and the LIVE/DEAD cell assay kit were from Invitrogen Corporation (Carlsbad, CA) and Fugene 6 was purchased from Roche (Rotkreuz, Switzerland). CAR siRNA duplex (sense sequence: 5′-GGUCAGAAGAAAUUGGAAATT-3′; antisense sequence: 5′-UUUCCAAUUUCUUCUGACCTT-3′) was synthesized by the USC/Norris Cancer Center DNA Core (Los Angeles, CA) and the siControl non-targeting siRNA was obtained from Dharmacon RNA Technologies (Lafayette, CO). Mouse monoclonal antibody to Cathepsin S protein was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DyLight 488-conjugated donkey anti-goat secondary antibody was purchased from Jackson ImmunoResearch (West Grove, PA). Rhodamine-phalloidin, Alexa Fluor 647-phalloidin, DAPI, and Prolong anti-fade medium were obtained from Molecular Probes/Invitrogen (Carlsbad, CA). The pmaxGFP plasmid is commercially available from Lonza. The cathepsin S-GFP plasmid was constructed using the full-length cDNA of mouse cathepsin S in plasmid pCMV-SPORT6 (Open Biosystems). In the recombinant fusion protein, GFP is located at the C-terminus.
Cell isolation and culture
Female New Zealand White rabbits weighing between 1.8 and 2.2 kg were obtained from Irish Farms (Norco, CA). LGAC were isolated and maintained in a laminin-based primary culture system for 2 days as described previously (Gierow et al. 1995; Hamm-Alvarez et al. 1997; da Costa et al. 1998). These culture conditions result in reconstitution of polarity, establishment of lumena, and formation of secretory vesicles (Gierow et al. 1995; Hamm-Alvarez et al. 1997; Jerdeva et al. 2005; da Costa et al. 1998; Xie et al. 2004). In some experiments, HeLa cells were utilized for comparison of transfection efficiency relative to LGAC. HeLa cells were obtained from ATCC and were split and cultured as recommended by the manufacturer.
Transfection of cells
Transfection was performed on LGAC on day 2 of culture. For transfection using Fugene 6, 1 × 106 cells were transfected with 3 or 6 μL of Fugene 6 reagent and either 1 or 2 μg pmaxGFP to achieve the recommended 3:1, 3:2, and 6:1 Fugene 6 reagent: DNA ratios in 2 mL of culture medium in a 6-well plate, according to the manufacturer’s protocol. For transfection using Lipofectamine™ 2000, 4 × 106 cells were transfected with 4 μg pmaxGFP and either 4, 10 or 20 μL of lipofectamine 2000 to achieve a 1:1, 1:2.5 and 1:5 DNA:Lipofectamine ratio in 2 mL of culture medium in a 6-well plate, according to the manufacturer’s protocol. Nucleofection was performed on LGAC on day 1 or 2 of culture using the Lonza Group nucleofector device and the Basic Nucleofector Kit for Primary Mammalian Epithelial Cells. For the optimized reaction, 6 × 106 cells were resuspended in 100 μL nucleofector solution with 3 μg either pmaxGFP or a cathepsin S-GFP. Program Z-001 was used for the electrical settings. Immediately after nucleofection, the cells were transferred into 3 mL pre-warmed medium in a 6-well plate. For imaging experiments, the cells were subsequently plated onto Matrigel™-coated glass coverlips in 12-well plates (2 × 106 cells/well). Transfection efficiency was measured after 24 h.
