Abstract
Human mesenchymal stromal cell (hMSC) is a potential target for cell and gene therapy-based approaches against a variety of different diseases. Whilst cationic lipofection has been widely experimented, the Nucleofector technology is a relatively new non-viral transfection method designed for primary cells and hard-to-transfect cell lines. Herein, we compared the efficiency and viability of nucleofection with cationic lipofection, and used the more efficient transfection method, nucleofection, to deliver a construct of minimalistic, immunologically defined gene expression encoding the erythropoietin (MIDGE-EPO) into hMSC. MIDGE construct is relatively safer than the viral and plasmid expression systems as the detrimental eukaryotic and prokaryotic gene and sequences have been eliminated. Using a plasmid encoding the luciferase gene, we demonstrated a high transfection efficiency using the U-23 (21.79 ± 1.09%) and C-17 (5.62 ± 1.09%) pulsing program in nucleofection. The cell viabilities were (44.93 ± 10.10)% and (21.93 ± 5.72)%, respectively 24 h post-nucleofection. On the other hand, lipofection treatment only yielded less than 0.6% efficiencies despite showing higher viabilities. Nucleofection did not affect hMSC renewability, immunophenotype and differentiation potentials. Subsequently, we nucleofected MIDGE-EPO using the U-23 pulsing program into hMSC. The results showed that, despite a low nucleofection efficiency with this construct, the EPO protein was stably expressed in the nucleofected cells up to 55 days when determined by ELISA or immunocytochemical staining. In conclusion, nucleofection is an efficient non-viral transfection approach for hMSC, which when used in conjunction with a MIDGE construct, could result in extended and stable transgene expression in hMSC.
Keywords: Bone marrow mesenchymal stromal cells, Nucleofection, Cationic lipofection, MIDGE, Erythropoietin
Introduction
Mesenchymal stromal cell (MSC) is an attractive source of adult stem cells for therapeutic applications in the clinics (Parekkadan and Milwid 2010). Genetic modifications of MSC with therapeutic genes make them more effective for therapeutic use (Reiser et al. 2005). These cells have been manipulated for the delivery of various genes including cytotoxic T lymphocyte antigen-imunoglobulin (CTLA-Ig), factor VIII, interleukin-2 and tumor necrosis factor apoptosis ligand (TRAIL) to treat graft versus host disease (GvHD), hemophilia A, glioma and glioblastoma, respectively (Deng et al. 2003; Doering 2008; Nakamura et al. 2004; Sasportas et al. 2009; Van Damme et al. 2003).
The most commonly used systems to deliver DNA into primary cells are viral-based techniques. Adenovirus, retrovirus and lentivirus are probably the most effective gene transfer vehicles that can provide high transduction efficiency, integration into the host cell genome, and high levels of expression (Chan et al. 2006). However, viral approaches are complicated by immune responses, insertional mutagenesis and cell specificity of transgene delivery (Chen et al. 2008; Donahue et al. 1992; Thomas et al. 2003; Vorburger and Hunt 2002). To circumvent biological detriments, many chemical and physical techniques have also been used to increase the uptake efficiency and expression of naked DNA.
It has now been well established that coating DNA with DEAE-dextran allows DNA to penetrate the cell membrane. Calcium phosphate and liposomes have later been found to be able to import DNA into cells with better efficiency and lower toxicity (Bonetta 2005). Commonly used transfection method employing physical techniques such as microinjection, ‘gene gun’ transfer and electroporation, can be very tedious and require highly skilled workers, and the procedures often cause high cell toxicity (Heller et al. 2000; Lim et al. 2010; Zhang 2007).
Derived from liposomes, the cationic-liposomes contain a mixture of cationic and neutral lipids organized into lipid bilayer structures. Transfection complex formation is based on the interaction of the positively charged liposome with the negatively charged phosphate groups of the nucleic acid. The uptake of the liposome-DNA complexes may be mediated by endocytosis (Maruyama 2005; Ross and Hui 1999). The Nucleofector technology is a relatively new non-viral transfection method especially designed for primary cells and hard-to-transfect cell lines. Nucleofection combines electrical parameters and cell-type-specific solutions to drive plasmid DNA, oligonucleotides, or siRNA directly into the cell nucleus (Bruenker 2006; Lenz et al. 2003; Nakashima et al. 2005). To date, there are few reports on the comparison of the transfection efficiency and cell viability of MSC following transfection using nucleofection and lipofection.
The current study aimed to evaluate the efficiency and viability of cells following two non-viral gene delivery methods, i.e. nucleofection and cationic lipofection using Lipofectamine 2000 to deliver a transgene into adult human MSC (hMSC). Since nucleofection showed high efficiency, it was then used to deliver the erythropoietin (EPO) gene via the novel minimalistic, immunologically defined gene expression (MIDGE) construct. The protocol developed was also tested against interference with renewability, immunophenotype properties and differentiation potentials of the nucleofected MSC.
Materials and methods
Source of bone marrow MSC
Four samples of hMSC were isolated from the bone marrow aspirate of patients with non-malignant blood disorder in Universiti Kebangsaan Malaysia (UKM) Medical Center after informed consent and using a protocol approved by the UKM Research Committee and Ethics Committee. One human MSC sample was also purchased from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD, USA) and used in our study.
Isolation and culture of bone marrow MSC
Five mL of bone marrow aspirate was layered on top of 3 mL Ficoll-Paque (Amersham Biosciences; Uppsala, Sweden) and centrifuged at 400 g for 30 min. The mononuclear cells (MNC) in the interface (density gradient 1.077 g/L) were extracted and washed twice with culture medium by centrifugation at 200 g for 10 min. The pelleted cells were then suspended in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco-Invitrogen; Grand Island, NY, USA) and the viability of cells was assessed by using a haematocytometer after trypan blue staining. The cells were then seeded at a density of 1 × 107 cells in a 25 cm2 plastic flask containing DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin antibiotics (Gibco-Invitrogen). The flask was then incubated in 5% CO2 and monitored daily. Once the cells reached confluency, they were detached by 1 mL of 0.25% trypsin–EDTA (Gibco-Invitrogen) and replated again into new flasks at the similar cell density. Characterization of DMEM-derived adherent cells was performed by using cells from the third and fourth passages after 4–5 weeks from the initial culture (Choong et al. 2007; Mok et al. 2003; Wong et al. 2008).
