Abstract
Mercury, a xenobiotic metal, is a highly deleterious environmental pollutant. Moreover, in any form mercury is reported to be toxic. On the other hand, Thymbra spicata L., a member of the Lamiaceae family, has long been investigated popularly of biological roles; mainly antimicrobial and antioxidant activities. However, there are very scarce data on the cytogenetic effects of thyme species. The purpose of this study was to investigate the genetic safety of different extracts from T. spicata (water extract, methanol extract, and ethanol extract) and the effects of T. spicata on mercury (as HgCl2) induced genotoxicity. Sister chromatid exchange (SCE) and micronucleus (MN) assays were performed to assess DNA damages in cultured human lymphocytes (n = 5). Our results clearly revealed that, the SCE and MN rates induced by HgCl2 were alleviated by the presence of T. spicata. As conclusion, this study demonstrated for the first time that the T. spicata provided increased resistance of DNA against HgCl2 induced genetic damage in human lymphocytes. Based on the results of this study, it may be concluded that the T. spicata is a nontoxic material that could be used as a suppressor of heavy metal-induced genotoxicity.
Keywords: Thymbra spicata, Mercuric chloride, Genotoxicity, Human lymphocyte culture, Antimutagenic activity
Introduction
In the environment, animals and humans are exposed to numerous chemical forms of mercury (Hg), including elemental mercury vapor, inorganic mercurous (Hg(I)), mercuric (Hg(II)) and organic mercuric compounds (Fitzgerald and Clarkson 1991). Hg, one of the most widely diffused and hazardous organ-specific environmental contaminants, exists in a wide variety of physical and chemical states, each of which with unique characteristics of target organ specificity (Aleo et al. 2002). Elemental, inorganic, and organic forms of mercury exhibit toxicologic characteristics including nephrotoxicity, neurotoxicity, and gastrointestinal toxicity with ulceration and hemorrhage (Zalups and Koropatnick 2000). Besides, the genotoxicity of mercuric compounds have been reported in both in vitro and in vivo model systems (Silva-Pereira et al. 2005; Grotto et al. 2010; Turkez et al. 2011). Recent findings elucidated that mechanism of mercury toxicity included production of reactive oxygen species (ROS) capable of damaging lipids in membrane, proteins or enzymes in tissues, and DNA to induce oxidative stress (Jan et al. 2011). Therefore, many efforts are being made to minimize the mercury toxicity by antioxidant featured agents such as fish oil (Grotto et al. 2010), selenium (Glaser et al. 2010), boron compounds (Turkez et al. 2011), melatonin (Rao et al. 2010), curcumin (Agarwal et al. 2010) and Allium sativum L. extract (Abdalla et al. 2010).
Thymbra spicata L. from Lamiaceae family is a perennial plant known as “Kekik, Zahter or Sater” in Turkey. The plant is naturally grown in Southeast Anatolia (especially in the Gaziantep, Kahramanmaraş and Şanlıurfa Cities) (Daneshvar-Royandezagh et al. 2009). Dried herbs of Thymbra are used as herbal tea, condiment and folk medicine for treating asthma, colic, bronchitis, coughs. In addition, it is also used in food industry for flavouring, as preservation agent and aroma in Turkey. Several previous studies reported that the essential oils of T. spicata, and their main phenolic constituents such as carvacrol and thymol that show remarkable antimicrobial, antibacterial and antifungal activities (Baytop 1984; Shelef 1983).
Many efforts are being made to investigate therapeutic substances capable of reducing the genotoxicity of man-made or natural mutagens in human life. These include frequently vitamins, minerals and plant products (Turkez and Geyikoglu 2010; Turkez et al. 2010; Haleagrahara et al. 2010; Aboul-Soud et al. 2011; Fujiwara et al. 2011; Turkez and Dirican, 2011). Concomitant treatment with the antioxidants and mutagens provided protection against tissue damages in experimental models (Takaishi et al. 2009; Turkez et al. 2011). To our best knowledge, there is no report on the effects of T. spicata extracts against mercury toxicity on human blood cells. Therefore, in this investigation it was aimed to explore the role of T. spicata water extracts, methanol extract and ethanol extract on HgCl2-induced genotoxicity in human lymphocyte cells by using SCE and MN tests. Hence, SCE and MN tests covering a wide range of induced genetic damage as primary DNA damage were performed on peripheral lymphocytes.