Analysis of plasmid transfection efficiency
For pmaxGFP, expression was detected by fluorescence microscopy and flow cytometry. Immediately following nucleofection, the cells were transferred into 3 mL pre-warmed media in 6-well plates and incubated at 37 °C. After 24 h, the cells were imaged and then collected and processed for flow cytometry. For Cathepsin S-GFP, expression was analyzed by immunofluorescence. As before, cells were transferred into 3 mL pre-warmed media, but were immediately seeded onto Matrigel®-coated coverslips in 12-well plates at a density of 2 × 106 cells/well and incubated at 37 °C. After 24 h, the cells were fixed in 100% ETOH (−20 °C) for 5 min and rinsed extensively with PBS prior to the addition of goat polyclonal antibody to Cathepsin S and appropriate fluorophore-conjugated secondary antibody. Rhodamine-phalloidin or Alexa Fluor 647-phalloidin was used to label actin filaments, while DAPI was used to label nuclei. Confocal fluorescence images were obtained using a Zeiss LSM 510 Meta NLO imaging system.
Delivery of small interfering RNA duplexes
For delivery of siRNAs using nucleofection, 6 × 106 cells were resuspended in 100 μL nucleofector solution with 2 μM siRNA duplexes directed against the coxsackie-adenovirus receptor (CAR) or the siControl non-targeting siRNA. Program Z-001 was used for the electrical settings. Immediately after nucleofection, the cells were transferred into 3 mL pre-warmed medium in a 6-well plate. For delivery of siRNAs using GeneSilencer® siRNA Transfection Reagent, 4 × 106 cells were transfected with 2 μg siRNA and 10 μL GeneSilencer reagent, according to protocol. For delivery of siRNAs using Lipofectamine 2000, 4 × 106 cells were transfected with 100 pmol siRNA duplexes and 5 μL Lipofectamine 2000 reagent, according to protocol.
Analysis of siRNA knockdown efficiency
Uptake of CAR-specific siRNAs was evaluated by semi-quantitative real-time PCR analysis of CAR mRNA. After nucleofection, cells were transferred into 3 mL pre-warmed media in 6-well plates and incubated at 37 °C. After 24 h, the cells were collected and the total RNA for both control and treated samples was isolated and used for cDNA synthesis. Relative expression levels were detected by using TaqMan® gene expression assays for the target, CAR, and the endogenous control, hypoxanthine phosphoribosyltransferase 1 (HPRT1). Results were normalized to HPRT1 mRNA.
Cytotoxicity assays
For analysis of cytotoxicity, LGAC were either mock-nucleofected, or nucleofected with either pmaxGFP or CAR-specific siRNA duplexes on day 2 of culture. Immediately following nucleofection, the cells were transferred into 3 mL pre-warmed medium in a 6-well plate and incubated at 37 °C. After 24 h, calcein (Ex 494/Em 517 nm) and ethidium homodimer-1 (Ex 528/Em 617 nm), both provided in the LIVE/DEAD cell assay kit (Invitrogen), were used to detect live/dead cells. Both were added to the cells (50 μL of 0.5 μM calcein AM and 3.0 μM EthD-1) prior to incubating at 37 °C for 15 min. Fluorescence intensity was measured using an Envision 2103 Multilabel Reader (Perkin-Elmer).
Statistics
Cells from each experiment were analyzed individually, and the results from different experiments were compared using the Student’s t test with p ≤ 0.05 to evaluate statistical significance of any observed changes.
Results and discussion
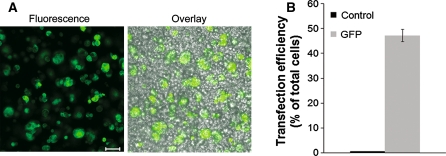
We have evaluated the efficiency of nucleofection technology for gene and siRNA delivery into primary rabbit LGAC. After an initial screening of several program settings, including S-005, K-033, Y-001, Y-002, and Z-001, it was determined that program Z-001 yielded the best transfection efficiency and cell viability balance. Fluorescence imaging and flow cytometry analysis of cells nucleofected with pmax-GFP, a control, GFP-encoding plasmid, indicated that 47 ± 2% of cells were GFP-positive after 24 h (Fig. 1).
Fig. 1.