Immunophenotyping of hMSC
To detect surface antigens, aliquots of DMEM culture-derived adherent cells were washed twice with phosphate-buffered saline (PBS) (Gibco-Invitrogen) pH 7.2 after detachment with 0.25% trypsin–EDTA. The cells were then diluted with PBS and incubated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated monoclonal antibodies for 20 min. The antibodies used were CD13, CD29, CD34, CD44, CD45, CD56, CD73, CD90, CD105, CD138, CD147 and CD166, all purchased from Becton–Dickinson (Ontario, Canada). After incubation, the cells were rediluted with PBS and subjected to flow cytometric analysis. Sample preparation for immunophenotyping was performed according to the procedure recommended by Becton–Dickinson. The CellQuest software and FacsCalibur (Becton–Dickinson) were used for flow cytometric analysis.
Plasmids used for delivering gene into hMSC
pRL-CMV plasmid encoding the Renilla luciferase gene (Promega; Madison, USA) was used to study the transfection efficiency and cell viability of nucleofection and cationic lipofection treatment. pmaxGFP plasmid encoding the green fluorescent protein (GFP) (Amaxa GmbH; Cologne, Germany) was used for the selection of transfected cells by limiting dilution and fluorescence microscopy, and for construction of MIDGE encoding the GFP (MIDGE-GFP).
Cell transfection using Lipofectamine 2000
Transfection with Lipofectamine 2000 was performed following the manufacturer’s guidelines (Gibco-Invitrogen). In brief, cells were plated in opaque 96-well microplates (Nunc A/S; Roskilde, Denmark) at a cell density of 10,000 cells per well and were allowed to grow overnight to achieve 80% confluency. Transfection complexes, consisting of 0.2 μg pRL-CMV plasmid DNA and different volumes of the Lipofectamine reagent, were added to the wells in serum-free medium to produce different ratios of plasmid DNA to Lipofectamine reagent (wt/vol). After 4 h, the media were discarded and to each well 100 μL of complete medium containing 10% FBS and 1% antibiotics in DMEM was added. Cells were analyzed 24 h after lipofection for transfection efficiency and viability. For each transfection complex ratio, a replicate of three wells was carried out.
Nucleofection
Nucleofection of the hMSC was done by using the Human MSC Nucleofector Kit and the Nucleofector (Amaxa GmbH). Prior to nucleofection, a petri-dish culture containing 1 mL of DMEM was incubated in the CO2 incubator at 37 °C. Early passages of MSC (P3 to P5) were trypsinized and neutralized with DMEM supplemented with 10% FBS. The cell number was then counted. An aliquot of 5 × 105 cells was transferred into an eppendorf tube and centrifuged at 200 g for 10 min. The pellet was then supplemented with 100 μL nucleofector solution and 2 μg pRL-CMV plasmid DNA. The mixture was resuspended slowly, transferred into a cuvette and inserted into the Nucleofector. For nucleofection, the program C-17 or U-23 was chosen. After nucleofection, the mixture in the cuvette was supplemented with 500 μL DMEM and transferred gently into a prepared petri dish. The culture was incubated in a CO2 incubator at 37 °C and monitored daily. For a no-DNA control, distilled water was used to replace the expression vector systems. A no-transfection control was also carried out by not pulsing the cells. Cells were analyzed 24 h post-nucleofection for transfection efficiency and viability.
Analysis of transfection efficiency and cell viability based on luminescence activity
Viviren Live Cell Substrates (Promega; Madison, USA) was suspended in dimethylsulfoxide (Amersham Pharmacia Biotech) to a concentration of 60 mM. To determine the lipofection efficiency, 1 μL suspended Viviren Live Cell Substrates was added directly to each well containing cells to achieve a concentration of 60 μM in the supernatant. Two minutes later, the luminescence was measured using a BioTek Synergy 2 SL Microplate Reader and Gen 5 software (BioTek Instruments Inc; Winooski, VT, USA). For cell viability, 100 μL constituted CellTiter Glo (Promega) was then added to each well of the microplate. The plate was shaken for 2 min on an orbital shaker and incubated in the dark for 10 min before measurement of luminescence activities.
For the analysis of nucleofection efficiency, the nucleofected cells were trypsinized with 300 μL 0.25% trypsin–EDTA and washed with DMEM by centrifuging the cells at 200 g for 10 min. The supernatant was discarded and the cell pellet was diluted to 10,000 cells per 100 μL complete medium. Aliquots of 100 μL of the diluted cells were seeded into a 96-well opaque microplate and transfection efficiency and cell viability were determined as described above for lipofection.
Renewability and phenotyping following nucleofection
Following nucleofection, a single cell was seeded into each well of a 96-well microplate by limiting dilution and under a fluorescence microscope. The single cells were then cultured and expanded before they were tested for positivity for immunocytochemical markers and for their capability to differentiate into adipocytes and osteoblasts.
The transfected cells were analyzed in situ for CD90 and CD105 (BD Biosciences Pharmingen; San Diego, CA, USA) surface markers. Cells were fixed in methanol at 4 °C for 30 min before subjected to immunocytochemical staining using the LSAB+ System-Horseradish Peroxidase (HRP) Kit. The staining was performed according to the manufacturer’s guidelines (Dako Cytomation; Glostrup, Denmark). For the detection of EPO protein in the transfected cells, an anti-EPO antibody (Santa Cruz Biotechnology, Inc; CA, USA) was used.
Induced differentiation into adipocytes
To induce adipogenesis, an adipogenic induction medium was prepared with DMEM supplemented with 1 μM dexamethasone, 0.2 mM indomethacin, 0.01 mg/mL insulin, 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma Aldrich; St. Louis, Missouri, USA), 10% FBS and 1% antibiotics. MSC was plated at a density of 4.0 × 104 cells per cm2 of plastic culture flasks and incubated in a humidified atmosphere at 37 °C with 5% CO2. After 80–90% confluency was achieved to provide cell–cell interaction, an adipogenic induction medium was added to the cells and the induction medium was changed every 3 days for 2–3 weeks. Oil Red O (Sigma Aldrich) was used as a histological stain to visualize the presence of lipid droplets.