Materials and methods
Experimental design
Blood samples were obtained by veinpuncture from five healthy non-smoking donors. Human peripheral blood lymphocyte cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (1975). The heparinized blood (0.5 ml) was cultured in 6 ml culture medium (Chromosome Medium B, Biochrom®, Leonorenstr. 2-6, D-12247, Berlin) with 5 μg/l of phytohemagglutinin (Biochrom®). HgCl2 were purchased from Sigma® (St. Louis, MO, USA; CAS No. 7487-94-7). T. spicata was purchased in ready package. For water extraction of T. spicata, 20 g leaves was mixed with 400 ml distilled and boiling water using magnetic stirrer for 15 min. Then extract was filtered over Whatman No. 1 paper. Then, HgCl2 (9 mg/L) and T. spicata extracts (0, 25, 50, 100 and 200 mg/L) were added into culture tubes separately and together. After supplementation of HgCl2 and plant extracts, the blood samples were incubated for 72 h at 37 °C to adjust body conditions. Each individual whole blood culture without HgCl2 or T. spicata extract was studied as a control group.
Preparation of methanol extract
Air-dried and powdered leaves (10 g) were extracted with 250 mL of methanol using a Soxhlet.
extractor (Isopad, Heidelberg, Germany) for 72 h at a temperature not exceeding the boiling point of the solvent. The extract was filtered using Whatman filter paper No. 1 and then concentrated in a vacuum at 40 °C using a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland), yielding a waxy material. The extract was then lyophilized and kept in the dark at 4 °C until tested.
Preparation of ethanol extract
About 50 g of air dried leaves were dissolved in 250 ml of ethanol and kept in an orbital shaker for overnight. The extracts obtained were filtered through Whatman No. 1 filter paper and the filtrate was collected. Fifty grams of powdered plant material was extracted in 250 ml of boiling water for 2 h and concentrated to half of the volume by boiling in a water bath. The extract was cooled and filtered using Whatmann No. 1 filter paper.
Genotoxicity testing
SCE assay
With the aim of providing successive visualization of SCEs, 5-bromo-2′-deoxyuridine (Sigma®) was added at culture initiation. The cultures were incubated in complete darkness for 72 h at 37 °C. Exactly 70 h and 30 min after beginning the incubations, demecolcine (N-Deacetyl-N-methylcolchicine, Sigma®) was added to the cultures. After hypotonic treatment (0.075 M KCl), followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease-free microscopic slides, air-dried, aged for 3 days, and then differentially stained for the inspection of the SCE rate according to fluorescence plus Giemsa (FPG) procedure. For each treatment condition, well-spread 25 s division metaphases containing 42–46 chromosomes in each cell were scored by one observer (by E. Dirican), and the values obtained were calculated as SCEs per cell.
MN assay
The MN test was performed by adding cytochalasin B (Sigma®) after 44 h of culture. At the end of the 72 h incubation period, the lymphocytes were fixed with ice-cold methanol/acetic acid (1:1, v/v). The fixed cells were put directly on slides using a cytospin, and stained with Giemsa solution. All slides were coded before scoring. The criteria for scoring MN were as described by Fenech (1993). At least 1,000 binucleated lymphocytes were examined per concentration for the presence of one, two or more MN by one observer (by E. Dirican).
Statistics
Statistical analysis was performed using SPSS Software (version 18.0, SPSS, Chicago, IL, USA). For statistical analysis of obtained data Duncan’s test was used. Statistical decisions were made with a significance level of 0.05.
