Abstract
Tuberculosis (TB) is associated with excessive production and bio-activation of transforming growth factor bets (TGF-β) in situ. Here, modification of expression of components of plasminogen/plasmin pathway in human monocytes (MN) by inhibitors of TGF-β signaling were examined. Smad3 siRNA effectively inhibited TGF-β induced urokinase plasminogen activator receptor (uPAR). Agents known to interfere with TGF-β signaling, including the Smad inhibitors SIS3 and Erythromycin derivatives, and ALK5 receptor inhibitor (SB 431542) in inhibition of uPAR expression in response to MTB were examined. Inhbibition by SIS 3 only sinhibited uPAR mRNA significantly. SIS3 may prove to be an effective adjunct to TB therapy.
Introduction
A prominent role for TGF-β in macrophage deactivation and suppression of T-cell responses to M. tuberculosis (MTB) is well-established (Reviewed in [1]). Excessive TGF-β activity is a feature of active pulmonary TB [2], and human mononuclear phagocytes that are infected or exposed to MTB or its components in vitro. Importantly, lung lavage from patients with active TB contain bioactive TGF-β [3], implicating that conditions for TGF-β signaling are present in situ. In a murine model of M. bovis pulmonary infection, administration of latency-associated peptide of TGF-β, modified TGF-β bioactivity in situ, and both decreased BCG growth in the lung and enhanced antigen-specific T-cell responses [4]. In vitro, MTB stimulation of human mononuclear phagocytes also leads to production of bioactive TGF-β [5]. Collectively these data implicate that both production of TGF-β itself and the molecular context necessary for its bio-activation are present at sites of MTB infection. Recently several inhibitors of TGF-β bio-activity have been developed. Whether TGF-β signaling can be aborted by any of these agents during MTB infection is currently unknown. Inhibitors of TGF-β signaling, however, may have a role as adjuncts to anti-tuberculosis therapy.
Binding of bioactive TGF-β to homodimeric type II TGF-β receptor, leads to recruitment and activation of homodimeric type 1 receptor (also known as activin-like receptor kinases [ALK]. This then leads to phosphorlation of Smads 2, and 3, which in turn form heterodimers with Smad 4, and then the complex translocates into the nucleus, ultimately leading to TGF-β bioactivity [6]. Of TGF-β signaling inhibitors developed to date, inhibitors of Alk5 actually have already been used in vivo models of conjunctival and intestinal fibrosis. However, the Alk5 kinase inhibitor (SB 431542) most studied to date has activity against other Alks (-2 to-7) [7]. On the other hand, the Smad 3 inhibitor (SIS 3) is very specific in that it even excludes inhibition of Smad 2 phosphorylation [8]. The macrolide, Erythromycin (EMA) and its derivative EM703 (that lacks any antibacterial activity) have also been shown to interfere with TGF-β induced Smad2/3 activation in bleomycin-induced pulmonary fibrosis in mice. In a human lung fibroblast cell line, inhibition of TGF-β signaling by EMA and EM703 was mediated by increased expression of Smad 7 (the cytoplasmic inhibitor of Smad 2/3) [9]. Studies to evaluate inhibitors of TGF-β signaling in human primary mononuclear phagocytes are currently limited, however, critical to start to apply any of these inhibitors to human diseases associated with TGF-β excess, such as TB.
Recently we found that an inhibitor of plasmin (bdelin), reduced MTB-induced bioactive TGF-β production in MN [5], implicating involvement of the plasmin/plasminogen pathway. Urokinase plasminogen activator receptor (uPAR), a molecule critical to activation of uPA which leads to conversion of plaminogen to plasmin [10], was increased in MTB activated MN. Further, neutralization of uPAR suppressed bioactive TGF-β in MTB activated MN [5]. TGF-β itself controls uPAR at both mRNA and protein levels [11]. Thus it appears that bioactivation of TGF-β though plasmin/plasminogen pathway is under TGF-β control.
Here, we investigated if inhibition of TGF-β signaling by siRNA, and receptor or post-receptor molecular inhbitors are useful in inhibition of its downstream effect in human primary mononuclear phagocytes. This study was focused on human blood MN because, the capacity to produce [12] and bio-activate [5] TGF-β by immature blood monocytes (MN) exceeds that of autologous terminally differentiated alveolar macrophages. This is important, as up to 30% of mononuclear phagocytes in bronchoalveolar lavage cells from patients with pulmonary TB are immature [13], likely comprised of newly recruited blood MN.