Induced differentiation into osteoblasts
To induce osteogenesis, an osteogenic induction medium was prepared by supplementing DMEM with 10% FBS, 50 μg/mL ascorbate-2-phosphate, 10 mM β-glycerophosphate, 100 nM dexamethasone (Sigma Aldrich) and 1% antibiotics. MSC were plated at a density of 2.4 × 104 cells per cm2 of plastic culture flasks and incubated in the osteogenic induction medium in a humidified atmosphere at 37 °C with 5% CO2. The medium was changed every 3 days for 2–3 weeks. Alizarin Red S (Sigma Aldrich) was used to stain matrix mineralization associated with osteoblasts.
Nucleofection with MIDGE construct carrying the EPO or GFP gene
Construction of MIDGE-EPO and MIDGE-GFP
First-strand cDNA was synthesized using a fetal liver total RNA preparation (Cell Applications, Inc.; San Diego, CA, USA) as the template and oligo-(dT) as the primer. Reverse transcription was performed using the SuperScript III RNase H-Reverse Transcriptase (Invitrogen). The polymerase chain reaction (PCR) amplification was carried out using this first-strand cDNA and an upstream oligonucleotide containing a BamHI restriction site (underlined) (5′-GCGGATCCACCATGGGGGTGCACGAATGTCCTGCC-3′) and a downstream primer containing a SacI site (underlined) (5′-GCGAGCTCTCATCTGTCCCCTGTCCTGCAGGC-3′) targeted at the 5′- and 3′-ends of the erythropoietin (EPO) gene. PCR was performed for 30 cycles of amplification (94 °C, 15 s; 58 °C, 45 s; and 72 °C, 30 s) using a LA Taq PCR mixture (Takara Bio Inc; Otsu, Shiga, Japan) and this was followed by a final extension of PCR product at 72 °C for 10 min.
pMCV1.2 plasmid DNA was obtained from Mologen (Berlin, Germany) as a source plasmid for synthesis of the MIDGE vectors. The full-length EPO gene was cloned into the plasmid via the BamHI and SacI restriction sites. The pMCV-EPO construct was then cleaved with Esp3I (Fermentas; Vilnius, Lithuania) to produce a linear double-stranded DNA molecule consisting solely of the expression cassette. Both ends of the expression cassette were then ligated with an oligonucleotide hairpin structure to produce an end-protected MIDGE recombinant containing the EPO gene (MIDGE-EPO). The sequence of the hairpin used at the 5′- and 3′- ends of the expression cassette were GCGTCTTTTGACGCAGGG and AGGGCGCAGTTTTCTGCG (Sigma Proligo; Biopolis Way, Helios, Singapore), respectively. Meanwhile, MIDGE-GFP was constructed using pmaxGFP plasmid which was restricted with Esp3I and then ligated with the same oligonucleotide hairpins as used for the MIDGE-EPO construction. The two MIDGE constructs were then purified with GeneJet DNA purification kit (Fermentas; Vilnius, Lithuania).
Determination of EPO Expression by ELISA
All the supernatants from the nucleofected and non-nucleofected cells were collected on every 5 days and changed with DMEM supplemented 10% FBS and 1% antibiotics. The collected supernatant was centrifuged at 200 g for 10 min to exclude dead cells that might affect the EPO expression as determined by Human Erythropoietin ELISA Immunoassay Kit (Stem Cell Technologies; Vancouver, Canada). A duplicate of wells containing 50 μL of supernatant from each test sample was performed and the average values of light absorbance at 450 nm were calculated. A correction wavelength of 650 nm was used to correct for optical imperfections. The corrected optical density (OD) was then subtracted with the corrected OD obtained from a background control containing culture medium only. The values of OD were used to determine the EPO expression from a standard curve prepared according to the procedures recommended by the manufacturer of the kit.
Flow cytometric analysis of GFP reporter gene
Cells nucleofected with vector pmaxGFP plasmid or linearised MIDGE-GFP were trypsinized with 0.25% Trypsin–EDTA and washed with PBS pH 7.2 (Gibco-Invitrogen). Approximately 10,000 cells were aliquoted into a Falcon tube and 10 μL 7-amino actinomycin D (Becton-Dickinson) was added. The cells were incubated for 20 min before they were washed with PBS and subjected to flow cytometric analysis using CellQuest software and FacsCalibur (Becton–Dickinson).
Statistical analysis
Results are expressed as mean ± S.E.M. The results were analyzed with Student’s T, one-way ANOVA or Kruskal–Wallis test, as appropriate. Two-sided P values less than 0.05 were considered statistically significant.