Results
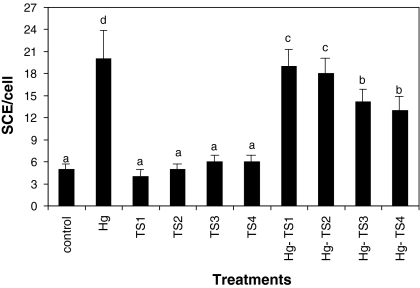
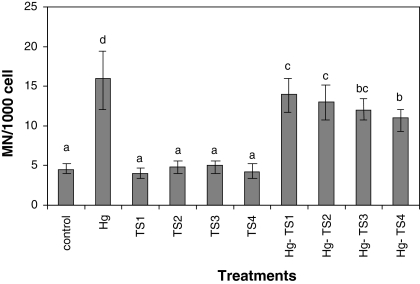
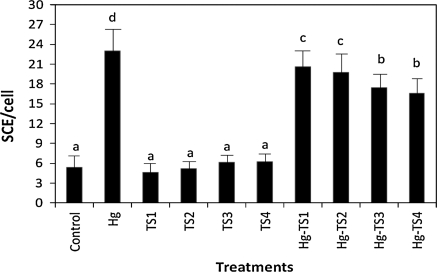
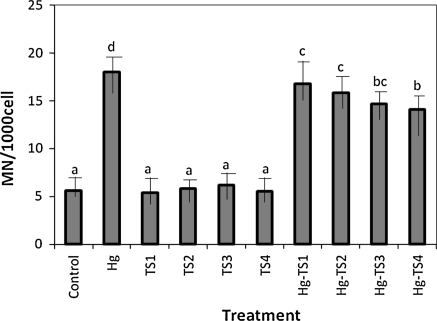
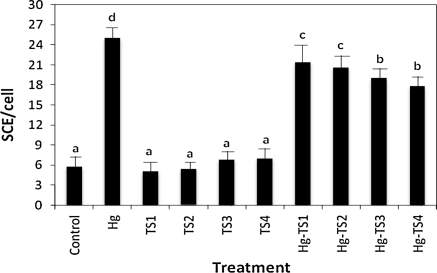
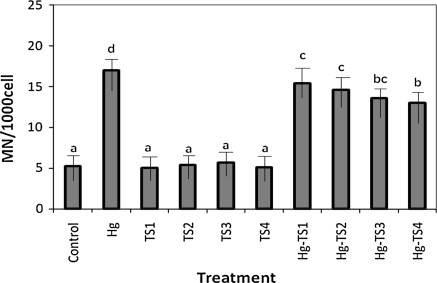
The effects of HgCl2 and aqueous, ethanol and methanol T. spicata extracts on the number of SCEs and MNs in human whole blood cultures are shown in Figs. 1, 2, 3, 4, 5 and 6 respectively. 9 mg/L HgCl2 caused significant increases of SCE and MN frequencies in human peripheral lymphocytes as compared with the controls. But, the plant extracts at four applied concentrations (25, 50, 100 and 200 mg/L) did not indicate statistically significant differences (p > 0.05) in the number of SCEs or MN rates. Moreover, the positive effect of T. spicata extracts was established on HgCl2-induced SCE and MN formations. The incidences for the SCE and MN values were decreased in comparison with HgCl2-treated group. In addition, the magnitude of the positive effect depended on the concentrations of T. spicata different extracts. Moreover, methanol extracts gave an higher effect than ethanol extract (shown in Figs 3, 4, 5 and 6).
Fig. 1.
Effect of T. spicata water extracts on mercury induced SCE formations in human peripheral lymphocytes. Values are expressed as mean for five cultures in each group; means in the figure followed by the different letters present significant differences at the p < 0.05 level; Hg: 9 mg/L of HgCl2; TS1: 25 mg/L T. spicata extract; TS2: 50 mg/L T. spicata extract; TS3: 100 mg/L T. spicata extract; TS4: 200 mg/L T. spicata extract
Fig. 2.
Effect of T. spicata water extracts on mercury induced MN formations in human peripheral lymphocytes. Abbreviations are as in Fig. 1
Fig. 3.
Effect of T. spicata ethanol extracts on mercury induced SCE formations in human peripheral lymphocytes. Abbreviations are as in Fig. 1
Fig. 4.
Effect of T. spicata ethanol extracts on mercury induced MN formations in human peripheral lymphocytes. Abbreviations are as in Fig. 1
Fig. 5.
Effect of T. spicata methanol extracts on mercury induced SCE formations in human peripheral lymphocytes. Abbreviations are as in Fig. 1
Fig. 6.
Effect of T. spicata methanol extracts on mercury induced MN formations in human peripheral lymphocytes. Abbreviations are as in Fig. 1
Discussion
Our results indicated that one of the targets of HgCl2 in human cells was DNA. Similar to our finding, a dose-related increase in DNA damage was observed in human cultured human peripheral blood lymphocytes exposed to HgCl2 for 72 h in SCE and MN assays (Turkez et al. 2011). Likewise, significant increases in the frequencies of chromosome aberrations (CAs) and SCEs were observed for HgCl2 in cultured human lymphocytes (Rao et al. 2001; Silva-Pereira et al. 2005; Halder et al. 2005). HgCl2 was found to be genotoxic in bacterial mutagenicity assays (Schurz et al. 2000). Again, Bonacker et al. (2004) reported that HgCl2 induced concentration-dependently MN formations in V79 cells. HgCl2 treatment was shown to produce CAs, MNs and sperm head anomaly in mice (Datta et al. 2004). HgCl2 genotoxicity in rats following oral exposure was also established by comet assay (Rozgaj et al. 2005). On the contrary, HgCl2 exhibited a lack of genotoxic activity in the wing spot assay of Drosophila melanogaster (Carmona et al. 2008).