Methods
Reagents
TGF-β receptor [ALK-5] inhibitor (SB- 431542) (Torcris Bioscience, Bristol, UK), and Smad 3 inhibitor (SIS 3) (Calbiochem, EMD Chemicals, Lajolla, CA) were purchased. Erythromycin, Clarythromycin, and EM703 were gifts from Dr Omura (Kitasato Institute, Tokyo, Japan). MTB H37Rv lysate (L), a French Press preparation of irradiated late log-phase organisms was provided by Colorado State University (NIH contract NOI-AI-75320). MTB purified protein derivative (PPD) (Serum Institute, Copenhagen, Denmark), and Qiagen RNA extraction buffer (Qiagen, Hilden, Germany) were purchased.
Isolation of PBMC and Negative selection of CD14 MN
PBMC from healthy subjects were prepared by Ficoll Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density gradient [2]. Viability was > 98% as assessed by trypan blue exclusion. PBMC contain 8–12% MN (CD14 reactive) by immunostaining and FACS analysis. Blood MN were separated from PBMC by negative isolation (Miltenyi, City, State). Cells obtained were 80% CD14 reactive. In some experiments MN were obtained by adherence to plastic. MN thus obtained are 75–90% CD14 reactive.
Inhbition of TGF-β signaling by siRNA
First, we assessed the efficacy of transfection of primary human MN by nucleofection. For this purpose, 3 ×106 MN were combined with 1 μg of pmaxGFP in 100 μl of nucleofection solution and then MN nucleofection was performed per protocol [Amaxa Inc (http://www.amaxa.com)]. Negative controls included MN in solution that underwent sham nucleofection. Direct microscopy showed that up to 15% of MN were highly flourecent, however by FACS analysis up to 80% of MN were successfully nucleofected with pmaxGFP. To inhibit TGF-β signaling, Smad-3 siRNA (100nM) was added to 0.5×106MN culture.
In control experiments of gene silencing studies, an un-related RNA construct was used was used as control. Smad3 and control siRNA were purchased (Dharmacon, Lafayette, CO). To quantify mRNA expression, real-time RT-PCR (Taqman) using ABI7700 thermocycler was used. Primers and probe for uPAR were as before [5]s, whereas those for uPA, PAI were purchased (ABI Biosystems, Foster City, CA). Quantities of mRNA were determined by using a dilution series of target cDNA in each assay, and expression of target mRNA copies were corrected to the copy numbers of R18 in the same sample.
Statistical analysis
Comparisons of multiple measures assessed using cells from the same groups of subjects were evaluated with paired t-tests. P-values of <0.05 were considered significant.
Results
Inhibition of TGF-β signaling by siRNA for SMAD3 in MN
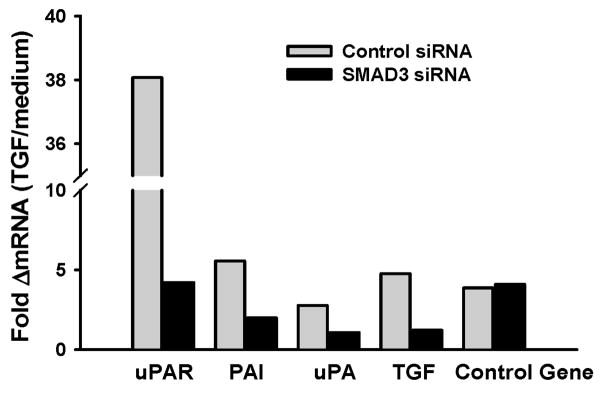
To investigate the role of TGF-β signaling in primary human MN, we used siRNA to Smad-3 and assessed for genes in the plasmin/plasminogen pathway [uPAR, plasminogen activator inhibitor (PAI), and urokinase plasminogen activator (uPA )] of TGF-β bioactivation. TGF-β mRNA was also assessed. All these genes are induced by TGF-β signaling through Smad3, however, to differing degrees and therefore are likely differently affected by inhibition of TGF-β signaling. A control gene, TNF-α, known not to be under TGF-β control was assessed as control. MN were transfected with si RNA for Smad 3 or a control si RNA construct. Four hrs later, recombinant (r) TGF-β (10 ng/ml) was added to wells. Cultures were harvested 24 hr later and total RNA harvested and assessed for uPAR, PAI, uPA, TGF-β, and TNF-α mRNA. Figure 1 shows a representative (of 4) experiments. In four experiments, whereas uPAR expression was induced about 4–30 fold by TGF-β, that of PA1 and uPA mRNA were induced very little (1.5–2 fold). Smad 3 siRNA inhbited all TGF-β induced genes including TGF-β itself. Inhibition of uPAR mRNA was most noticeable. In the control experiment, TNF-α, was neither induced by TGF-β nor inhibited by Smad 3 siRNA.