Results
Isolation and identification of mesenchymal stromal cells from human bone marrow aspirates
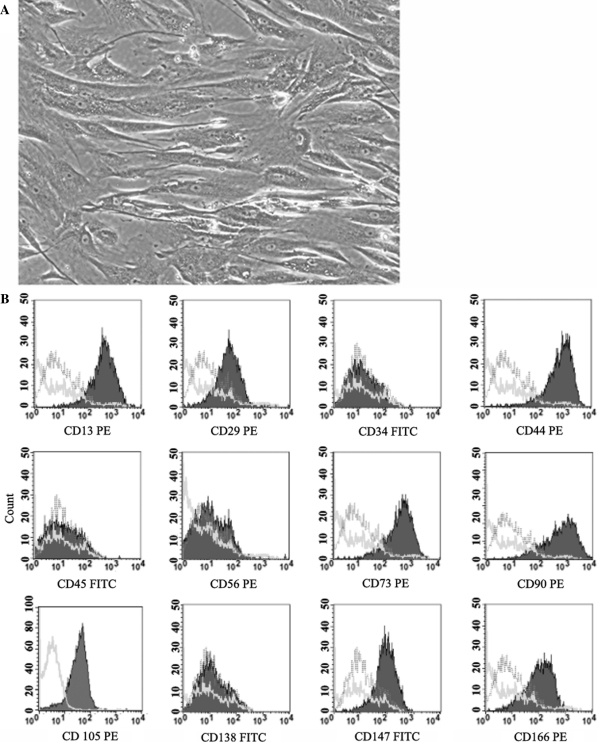
hMSCs were successfully expanded in culture from the bone marrow of all four donors. The MNCs extracted from the bone marrow samples showed a viability of more than 99%. When cultured in the plastic flask, the cells adhered to form fibroblast-like cells (Fig. 1A). These cells propagated rapidly and when cells at passage 3 were immunophenotyped, they showed abundant expression of CD13, CD29, CD44, CD73, CD90, CD105, CD147 and CD166. However, the cells did not show the expression of CD34, CD45, CD56 and CD138 indicating the absence of contaminating hematopoietic cells (Fig. 1B). When incubated in specific culture conditions, the fibroblast-like cells were capable of being induced to differentiate into adipocytes, chondrocytes and osteoblasts as assayed by Oil Red O, Alcian Blue-PAS and Alizarin Red S stain, respectively. Furthermore, transcripts of genes specific for adipogenesis, chondrogenesis and osteogenesis were also detected by RT-PCR (as were shown in our previous paper) (Mok et al. 2008a).
Fig. 1.
Culture of hMSC derived from bone marrow MNC. A The adherent cells were fibroblast-like and they showed a high proliferative rate in culture (50X). B Representative flow cytometric analysis of cultured hMSC. Filled purple histograms indicate the surface expression of the markers by cultured cells. For each antibody, isotype-matched mouse immunoglobulin G antibody is shown with a green line. The pink lines indicate unstained controls. (color figure online)
Nucleofection showed higher efficiency without cellular impairment and phenotypic changes
Different non-viral transfection methods have been developed for gene delivery into mammalian cells (Patil et al. 2005). An ideal gene delivery method needs to meet three major criterias: (1) it should protect the transgene against degradation by nucleases in intercellular matrices, (2) it should be able to mobilize the transgene across the plasma membrane and into the nucleus of target cells, and (3) it should have no detrimental effects (Gao et al. 2007). The transfection yield and toxicity of these methods vary for different cell types. Thus, we compared the efficiency and cell viability of hMSC after lipofection or nucleofection.
For this purpose, a pRL-CMV plasmid encoding the luciferase reporter gene was used for transfection in hMSC. The amount of expressed luciferase gene in the hMSC was determined by measuring the luminescence activity of viable cells following addition of Viviren Live Cell Substrates. Living cells are metabolically active and produce adenosine triphosphate (ATP). Addition of CellTiter Glo would result in cell lysis and generation of a luminescent signal proportional to the amount of ATP present. Thus, transfection efficiency was determined by normalizing the luminescence activity of the luciferase reporter gene with the luminescence activity exhibited by the viable cells.
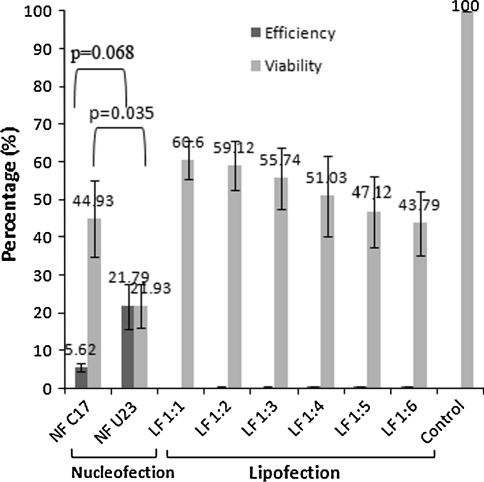
Our results showed that nucleofection with the pRL-CMV plasmid using U-23 and C-17 pulsing programs yielded viabilities of (21.93 ± 5.72)% and (44.93 ± 10.10)%, respectively, both P = 0.035, assayed 24 h after nucleofection. The nucleofection efficiencies of both programs were (21.79 ± 1.09)% and (5.62 ± 1.09)%, respectively, both P = 0.068. Transfection of 0.2 μg plasmid DNA complexed with Lipofectamine 2000 at a ratio of 1:1 (w/v) yielded a higher viability of (60.64 ± 5.18)% and efficiency of (0.19 ± 0.08)%. Increasing Lipofectamine 2000 to achieve a complex ratio of 1:6 reduced the cell viability to (43.79 ± 8.5)% and resulted only in an insignificant increase in efficiency to (0.56 ± 0.14)% (Fig. 2). When the amount of plasmid DNA was increased to 0.4 μg or 0.8 μg in lipofection, there was not much improvement in the efficiency (data not shown).
Fig. 2.