Nucleofection of rabbit lacrimal gland acinar cells. Rabbit LGAC were seeded in 150-mm petri dishes at a density of 2 × 106 cells/mL. On day 1 of culture, cells were nucleofected with 3 μg pmaxGFP. Nucleofected cells were analyzed for expression of GFP by fluorescence microscopy 24 h post-nucleofection (a) Bar 50 μm. Following microscopy imaging, the cells were prepared for FACS analysis and assayed by such (b). Overlay refers to the composite image including fluorescence and phase contrast. (For GFP, n = 4)
To examine whether the nucleofection process resulted in morphological differences, cells nucleofected with a plasmid encoding Cathepsin-S, a cysteine protease enriched in lysosomes and traces in secretory vesicles, were seeded on glass coverslips following transfection and were processed for confocal fluorescence microscopy after 24 h. Detection of actin filaments using fluorescent phalloidin was used to define the characteristic membrane organization and positioning of basolateral and apical membranes within these LGAC, as has been previously described (da Costa et al. 1998). As shown in Fig. 2a, nucleofected cells showed the normal organization of apical and basolateral membranes relative to cells in the absence of nucleofection, despite the obviously increased expression of cathepsin S. The nucleofected LGAC were clearly able to overexpress and accurately localize this luminal vesicle protease to a series of punctate intracellular compartments. The major localization of the punctate staining to basolateral regions is consistent with targeting to lysosomes. For better understanding of the LGAC morphology, Fig. 2b shows control cells that have been fixed and processed to label the actin filaments that help us visualize the polarity of the cells, as well as the nuclei.
Fig. 2.
Nucleofected LGAC are able to accurately localize a vesicular compartment protein. a Rabbit LGAC were seeded in 150-mm petri dishes at a density of 2 × 106 cells/mL. On day 2 of culture, cells were or were not nucleofected with 3 μg of a Cathepsin-S-GFP plasmid and seeded on Matrigel-coated coverslips in 12-well plates as described in Materials and Methods. After 24 h, the cells were fixed and processed to fluorescently label Cathepsin (green) and actin filaments (red). A schematic is also included in which lumena are marked with an “L”. Note the expression of Cathepsin S-GFP localized to punctate organelles. Bar10 μm. b Control rabbit LGAC were fixed on day 3 and processed to fluorescently label actin (red) and nuclei (blue), which are basolaterally located. Bar 10 μm
Since there are a number of available transfection reagents widely in use, we compared the optimized nucleofection method to two other non-viral techniques: a lipid-based transfection reagent, Fugene 6, and a cationic lipid-mediated transfection reagent, Lipofectamine™ 2000, using pmax-GFP. Transfection of LGAC using Fugene 6 yielded very poor efficiency across the suggested reagent-to-DNA ratios, (Fig. 3a) making this reagent clearly unsuitable for use in LGAC. Similarly, transfection of LGAC using Lipofectamine™ 2000 also resulted in poor transfection efficiency with the highest efficiency of ~2% of total cells achieved using a 1:2.5 reagent-to-DNA ratio (Fig. 3b). For comparison, HeLa cells were transfected in parallel and resulted in a 16.9 ± 1.7% transfection efficiency with the same reagent-to-DNA ratio of 1:2.5 indicating that while it may be useful for some cells, it would not be useful for transfection of LGAC.
Fig. 3.