It was reported that the genotoxicity of mercuric compounds including HgCl2 indicated by the CA and MN tests in peripheral blood lymphocytes could be partly due either to the disturbance of the spindle mechanism, or to the elevated level of 8-OH-dG (a product of oxidatively damaged DNA) brought by the generation of ROS. In addition, some authors have suggested that ROS may be implicated in the production of high basal SCE frequencies in chromosome instability syndromes (Lee et al. 1990; Therman and Susman 1993; Cinkilic et al. 2009). Similarly, oxidative stress could lead to MN formations (Tsangaris et al. 2010). Thus, the increases of SCE and MN rates after HgCl2 exposure could be explained by possible prooxidant effect of this compound.
The results of the current study also revealed that treatment with T. spicata extracts (water extract, methanol extract and ethanol extract) at different concentrations provided antigenotoxic effects against HgCl2. Also, there is considerable evidence that Thyme presents positive effects with increasing concentrations without leading to any genetic damage in human blood cells. Avci et al. (2006) have reported that T. spicata had antihypercholesterolaemic and antioxidant activities. Likewise Dorman et al. (2004) found antioxidant properties of aqueous T. spicata extracts (Dorman et al. 2004). Especially, proteins and nucleic acids in mammalian cells defend themselves with antioxidants (Kedziora et al. 2004). In this study, T. spicata could support the antioxidant defense mechanism against HgCl2. In fact, the previous studies revealed that many Lamiaceae family species such as Teucrium polium L., Thymbra spicata L., Ocimum basilicum L. and Foeniculum vulgar included pharmacologically and biologically active essential oils (Toroğlu et al. 2005). A recent finding indicated that the essential oils from T. spicata had antibacterial or antifungal activity on tested bacteria and fungi (Toroğlu et al. 2005). Thus, T. spicata essential oil was considered as a natural antimicrobial source (Unlü et al. 2009). The main components of this plant were determined as carvacrol, p-cymene, beta-myrcene, gamma-terpinene, alpha-terpinene and trans-caryophyllene (Kiliç 2006). Recent findings revealed that these components exhibited more or less antioxidant properties (El Babili et al. 2011; Asbaghian et al. 2011).
In conclusion, the findings of this research clearly indicated that T. spicata modulated HgCl2-induced genetic damage in human blood cultures due to its antioxidant and detoxifying nature. So, T. spicata can be a new resource of therapeutics against oxidative DNA damages as recognized in this study. Further investigation will be done for developing commercial formulation based on field trail and toxicological experiment. It is an important observation that all the biomolecules are polar in nature with a higher solubility in water, methanol, and ethanol.
References
- Abdalla FH, Belle LP, Bona KS, Bitencourt PE, Pigatto AS, Moretto MB. Allium sativum L. extract prevents methyl mercury-induced cytotoxicity in peripheral blood leukocytes (LS) Food Chem Toxicol. 2010;48:417–421. doi: 10.1016/j.fct.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Aboul-Soud MAM, Al-Othman AM, El-Desoky GE, Al-Othman ZA, Yusuf K, Ahmad J, Al-Khedhairy AA. Hepatoprotective effects of vitamin E/Selenium against Malathion—ınduced injuries on the antioxidant status and apoptosis-related gene expression in rats. J Toxicol Sci. 2011;36:285–296. doi: 10.2131/jts.36.285. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Goel SK, Behari JR. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J Appl Toxicol. 2010;30:457–468. doi: 10.1002/jat.1517. [DOI] [PubMed] [Google Scholar]
- Aleo MF, Morandini F, Bettoni F, Tanganelli S, Vezzola A, Giuliani R, Steimberg N, Boniotti J, Bertasi B, Losio N, Apostoli P, Mazzoleni G (2002) In vitro study of the nephrotoxic mechanism of mercuric chloride. Med Lav 93:267–278 [PubMed]