Transfection efficiency and cell viability following nucleofection (NF) and lipofection (LF) treatment. Two nucleofection pulsing programs, C-17 and U-23, were used to nucleofect 2 μg of pRL-CMV plasmid DNA into 5 × 105 cells. Efficiency and viability of nucleofection were compared with lipofection using different ratios of 0.2 μg of pRL-CMV plasmid to volumes of Lipofectamine 2000 (1:1 to 1:6) in 10,000 cells. The percentage of cell viability (grey bar) was determined by normalizing the luminescence activity of cells following transfection with plated cells (no transfection control) using CellTiter Glo. Efficiency (dark bar) was calculated by normalizing the luminescence activity of the luciferase reporter gene as measured by the Viviren Live Cell Substrates with luminescence activity exhibited by the viable cells and plated cell (no transfection control)
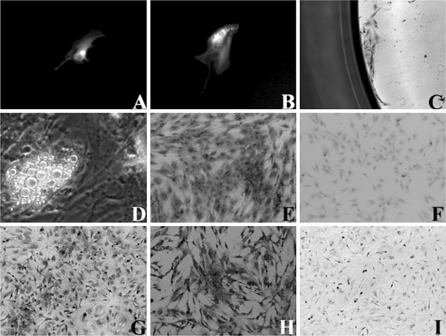
Since nucleofection using U-23 pulsing program yielded the higher nucleofection efficiency, we next determined the effect of this program on renewability, immunophenotype properties and differentiation potentials of the nucleofected cells. hMSC was nucleofected with pmaxGFP plasmid DNA and single cells were isolated by limiting dilution into a 96-well microplate. The single cells adhered and showed green fluorescence 24 h post-nucleofection (Fig. 3A). After 5 days, the single cells divided and the daughter cells also expressed the green fluorescence (Fig. 3B). A week later, the cells formed a small colony (Fig. 3C). The cells were expanded and tested for differentiation capabilities. The results showed that after 2 weeks of incubation in an adipogenic induction medium, the cells showed lipid accumulation indicating differentiation into adipocytes (Fig. 3D). The morphological observation by phase contrast microscopy showed that the lipid droplets increased in size and numbers during incubation with adipogenic induction medium and no appearance of apoptotic bodies. The cells were also stained positively with Alizarin Red S after 2–3 weeks of incubation in an osteogenic induction medium indicating differentiation into osteogenic lineage (Fig. 3E). The control for non-induced cells showed negative staining to Alizarin Red S (Fig. 3F). The expanded cells also showed positive staining to immunocytochemical markers of CD90 and CD105 (Fig. 3G, H). A negative control for immunocytochemical staining was shown in Fig. 3I.
Fig. 3.
Renewability, immunophenotype and differentiation of hMSC upon nucleofection. A hMSC nucleofected with plasmid encoding GFP was serially diluted to obtain a single cell. B A GFP-expressing cell divided after 5 days and C formation of a colony. D GFP-expressing cells showed lipid accumulation following adipogenic induction. E Expanded cells were stained positively with Alizarin Red S following osteogenic induction. F The control for non-induced cells showing negative staining with Alizarin Red S. The expanded cells also showed positive result to immunocytochemical markers of G CD105 and H CD90. I Negative control for immunocytochemical staining
MIDGE-EPO resulted in extended and stable EPO expression in hMSC despite low nucleofection efficiency
Our initial results prompted us to transfect hMSC using the Nucleofector technology to induce the expression of the EPO gene carried in a MIDGE construct. MIDGE consists solely of the expression cassette, capped with hairpin structures at the ends for protection against exonuclease degradation. The expression cassette includes CMV immediate-early enhancer promoter region providing strong constitutive expression. The construct of MIDGE has been described previously (Mok et al. 2008b).
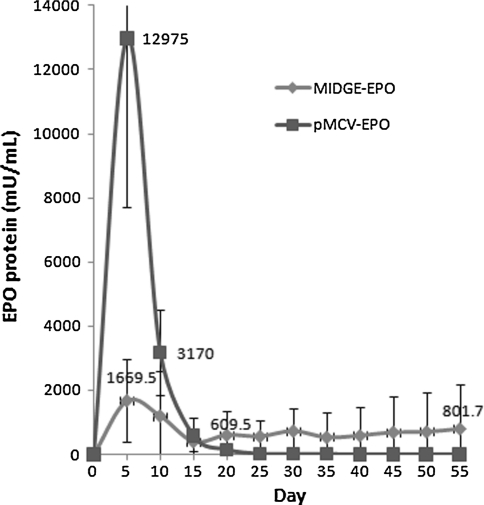
The MIDGE-EPO was next nucleofected into hMSC using U-23 pulsing program. The EPO protein level in the supernatant collected from cells nucleofected with MIDGE-EPO was determined by ELISA and compared with that of cells nucleofected with the corresponding pMCV-EPO plasmid DNA. It was observed that the EPO expression reached the highest level of (12.98 ± 5.26) U/mL in the first 5 days following nucleofection with the plasmid and (1.70 ± 1.30) U/mL with the MIDGE construct. The protein level in the supernatant on day 10, however, dropped sharply to (3.17 ± 1.34) U/mL and (1.20 ± 1.41) U/mL in both cells transfected with the plasmid and the MIDGE construct, respectively. The EPO level expressed by the plasmid-nucleofected cells continued to drop on day 15 and 35 to a level of (581.5 ± 577.6) mU/mL and (13.5 ± 13.5) mU/mL, respectively. However, in the supernatant of cells transfected with MIDGE-EPO, EPO protein was found to be consistently expressed. The EPO protein level was maintained at (0.41 ± 0.32) U/mL to (0.80 ± 1.37) U/mL from day 15 to day 50. There was no significant difference in the mean level of EPO expression in the MIDGE-nucleofected cells on different days of measurement (Fig. 4).
Fig. 4.
Nucleofection of MIDGE-EPO resulted in stable EPO expression in hMSC. Analysis of EPO level in the supernatant of nucleofected cells was determined by ELISA. Blue line represents the EPO expression by the MIDGE-EPO-nucleofected cells (N = 5), red line represents EPO expression by the pMCV-EPO-nucleofected cells (N = 2). (color figure online)
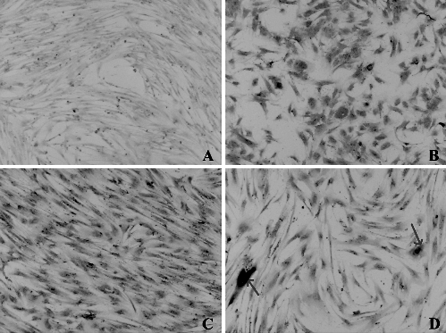
The cells nucleofected with MIDGE-EPO were also immunocytochemically stained for EPO protein. The staining of hMSC without nucleofection is shown in Fig. 5A. EPO staining on cells nucleofected with MIDGE-EPO following 24 h of nucleofection is shown in Fig. 5B. Most of the cells were stained intensely for the EPO protein. The nucleofected cells were found to be positively stained (as indicated by arrow) even after 3 months of nucleofection (Fig. 5C, D). The results obtained were consistent with the result of protein expression determined by ELISA.
Fig. 5.