Transfection of rabbit lacrimal gland acinar cells. Rabbit LGAC were seeded in 150-mm petri dishes at a density of 2 × 106 cells/mL. On day 2 of culture, cells were transfected with either Fugene 6 (a) or Lipofectamine 2000 (b) as described in “Materials and methods”. After 24 h, control and transfected cells were collected and prepared for analysis by flow cytometry. For comparison, HeLa cells were also transfected and analyzed in parallel (n = 3 for HeLa cells and n = 4 for LGAC)
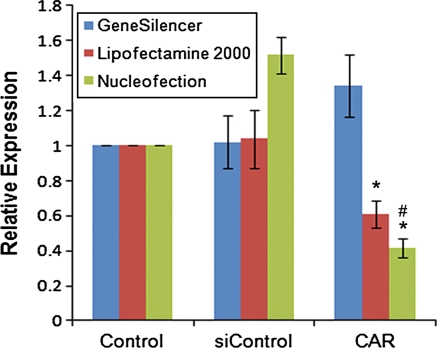
We also investigated the efficiency of siRNA delivery to LGAC via nucleofection. We performed knockdown of CAR, a protein highly expressed in LGAC, and evaluated the efficiency of its knockdown by real-time PCR analysis as previously described (Xie et al. 2006). Furthermore, we performed parallel knockdown experiments using either GeneSilencer or Lipofectamine™ 2000 for delivery of the siRNA duplexes. As shown in Fig. 4, nucleofection provided the highest knockdown efficiency (almost 60%) compared to the other two techniques. Surprisingly, Lipofectamine™ 2000 was shown to be quite efficient for siRNA delivery despite its poor efficiency in the delivery of plasmid DNA. Interestingly, delivery of the siControl non-targeting sequence by nucleofection resulted in an increase in CAR expression levels. This may be attributed, at least in part, to what is believed to be the physiological function of CAR, mainly as a cell adhesion molecule (Cohen et al. 2001; Coyne and Bergelson 2005). It is possible that the nucleofection itself, in essence a type of electroporation, triggered increased expression of the protein in order to re-establish the cell–cell contacts necessary for proper acinar structure of these cells. These cell-to-cell junctions have been shown to be important for the establishment of proper epithelial cell polarity (Aijaz et al. 2006; Shin et al. 2006).
Fig. 4.
Effective gene expression knockdown. Rabbit LGAC were seeded in 150-mm petri dishes at a density of 2 × 106 cells/mL. On day 2 of culture, cells were either transfected with 100 pmol CAR siRNA and 5 μL Lipofectamine, 2 μg CAR siRNA and 10 μL GeneSilencer, or nucleofected with 2 μM CAR siRNA or a non-targeting sequence, siControl. Afer 24 h, the total RNA from control and experimental samples was isolated, purified, and used for cDNA synthesis. Relative expression levels were determined by quantitative real-time PCR. Results were normalized to HPRT1 mRNA and are expressed relative to control, untreated cells (n = 4). *significant at p ≤ 0.05 relative to control. # significant at p ≤ 0.05 relative to Lipofectamine 2000
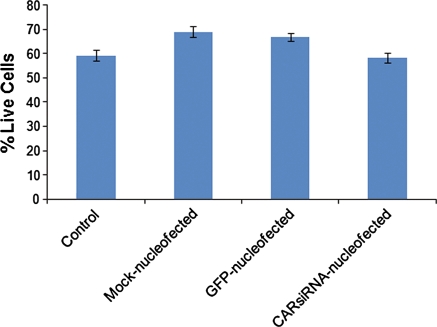
To effectively evaluate nucleofection as a technique, it was important to examine its effect on cell viability. We did this by assaying control or treated LGAC using a viability/cytotoxicity kit. We found that after 24 h, nucleofected cells retained viability comparable to control cells and did not vary with cargo, e.g., plasmid DNA or siRNA (Fig. 5). Viability is normally in the range of 60–70% in these cells which are primary reconstituted cells that have undergone a substantial digestion procedure during the cell culturing process. These data indicate that the nuclefection process and parameters do not significantly affect cell viability.
Fig. 5.
Nucleofection does not affect cell viability. Rabbit LGAC were seeded in 150-mm petri dishes at a density of 2 × 106 cells/mL. On day 2 of culture, cells were either mock-nucleofected or nucleofected with either 3 μg pmaxGFP or 2 μM siRNA. After 24 h, the cells were assayed using the LIVE/DEAD viability/cytotoxicity kit for mammalian cells (n = 4)
Here we have optimized a method for transfection of primary cultured LGAC. We determined that the most effective transfection of these cells is achieved using program Z-001 and the nucleofector solution found in the basic nucleofector kit for primary mammalian epithelial cells. With these parameters, we were able to detect nearly 50% transfection efficiency of these previously difficult-to-transfect primary differentiated epithelial cells while retaining their unique morphology. Additionally, we were able to effectively knockdown CAR expression by nearly 60%. This level of transfection and knockdown has not previously been reported for this cell type. Lastly, we were able to determine that nucleofected cells retain viability comparable to control cells. This technology offers a powerful, non-viral tool for further understanding of the lacrimal gland and its associated diseases, and may be further relevant to studies of other primary exocrine cells such as pancreatic acini.