- Asbaghian S, Shafaghat A, Zarea K, Kasimov F, Salimi F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Nat Prod Commun. 2011;6:137–140. [PubMed] [Google Scholar]
- Avci G, Kupeli E, Eryavuz A, Yesilada E, Kucukkurt I. Antihypercholesterolaemic and antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2006;107:418–423. doi: 10.1016/j.jep.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Baytop T (1984) Therapy with medicinal plants in Turkey. Publication of Istanbul University, Istanbul, No. 2355, Faculty of pharmacy, No. 40
- Bonacker D, Stoiber T, Wang M, Böhm KJ, Prots I, Unger E, Their R, Bolt HM, Dege GH. Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol. 2004;78:575–583. doi: 10.1007/s00204-004-0578-8. [DOI] [PubMed] [Google Scholar]
- Carmona ER, Kossatz E, Creus A, Marcos R. Genotoxic evaluation of two mercury compounds in the Drosophila wing spot test. Chemosphere. 2008;70:1910–1914. doi: 10.1016/j.chemosphere.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Cinkilic N, Kiyici S, Celikler S, Vatan O, Gul OO, Tuncel E, Bilaloglu R. Evaluation of chromosome aberrations, sister chromatid exchange and micronuclei in patients with type-1 diabetes mellitus. Mutat Res. 2009;676:1–4. doi: 10.1016/j.mrgentox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Daneshvar-Royandezagh S, Khawar KM, Ozcan S. In vitro micropropagation of garden thyme (Thymbra spicata L. var. Spicata L.) collected from Southeastern Turkey using cotyledon node. Biotechnol Biotechnol Eq. 2009;23:1319–1321. [Google Scholar]
- Datta S, Biswas SJ, Khuda-Bukhsh AR. Comparative efficacy of pre-feeding, post- feeding and combined pre- and post-feeding of two microdoses of a potentized homeopathic drug, mercurius solubilis, in ameliorating genotoxic effects produced by mercuric chloride in mice. Evid Based Complem Altern Med. 2004;1:291–300. doi: 10.1093/ecam/neh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected lamiaceae species grown in Turkey. J Agric Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- El Babili F, Bouajila J, Souchard JP, Bertrand C, Bellvert F, Fouraste I, Moulis C, Valentin A. Oregano: chemical analysis and evaluation of ıts antimalarial, antioxidant, and cytotoxic activities. J Food Sci. 2011;76:512–518. doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis blocks micronucleus technique: a detailed description on the method and its application to genotoxicity studies in human population. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Clarkson TW. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Banno H, Shinkai Y, Yamamoto C, Kaji T, Satoh M. Protective effect of pretreatment with cilostazol on cytotoxicity of cadmium and arsenite in cultured vascular endothelial cells. J Toxicol Sci. 2011;36:155–161. doi: 10.2131/jts.36.155. [DOI] [PubMed] [Google Scholar]
- Glaser V, Nazari EM, Müller YM, Feksa L, Wannmacher CM, Rocha JB, Bem AF, Farina M, Latini A. Effects of inorganic selenium administration in methylmercury induced neurotoxicity in mouse cerebral cortex. Int J Dev Neurosci. 2010;28:631–637. doi: 10.1016/j.ijdevneu.2010.07.225. [DOI] [PubMed] [Google Scholar]
- Grotto D, Vicentini J, Angeli JP, Latorraca EF, Monteiro PA, Barcelos GR, Somacal S, Emanuelli T, Barbosa Jr F (2010) Evaluation of protective effects of fish oil against oxidative damage in rats exposed to methylmercury. Ecotoxicol Environ Saf. (doı:10.1016/j.ecoenv.2010.10.012) (in press) [DOI] [PubMed]
- Halder A, Patra M, De M. Evaluation of mercury toxicity by some cytological indices in leucocyte cultures. Indian J Exp Biol. 2005;43:737–739. [PubMed] [Google Scholar]
- Haleagrahara N, Jackie T, Chakravarthi S, Rao M, Kulur A. Protective effect of Etlingera elatior (torch ginger) extract on lead acetate–induced hepatotoxicity in rats. J Toxicol Sci. 2010;35:663–671. doi: 10.2131/jts.35.663. [DOI] [PubMed] [Google Scholar]
- Jan AT, Ali A, Haq Q (2011) Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J Postgrad Med (in press) [DOI] [PubMed]