Immunocytochemistry staining for EPO protein on hMSC nucleofected with MIDGE-EPO. Cells nucleofected with MIDGE-EPO were stained for EPO protein using monoclonal antibody anti-human EPO with a Dako LSAB kit. A Staining on cells without nucleofection. B Cells were positively stained 24 h post-nucleofection and C 3 months later. D Some cells showed very strong positive staining for EPO protein (arrows). Magnification ×40
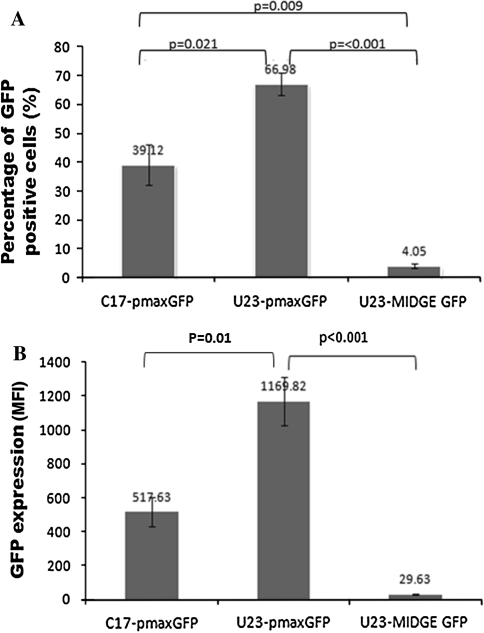
To confirm that the plasmid had a better transfection efficiency than the MIDGE construct in hMSC, we nucleofected both constructs encoding the GFP reporter gene and analyzed the expression by flow cytometry 24 h post-nucleofection. The MIDGE construct resulted in a very low yield of GFP-positive hMSC (4.05 ± 0.82)% when nucleofected with U-23 pulsing program. Nucleofection using U-23 or C-17 program to deliver the corresponding GFP-encoded plasmid showed yields of (66.98 ± 3.84)% and (39.12 ± 6.99)%, respectively. There was a significant difference (P < 0.001) in the percentage of cells expressing GFP in MIDGE compared with plasmid nucleofection using U-23 program (Fig. 6A). The mean GFP expression in viable nucleofected cells is shown in Fig. 6B. There was a significant difference (P < 0.001) in GFP expression in MIDGE-nucleofected cells compared with plasmid-nucleofected cells using the U-23 pulsing program (29.63 ± 2.04 MFI vs. 1,169.82 ± 140.92 MFI).
Fig. 6.
Nucleofection efficiency of plasmid and MIDGE into hMSC using different pulsing programs (C-17 or U-23). A Both GFP-encoding plasmid and MIDGE were nucleofected into hMSC. The bars show the percentage of viable cells expressing GFP as determined by flow cytometry. B Fluorescence intensities (MFI—Median Fluorescence Intensity) exhibited by the nucleofected cells. Each bar represents data from six independent experiments
Discussion
Mesenchymal stromal cells are useful in delivering therapeutic protein to specific tissues as they can migrate to tumor or injury sites (Khakoo et al. 2006; Kidd et al. 2010; Pittenger and Martin 2004; Tomita et al. 2002; Yen and Yen 2008). This offers an alternative treatment in lieu of recombinant protein therapy, as it reduces the cost of treatment, development of auto-antibody, and risks related to high concentration of recombinant proteins in the blood stream (Bennett et al. 2004). To date, most of the methods used to deliver genes into hMSC have used the viral approaches. Non-viral methods using chemicals, for instance, Lipofectamine 2000 (cationic lipofection) has reported the highest efficiency compared with Superfect, Polyfect, Effecten (all Qiagen) and Fugene HD (Roche; Mannheim, Germany) (Gheisari et al. 2008; Helledie et al. 2008).
Consistent with previous reports, we showed a far more effective way of transferring a gene into hMSC using the Nucleofector technology rather than cationic lipofection. While others showed the percentage of cells exhibiting green fluorescent protein reporter gene (Aluigi et al. 2006; Zarogasi et al. 2007), we measured the mean intensity of luciferase reporter gene in viable cells using the pRL-CMV plasmid DNA. Our results showed that the mean expression in viable hMSC was four times higher (21.79%) when the U-23 pulsing program was used compared with C-17 (5.62%). On the other hand, lipofection treatment only yielded less than 0.6% efficiencies. We also demonstrated that positively nucleofected cells retained renewability in being able to proliferate to form single colonies; expanded cells also exhibited capability to differentiate into adipocytes and osteocytes, and showed the expression of CD90 and CD105 surface markers. Taken together, these experiments showed that Nucleofector technology is an efficient method for gene delivery into bone marrow-derived hMSC and does not impair the renewability, immunophenotype and differentiation potentials of the nucleofected hMSC.
We also investigated the ability of the nucleofected hMSC to secrete a functional, transgene-encoded protein by nucleofecting hMSC with the human EPO gene via a linearized MIDGE construct using U-23 pulsing program. MIDGE constructs have shown promising results for in vitro transfection of human colon adenocarcinoma cell lines (Schakowski et al. 2001) and in vivo enhancement of immunization and vaccination (Leutenegger et al. 2000; López-Fuertes et al. 2002; Moreno et al. 2004). However, MIDGE transfection into stem cells has never been explored. Here, we reported on the nucleofection of MIDGE into hMSC and confirmed that, despite the fact that MIDGE exhibited lower nucleofection efficiency into hMSC compared with corresponding plasmid, MIDGE-nucleofected hMSC manifested extended and stable EPO gene expression in vitro up to day 55. On the other hand, plasmid-nucleofected hMSC showed transient expression up to day 25 only.
Immunohistochemistry staining on the MIDGE-nucleofected cells showed that some cells were strongly stained for EPO protein after 3 months of nucleofection, and these cells could have contributed to the extended and stable expression of EPO as determined by ELISA. The possibility of MIDGE driven integration of genes into the genome of hMSC could not be ruled out in this study as currently there is lack of data on the intracellular distribution of MIDGE in vitro. Thus, future study should isolate these positively stained cells either by using a cell sorter, laser microdissector or other means to investigate the site of integration in the MSC genome by a sensitive detection technique. Factors that could theoretically affect integration include the construct sequence (by virtue of recombination signals), mode and route of administration into cells, use of adjuvants, and the nature of the transgene protein product. Integration of a plasmid encoding the EPO gene has been reported previously in Balb/c mice following electroporation in quadriceps muscle by repeat-anchored integration capture PCR (RAIC PCR) assay (Wang et al. 2004). Additionally, future study should also increase the initial amount of MIDGE-EPO to improve the delivery of MIDGE-EPO by nucleofection into MSC and the gene expression.