Figure 1. Effect of Smad 3 gene silencing on expression of TGF-β induced mRNA in MN.
MN were transfected by siRNA for Smad3 (black) or control construct (gray). MN cultures received recombinant (r) TGF-β (10 ng/ml) after 4 hrs. Expression of uPAR, uPA, PA-1 and TGF-β mRNA were assessed in cell lysates after 24 hr. Expression of TNF a, not known to be under Smad3 pathway of TGF-β signaling, was also assessed as control gene (last panel). Inhibition of Smad3 reduced mRNA for all genes under this classical pathway of cell signaling. Induction of uPAR and its inhibition by Smad3 siRNA was most dramatic.
The effect of TGF signaling inhibition on uPAR expression
The effect of known inhibitors of TGF-β signaling, Smad 3 inhibitor (SIS3), ALK-5 inhibitor (SB-431542), and macrolides (Erythromycin, Clarythromycin, and EM703) on TGF-β signaling and induction of uPAR was assessed next. MN were cultured in Accel medium at 1.5×105/well in presence and absence of inhibitors of TGF-β signaling. MTB H37RvL (10 μg/ml) or PPD (10 μg/ml) were then added and cells harvested 24 hr later in Qiagen RNA buffer. Total RNA was isolated and assessed for uPAR mRNA.
In initial dose response experiments (n=4), we did not find any effect of erythromycin or its derivatives (tested at 50–300 μM) on inhibition of uPAR mRNA, whereas both SIS3 and SB-431542 were effective at 1–10 μM (data not shown).
Figure 2 shows the results of 12 experiments of induction of uPAR mRNA by MTB H37RvL (10 μM) (Fig 2A) or PPD (10 μM) (Fig 2B) and inhibition of TGF signaling by SIS3 (1 and 5 μM) and SB-431542 (1 and 5 μM). Results shown are mean ± SEM experiments. Induction of uPAR mRNA by PPD was lower in every experiment as compared to MTB H37RvL (p < 0.001) (comparison of first panel from Fig. 2A and B). Whereas SIS3 at both doses effectively inhibited uPAR mRNA induced by MTB H37Rv L (p<0.01 and 0.05 respectively), inhibition of induction of uPAR mRNA by either dose of SB-431542 was more variable and only significant at 5 μM of the inhibitor (p<0.01). The inhibitory effect of both SIS3 and SB-431542 on PPD-induced uPAR expression was also very variable and only significant at 5μM of SB-431542 (p<0.05).
Figure 2. Effect of Smad 3 inhibitor (SIS 3) and ALK5 inhibitor (SB 431542) on MTB induced uPAR expression in MN.
MN were prepared and received SIS3 (1 and 5μM) or SB431542 (1 and 5 μM) for 1 hr. Then wells received MTB H37Rvlysate (L) (A) or PPD (B) at 10 μg/ml. After 24 hrs cell lysates were prepared, RNA extracted and assessed for uPAR mRNA. Fold inuction of uPAR (that induced by MTB H37RvL or PPD over that induced in medium alone) is shown. Means ± SEM of 12 experiments is shown.
Discussion
At sites of TB, a major determinant of TGF-β activity is the molecular context that allows its bioactivation and signaling. Studies to date implicate that 10–20% of TGF-β in situ is in it’s bioactive state [3]. Further, uPAR mRNA levels were significantly elevated in TB involved as compared to TB uninvolved lung lavage from patients with smear negative pulmonary TB (Unpublished data). Collectively, these data are supportive of use of TGF-β signaling inhibitors as adjuncts to anti-tuberculosis therapy. A spectrum of activity of the inhibitors of bioactive TGF-β was found here; whereas the potency of SIS3 was notable, the better studied SB-431542 was less active. None of the macrolides used were effective in inhibition of TGF-β signaling in induction of uPAR mRNA in human MN. This is disappointing because of lack of toxicity of Erythromycin and Clarythromycin which are already in clinical use.