- Kedziora K, Natowska K, Czuczejko J, Pawluk H, Kornatowski T, Motyl J, Szadujkis-Szadurski L, Szewczyk-Golec K, Kedziora J. The markers of oxidative stress and activity of the antioxidant system in the blood of elderly patients with essential arterial hypertension. Cell Mol Biol Lett. 2004;9:635–641. [PubMed] [Google Scholar]
- Kiliç T. Analysis of essential oil composition of Thymbra spicata var. spicata: antifungal, antibacterial and antimycobacterial activities. Z Naturforsch C. 2006;61:324–328. doi: 10.1515/znc-2006-5-604. [DOI] [PubMed] [Google Scholar]
- Lee KH, Abe S, Yanabe Y, Matsuda I, Yoshida MC. Superoxide-dismutase activity and chromosome damage in cultured chromosome instability syndrome cells. Mutat Res. 1990;244:251–256. doi: 10.1016/0165-7992(90)90137-9. [DOI] [PubMed] [Google Scholar]
- Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI. Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro. 2001;15:649–654. doi: 10.1016/S0887-2333(01)00081-9. [DOI] [PubMed] [Google Scholar]
- Rao MV, Purohit A, Patel T. Melatonin protection on mercury-exerted brain toxicity in the rat. Drug Chem Toxicol. 2010;33:209–216. doi: 10.3109/01480540903349258. [DOI] [PubMed] [Google Scholar]
- Rozgaj R, Kasuba V, Blanusa M. Mercury chloride genotoxicity in rats following oral exposure, evaluated by comet assay and micronucleus test. Arh Hig Rada Toksikol. 2005;56:9–15. [PubMed] [Google Scholar]
- Schurz F, Sabater-Vilar M, Fink-Gremmels J. Mutagenicity of mercury chloride and mechanisms of cellular defense: the role of metal-binding proteins. Mutagenesis. 2000;15:525–530. doi: 10.1093/mutage/15.6.525. [DOI] [PubMed] [Google Scholar]
- Shelef LA. Antimicrobial effects of spices. J Food Saf. 1983;6:29–44. doi: 10.1111/j.1745-4565.1984.tb00477.x. [DOI] [Google Scholar]
- Silva-Pereira LC, Cardoso PC, Leite DS, Bahia MO, Bastos WR, Smith MA, Burbano RR. Cytotoxicity and genotoxicity of low doses of mercury chloride an methylmercury chloride on human lymphocytes in vitro. Braz J Med Biol Res. 2005;38:901–907. doi: 10.1590/S0100-879X2005000600012. [DOI] [PubMed] [Google Scholar]
- Takaishi M, Sawada M, Shimada A, Suzuki JS, Satoh M, Nagase H. Protective role of metallothionein in benzo[a]pyrene-induced DNA damage. J Toxicol Sci. 2009;34:449–458. doi: 10.2131/jts.34.449. [DOI] [PubMed] [Google Scholar]
- Therman E, Susman M. Human chromosomes: structure, behaviour, and effects. J Med Genet. 1993;29:699–703. [Google Scholar]
- Toroğlu S, Dığrak M, Kocabaş YA. Çay veya Baharat Olarak Tüketilen Teucrium polium L., Thymbra spicata L. var. spicata, Ocimum basilicum L. ve Foeniculum vulgare Miller’in Uçucu Yağlarının In Vitro Antimikrobiyal Aktivitesi ve Bazı Antibiyotiklerle Etkileşimleri. KSÜ Fen ve Mühendislik Dergisi. 2005;8:36–42. [Google Scholar]
- Tsangaris C, Vergolyas M, Fountoulaki E, Nizheradze K (2010) Oxidative stress and genotoxicity biomarker responses in grey mullet (Mugil cephalus) from a polluted environment in Saronikos Gulf, Greece. Arch Environ Contam Toxicol. (doi:10.1007/s00244-010-9629-8) (in press) [DOI] [PubMed]
- Turkez H, Dirican E (2011) A modulator against mercury chloride-induced genotoxic damage: Dermatocarpon intestiniforme (L.). Toxicol Ind Health. (doı:10.1177/0748233711404036) (in press) [DOI] [PubMed]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b1 toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Aslan A, Karagöz Y, Turkez O, Anar M. Antimutagenic effects of lichen Pseudovernia furfuracea (L.) Zoph. extracts against the mutagenicity of aflatoxin B1 in vitro. Toxicol Ind Health. 2010;26:625–631. doi: 10.1177/0748233710377779. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I (2011) The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. (doi:10.1016/j.etp.2010.06.011) (in press) [DOI] [PubMed]
- Unlü M, Vardar-Unlü G, Vural N, Dönmez E, Ozbaş ZY. Chemical composition, antibacterial and antifungal activity of the essential oil of Thymbra spicata L. from Turkey. Nat Prod Res. 2009;23:572–579. doi: 10.1080/14786410802312316. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Koropatnick J. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther. 2000;295:74–82. [PubMed] [Google Scholar]