Stable expression of hMSC nucleofected with MIDGE-EPO has significant benefit for correction of anemia in patients suffering from chronic kidney disease (Brines and Cerami 2008). Our previous study has shown successful induction of erythropoiesis when hematopoietic stem cells were incubated with the supernatants from nucleofected cells (Mok et al. 2008b). In neurodegeneration, hMSC could serve as a vehicle to deliver EPO protein into injured tissues as recombinant EPO could not pass through the blood–brain barrier. While high concentrations of recombinant EPO could be administered intravenously to enable some protein to reach the injured sites, there are also risks of thrombosis and hypertension to be considered (Baskin and Lasker 1990; Ghezzi and Brines 2004; Lieutaud et al. 2008; Loo and Beguin 1999). Local delivery of EPO by MSC has manifested a more potent therapeutic effect for treatment of myocardium infarct (Copland et al. 2008) and cerebral ischemia (Esneault et al. 2008) than with delivery of MSC or EPO alone in in vivo studies. EPO could trigger the phosphorylation of Janus associated kinase 2 (JAK2) and thus enhance the survivability of MSC in hypoxic tissue (Mangi et al. 2003; Rossert and Eckardt 2005).
In conclusion, our results demonstrated that Nucleofector technology is an efficient method to induce high levels of transgene expression in bone marrow-derived mesenchymal stromal cells without impairing the immunophenotype, renewability and differentiation potentials. Nucleofection is an efficient non-viral transfection technique for human MSC using plasmid compared to MIDGE construct. However, MIDGE nucleofection could result in a longer term of EPO transgene expression in MSC.
Acknowledgments
We acknowledge the generous support from Malaysia Toray Science Foundation (MTSF), Scientex Foundation and Majlis Kanser Nasional (MAKNA). We thank the staff and fellow researchers from the Cell Banking Unit, Genetic Cancer Laboratory, Hemostasis Laboratory, Tissue Engineering Laboratory, Hematology Laboratory and Histopathology Laboratory of Universiti Kebangsaan Malaysia Medical Center for their technical guidance and assistance. We thank Professor Choo Kong Bung of Universiti Tunku Abdul Rahman for comments on the manuscript.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
P. L. Mok, Email: rachelmok2005@gmail.com
S. K. Cheong, Email: cheongsk@utar.edu.my
C. F. Leong, Email: cfleong@gmail.com
K. H. Chua, Email: ckienhui@hotmail.com
O. Ainoon, Email: ainoon@usim.edu.my
References
- Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, Colombo MP, Versura P, D’Errico-Grigioni A, Ferri E, Baccarani M, Lemoli RM. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:454–461. doi: 10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- Baskin S, Lasker N. Erythropoietin-associated hypertension. New Engl J Med. 1990;323:999. doi: 10.1056/NEJM199010043231418. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, Mc Williams N, McKoy JM, Kim B, Lyons EA, Trifilio SM, Rasich DW, Evens AM, Kuzel TM, Schumock GT, Belknap SM, Locatelli F, Rossert J, Casadevall N. Pure red-cell aplasia and epoetin therapy. New Engl J Med. 2004;351:1403–1408. doi: 10.1056/NEJMoa040528. [DOI] [PubMed] [Google Scholar]
- Bonetta L. The inside scoop-evaluating gene delivery methods. Nat Methods. 2005;2:875–883. doi: 10.1038/nmeth1105-875. [DOI] [Google Scholar]
- Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Bruenker HG. Efficiency transfection of primary cells for basic research and gene therapy. Mol Ther. 2006;13:215. doi: 10.1016/j.ymthe.2006.08.630. [DOI] [Google Scholar]
- Chan J, O’Donoghue K, Fuente JDL, Roberts IA, Kumar S, Morgan JE, Fisk NM. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2006;23:93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- Chen HH, Cawood R, Seymour LW. Toward more effective gene delivery. Genome Biol. 2008;9:301. doi: 10.1186/gb-2008-9-1-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong PF, Mok PL, Cheong SK, Leong CF, Then KY. Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytother. 2007;9:170–183. doi: 10.1080/14653240701196829. [DOI] [PubMed] [Google Scholar]
- Copland IB, Jolicoeur EM, Gilis M, Cuerquis J, Eliopoulos N, Annabi B, Calderone A, Tanguay J, Ducharme A, Galipeau J. Coupling erythropoietin secretion to mesenchymal stromal cells enhances their regenerative properties. Cardiovasc Res. 2008;79:405–415. doi: 10.1093/cvr/cvn090. [DOI] [PubMed] [Google Scholar]
- Deng Y, Guo X, Yuan Q, Li S. Efficiency of adenoviral vector mediated CTLA4Ig gene delivery into mesenchymal stem cells. Chin Med J. 2003;116:1649–1654. [PubMed] [Google Scholar]
- Doering CB. Retroviral modification of mesenchymal stem cells for gene therapy of hemophilia A. Methods Mol Biol. 2008;433:203–212. doi: 10.1007/978-1-59745-237-3_12. [DOI] [PubMed] [Google Scholar]
- Donahue RE, Kessler SW, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo KM, Nienhuis AW. Helper virus induced T-cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esneault E, Pacary E, Eddi D, Freret T, Tixier E, Toutain J, Touzani O, Schumann-Bard P, Petit E, Roussel S, Bernaudin M. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cerebr Blood Flow Metab. 2008;28:1552–1563. doi: 10.1038/jcbfm.2008.40. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheisari Y, Soleimani M, Azadmanesh K, Zeinali S. Multipotent mesenchymal stromal cells: optimization and comparison of five cationic polymer-based gene delivery methods. Cytother. 2008;10:815–823. doi: 10.1080/14653240802474307. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11:S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Helledie T, Nurcombe V, Cool SM. A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem Cells Dev. 2008;17:837–848. doi: 10.1089/scd.2007.0209. [DOI] [PubMed] [Google Scholar]