Recently, blockade of TGF-β signaling by an orally available type I receptor (Alk5/4) inhibitor augmented efficacy of immunogen therapy in a murine model of prostate cancer [14]. In the current work, ALK5 inhibitor SB431542 did not effectively inhibit induction of uPAR expression in human mononuclear phagocytes. This may be because of the inefficiency of this inhibitor in human macrophages. The more recently developed small molecular inhibitor of ALK 5, SB-505124, which has been shown to be significantly more potent and less cytotoxic [15], may prove to be useful in inhibition of MTB-induced uPAR and thereby TGF-β signaling in primary MN.
While here SIS3 was potent in inhibition of MTB induced uPAR mRNA, and thereby TGF-β signaling in human MN, review of the current literature fails to reveal SIS3 application to animal models of human diseases. As a result no efficacy or safety information is available regarding this more specific modality of TGF-β signaling inhibition. Here, SIS3 at either dose was very effective in inhibition of MTB H37RvL induced, but not PPD-induced uPAR mRNA. The molecular nature of MTB H37Rv L is clearly more complex than PPD, but the finding that it induced uPAR significantly more than PPD suggests an effect of lipids and/or lipoproteins of MTB in induction of TGF-β. Both MTB ManLAM [12] and 19kda induce TGF-β and presumably its signaling, however, other predominant MTB lipid components and ultimately the organism itself have to be tested in this respect. However, to establish any usefulness of SIS3 in MTB infection, the mouse models of aerosolized virulent MTB infection need to be employed. One caveat in use of any Smad inhibitor of TGF-β signaling is the more recent identification and characterization of non-Smad signaling pathways in TGF-β bioactivity.
Acknowledgments
This work was supported by funding from NHLBI (HL-51636), NIAID (AI-45244/AI-95383, Tuberculosis Research Unit) and NIAID (AI-36219, Center for AIDS Research), and a Merit Review grant from Department of Veterans Affairs.
Footnotes
None of the authors have any commercial or other association that may pose a conflict of interest.
References
- 1.Toossi Z, Ellner JJ. The role of TGF beta in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol. 1998 May;87:107–14. doi: 10.1006/clin.1998.4528. [DOI] [PubMed] [Google Scholar]
- 2.Aung H, Toossi Z, McKenna SM, et al. Expression of transforming growth factor-beta but not tumor necrosis factor-alpha, interferon-gamma, and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber Lung Dis. 2000;80:61–7. doi: 10.1054/tuld.2000.0235. [DOI] [PubMed] [Google Scholar]
- 3.Bonecini-Almeida MG, Ho JL, Boechat N, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect Immun. 2004 May;72:2628–34. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson KA, Martin TD, Reba SM, et al. Latency-associated peptide of transforming growth factor beta enhances mycobacteriocidal immunity in the lung during mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2000;68:6505–8. doi: 10.1128/iai.68.11.6505-6508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aung H, Wu M, Johnson JL, Hirsch CS, Toossi Z. Bioactivation of latent transforming growth factor beta1 by Mycobacterium tuberculosis in human mononuclear phagocytes. Scand J Immunol. 2005 Jun;61:558–65. doi: 10.1111/j.1365-3083.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AB. TGF-beta signaling from receptors to the nucleus. Microbes Infect. 1999;1:1265–73. doi: 10.1016/s1286-4579(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 7.Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002 Jul;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 8.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006 Feb;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Azuma A, Li Y, et al. EM703, a new derivative of erythromycin, inhibits transforming growth factor-beta signaling in human lung fibroblasts. Exp Lung Res. 2008 Aug;34:343–54. doi: 10.1080/01902140802093238. [DOI] [PubMed] [Google Scholar]
- 10.Khalil N. TGF-beta: from latent to active. Microbes Infect. 1999;1:1255–63. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 11.Lund LR, Ellis V, Ronne E, Pyke C, Dano K. Transcriptional and post-transcriptional regulation of the receptor for urokinase-type plasminogen activator by cytokines and tumour promoters in the human lung carcinoma cell line A549. Biochem J. 1995 Aug 15;310( Pt 1):345–52. doi: 10.1042/bj3100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch CS, Yoneda T, Averill L, Ellner JJ, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994;170:1229–37. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 13.Schwander SK, Torres M, Sada E, et al. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J Infect Dis. 1998;178:1434–45. doi: 10.1086/314454. [DOI] [PubMed] [Google Scholar]
- 14.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008 May 1;68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 15.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004 Mar;65:744–52. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]