Acknowledgments
We thank the National Institutes of Health for support for this work through RO1 EY017293 awarded to SHA and a Ruth L. Kirchstein Predoctoral Fellowship (F31 EY08807) to JC.
Conflict of interest The authors declare no competing interests.
References
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Glass JD, Walton SC, Laurie GW. Role of laminin-1, collagen IV, and an autocrine factor(s) in regulated secretion by lacrimal acinar cells. Am J Physiol. 1998;275:C278–C284. doi: 10.1152/ajpcell.1998.275.1.C278. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869–882. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- da Costa SR, Yarber FA, Zhang L, Sonee M, Hamm-Alvarez SF. Microtubules facilitate the stimulated secretion of beta-hexosaminidase in lacrimal acinar cells. J Cell Sci. 1998;111:1267–1276. doi: 10.1242/jcs.111.9.1267. [DOI] [PubMed] [Google Scholar]
- da Costa SR, Wu K, Veigh MM, Pidgeon M, Ding C, Schechter JE, Hamm-Alvarez SF. Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development. Exp Eye Res. 2006;82:33–45. doi: 10.1016/j.exer.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen MT, Wegner F, Schwarz SC, Milosevic J, Schneider B, Busch M, Römuss U, Brandt A, Storch A, Schwarz J (2009) Non-viral gene transfer by nucleofection allows stable gene expression in human neural progenitor cells. J Neurosci Methods 178:15–23 [DOI] [PubMed]
- Distler JH, Jungel A, Kurowska-Stolarska M, Michel BA, Gay RE, Gay S, Distler O. Nucleofection: a new, highly efficient transfection method for primary human keratinocytes*. Exp Dermatol. 2005;14:315–320. doi: 10.1111/j.0906-6705.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Gierow JP, Lambert RW, Mircheff AK. Fluid phase endocytosis by isolated rabbit lacrimal gland acinar cells. Exp Eye Res. 1995;60:511–525. doi: 10.1016/S0014-4835(05)80066-1. [DOI] [PubMed] [Google Scholar]
- Gierow JP, Yang T, Bekmezian A, Liu N, Norian JM, Kim SA, Rafisolyman S, Zeng H, Okamoto CT, Wood RL, Mircheff AK (1996) Na-K-ATPase in lacrimal gland acinar cell endosomal system: correcting a case of mistaken identity. Am J Physiol 271:C1685–C1698 [DOI] [PubMed]
- Hamm-Alvarez SF, Da Costa S, Yang T, Wei X, Gierow JP, Mircheff AK. Cholinergic stimulation of lacrimal acinar cells promotes redistribution of membrane-associated kinesin and the secretory protein, beta-hexosaminidase, and increases kinesin motor activity. Exp Eye Res. 1997;64:141–156. doi: 10.1006/exer.1996.0198. [DOI] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Xie J, Wang Y, Medina-Kauwe LK. Modulation of secretory functions in epithelia by adenovirus capsid proteins. J Control Release. 2003;93:129–140. doi: 10.1016/j.jconrel.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Raddassi I, Zoukhri D, Toker A, Kazlauskas A, Dartt DA. Effect of overexpression of constitutively active PKCalpha on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 2004;45:3974–3981. doi: 10.1167/iovs.04-0508. [DOI] [PubMed] [Google Scholar]
- Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118:4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmipathy U, Buckley S, Verfaillie C. Gene transfer via nucleofection into adult and embryonic stem cells. Methods Mol Biol. 2007;407:115–126. doi: 10.1007/978-1-59745-536-7_9. [DOI] [PubMed] [Google Scholar]