- Heller L, Jaroszeski MJ, Coppola D, Pottinger C, Gilbert R, Heller R. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo. Gene Ther. 2000;7:826–829. doi: 10.1038/sj.gt.3301173. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M, Marini FC. Mesenchymal stromal cells alone or expessing interferon-β suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytother. 2010;12:615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- Lenz P, Bacot SM, Frazier-Jessen MR, Feldman GM. Nucleofection of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett. 2003;538:149–154. doi: 10.1016/S0014-5793(03)00169-8. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Boretti FS, Mislin CN, Flynn JN, Schroff M, Habel A, Junghans C, Koenig-Merediz SA, Sigrist B, Aubert A, Pedersen NC, Wittig B, Lutz H. Immunization of cats against feline immunodeficiency virus (FIV) infection by using minimalistic immunogenic defined gene expression vector vaccines expressing FIV gp140 alone or with feline Interleukin-12 (IL-12), IL-16, or a CpG motif. J Virol. 2000;74:10447–10457. doi: 10.1128/JVI.74.22.10447-10457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieutaud T, Andrews PJ, Rhodes JK, Williamson R. Characterization of the pharmacokinetics of human recombinant erythropoietin in blood and brain when administered immediately after lateral fluid percussion brain injury and its pharmacodynamic effects on IL-1 beta and MIP-2 in rats. J Neurotrauma. 2008;25:1179–1185. doi: 10.1089/neu.2008.0591. [DOI] [PubMed] [Google Scholar]
- Lim JY, Park SH, Jeong CH, Oh JH, Kim SM, Ryu CH, Park SA, Ahn JG, Oh W, Jeun SS, Chang JW. Microporation is a valuable transfection method for efficient gene delivery into human umbilical cord blood-derived mesenchymal stem cells. BMC Biotechnol. 2010;10:38. doi: 10.1186/1472-6750-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo M, Beguin Y. The effect of recombinant human erythropoietin on platelet counts is strongly modulated by the adequacy of iron supply. Blood. 1999;93:3286–3293. [PubMed] [Google Scholar]
- López-Fuertes L, Pérez-Jiménez E, Vila-Coro AJ, Sack F, Moreno S, Konig SA, Junghans C, Wittig B, Timón M, Esteban M. DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine. 2002;21:247–257. doi: 10.1016/S0264-410X(02)00450-4. [DOI] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infracted hearts. Nature. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Liposomes. In: Taira K, Kataoka K, Niidome T (eds) Non-viral gene therapy, Springer-Verlag, Tokyo, pp:19–34; 2005. [Google Scholar]
- Mok PL, Leong CF, Cheong SK. Isolation and identification of mesenchymal stem cells from human bone marrow. Malays J Pathol. 2003;25:121–127. [PubMed] [Google Scholar]
- Mok PL, Cheong SK, Leong CF. In vitro differentiation study on isolated human mesenchymal stem cells. Malays J Pathol. 2008;30:11–19. [PubMed] [Google Scholar]
- Mok PL, Cheong SK, Leong CF, Othman A. In vitro expression of erythropoietin by transfected human mesenchymal stromal cells. Cytother. 2008;10:116–124. doi: 10.1080/14653240701816996. [DOI] [PubMed] [Google Scholar]
- Moreno S, López-Fuertes L, Vila-Coro AJ, Sack F, Smith CA, Konig SA, Wittig B, Schroff M, Juhls C, Junghans C, Timón M. DNA immunization with minimalistic expression constructs. Vaccine. 2004;22:1709–1716. doi: 10.1016/j.vaccine.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumour effect of genetically engineered mesenchymal stromal cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Matsuyama Y, Nitta A, Sakai Y, Ishiguro N. Highly efficient transfection of human marrow stromal cells by nucleofection. Transplant Proc. 2005;37:2290–2292. doi: 10.1016/j.transproceed.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, Russa VFL. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5:1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PC, Hui SW. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999;6:651–659. doi: 10.1038/sj.gt.3300863. [DOI] [PubMed] [Google Scholar]
- Rossert J, Eckardt KU. Erythropoietin receptors: their role beyond erythropoiesis. Nephrol Dial Transplant. 2005;20:1025–1028. doi: 10.1093/ndt/gfh800. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, Water JAJM, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakowski F, Gorschlüter M, Junghans C, Schroff M, Buttgereit P, Ziske C, Schöttker B, König-Merediz SA, Sauerbruch T, Wittig B, Schmidt-Wolf IGH. A novel minimal-size vector (MIDGE) improves transgene expression in colon carcinoma cells and avoids transfections of undesired DNA. Mol Ther. 2001;3:793–800. doi: 10.1006/mthe.2001.0322. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H, Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20:279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- Damme A, Chuah KL, Dell’accio F, Bari D, Luyten F, Collen D, VandenDriessche T. Bone marrow mesenchymal cells for haemophilia A gene therapy using retroviral vectors with modified long-terminal repeats. Haemophilia. 2003;9:94–103. doi: 10.1046/j.1365-2516.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- Wang Z, Troilo PJ, Wang X, Griffiths II TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, Follmer TT, Rizzuto G, Ciliberto G, Fattori E, Monica NL, Manam S, Ledwith BJ. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Wong CY, Cheong SK, Mok PL, Leong CF. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52–57. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- Yen BL, Yen ML. Mesenchymal stem cells and cancer—for better or for worse? J Cancer Mol. 2008;4:5–9. [Google Scholar]
- Zarogasi LE, Billon N, Ailhaud G, Dani C. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells. 2007;25:790–797. doi: 10.1634/stemcells.2006-0235. [DOI] [PubMed] [Google Scholar]
- Zhang Y (2007) Microinjection technique and protocol to single cells. http://www.nature.com/protocolexchange/protocols/376. Accessed 27 May 2011