- Marchelletta RR, Jacobs DT, Schechter JE, Cheney RE, Hamm-Alvarez SF. The class V myosin motor, myosin 5c, localizes to mature secretory vesicles and facilitates exocytosis in lacrimal acini. Am J Physiol Cell Physiol. 2008;295:C13–C28. doi: 10.1152/ajpcell.00330.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mircheff AK, Gierow JP, Yang T, Zhang J, Wood RL, Azzarolo AM, Warren DW, Zeng H, Guo Z, Kaslow HR, Hamm-Alvarez SF, Okamoto CT, Bachmann M (1998) Sjögren’s autoimmunity: how perturbation of recognition in endomembrane traffic may provoke pathological recognition at the cell surface. J Mol Recognit 11:40–48 [DOI] [PubMed]
- Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, Zoukhri D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80:477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Walton S, Laurie GW. Quantitation of rat lacrimal secretion: a novel sandwich ELISA with high sensitivity. Exp Eye Res. 2000;70:651–658. doi: 10.1006/exer.2000.0833. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Siemen H, Nix M, Endl E, Koch P, Itskovitz-Eldor J, Brustle O. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005;14:378–383. doi: 10.1089/scd.2005.14.378. [DOI] [PubMed] [Google Scholar]
- Siemen H, Nolden L, Terstegge S, Koch P, Brustle O. Nucleofection of human embryonic stem cells. Methods Mol Biol. 2008;423:131–138. doi: 10.1007/978-1-59745-194-9_8. [DOI] [PubMed] [Google Scholar]
- Thiel C, Nix M. Efficient transfection of primary cells relevant for cardiovascular research by nucleofection. Methods Mol Med. 2006;129:255–266. doi: 10.1385/1-59745-213-0:255. [DOI] [PubMed] [Google Scholar]
- Uchida E, Mizuguchi H, Ishii-Watabe A, Hayakawa T. Comparison of the efficiency and safety of non-viral vector-mediated gene transfer into a wide range of human cells. Biol Pharm Bull. 2002;25:891–897. doi: 10.1248/bpb.25.891. [DOI] [PubMed] [Google Scholar]
- Xie J, Qian L, Hamm-Alvarez SF, Mircheff AK. Epidermal growth factor traffic in lacrimal acinar cells. Adv Exp Med Biol. 2002;506:213–217. doi: 10.1007/978-1-4615-0717-8_28. [DOI] [PubMed] [Google Scholar]
- Xie J, Qian L, Wang Y, Hamm-Alvarez SF, Mircheff AK. Role of the microtubule cytoskeleton in traffic of EGF through the lacrimal acinar cell endomembrane network. Exp Eye Res. 2004;78:1093–1106. doi: 10.1016/j.exer.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Xie J, Chiang L, Contreras J, Wu K, Garner JA, Medina-Kauwe L, Hamm-Alvarez SF. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J Virol. 2006;80:11833–11851. doi: 10.1128/JVI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitelhofer M, Vessey JP, Xie Y, Tubing F, Thomas S, Kiebler M, Dahm R. High-efficiency transfection of mammalian neurons via nucleofection. Nat Protoc. 2007;2:1692–1704. doi: 10.1038/nprot.2007.226. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Vessey JP, Thomas S, Kiebler M, Dahm R (2009) Transfection of cultured primary neurons via nucleofection. Curr Protoc Neurosci Chapter 4:Unit4 32 [DOI] [PubMed]
- Zoukhri D, Kublin CL. Impaired neurotransmission in lacrimal and salivary glands of a murine model of Sjogren’s syndrome. Adv Exp Med Biol. 2002;506:1023–1028. doi: 10.1007/978-1-4615-0717-8_42. [DOI] [PubMed] [Google Scholar]