Abstract
In the mammalian central nervous system, the majority of fast excitatory synaptic transmission is mediated by glutamate acting on AMPA-type ionotropic glutamate receptors. The abundance of AMPA receptors at the synapse can be modulated through receptor trafficking, which dynamically regulates many fundamental brain functions, including learning and memory. Reversible posttranslational modifications, including phosphorylation, palmitoylation and ubiquitination of AMPA receptor subunits are important regulatory mechanisms for controlling synaptic AMPA receptor expression and function. In this review, we highlight recent advances in the study of AMPA receptor posttranslational modifications and discuss how these modifications regulate AMPA receptor trafficking and function at synapses.
Introduction
The amino acid glutamate is the predominant excitatory neurotransmitter in the brain. After release from presynaptic nerve terminals, glutamate binds to postsynaptic ionotropic glutamate receptors to mediate excitatory synaptic transmission. These ionotropic glutamate receptors are tetrameric cation channels consisting of three distinct subtypes: AMPA, NMDA and kainate receptors [1]. Among them, AMPA receptors conduct the majority of fast, moment-to-moment synaptic transmission and are the primary driving force for postsynaptic depolarization. The abundance of AMPA receptors at synapses is a crucial factor in determining synaptic efficacy. Convincing evidence has demonstrated that AMPA receptors undergo activity-dependent recruitment to or removal from synapses during synaptic plasticity, including long-term potentiation (LTP), long-term depression (LTD) or homeostatic plasticity, to ensure proper synaptic communication [2-6]. In addition, channel biophysical properties of AMPA receptors can be modulated to influence synaptic transmission[1,7]. It has been demonstrated that both trafficking and functional properties of the AMPA receptor depend on subunit composition, subunit-specific protein-protein interactions and posttranslational modifications[1,3-6].
AMPA receptors are assembled from four subunits, GluA1 - A4 (previously termed GluR1-4 or GluR-A to -D) to form homomeric or heteromeric channels [1,8,9]. In the adult hippocampus, the majority of AMPA receptors are GluA1/A2 heteromers with a relatively minor fraction consisting of GluA2/A3 heteromers [10,11]. Each subunit is an integral membrane protein with an extracellular amino terminal domain, three membrane-spanning domains (M1, 3 and 4), a hydrophobic hairpin domain (M2), which forms the channel pore, and three intracellular domains, Loop1, Loop2 and carboxyl-tail (C-tail) (Figure 1). C-tails of the AMPA receptor subunits have long been shown to play critical roles in receptor trafficking and function [3-6]. Recent studies have also implicated other intracellular domains in the regulation of AMPA receptor-mediated synaptic transmission. Both the Loop1 and Loop2 domains regulate AMPA receptor trafficking [12,13]. The pore lining domain also functions in the trafficking of the AMPA receptor early in the secretory pathway [14,15]. The function of these domains in receptor trafficking and channel properties is subject to tight regulation through reversible posttranslational modifications, which influence exocytosis, endocytosis, degradation and channel gating of the AMPA receptor.
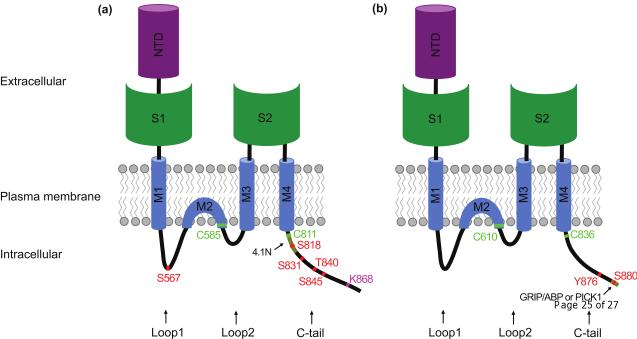
Figure 1.
Topology and posttranslational modification sites of AMPA receptor subunits
Each AMPA receptor subunit is an integral membrane protein with an extracellular amino terminal domain (NTD), three transmembrane domains (M1, 3 and 4), a hydrophobic hairpin domain (M2), which underlies the formation of the channel pore, and three intracellular domains, Loop1, Loop2 and the carboxyl-tail (C-tail). The S1 domain in the N-terminus and the S2 domain that links M3 and M4 underlie the formation of glutamate binding domain. (a) Schematic drawings of the GluA1 subunit. The residues that are palmitoylated (in green), phosphorylated (in red) and ubiquitinated (in purple) are highlighted. (b) Schematic drawings of the GluA2 subunit. The residues that are palmitoylated (in green) and phosphorylated (in red) are highlighted. Sub-domains that mediate the interaction with 4.1N protein (a) or GRIP/ABP and PICK1 (b) are also indicated (in yellow).
Here we will review the latest developments of AMPA receptor phosphorylation, palmitoylation and ubiquitination, in which there has been significant progress over the past few years. We will discuss the modulation of channel biophysical properties by AMPA receptor phosphorylation and examine the role of phosphorylation of individual AMPA receptor subunits in receptor trafficking and synaptic plasticity. In addition, we will review recent studies on AMPA receptor palmitoylation and ubiquitination. Finally, we will highlight how different posttranslational modifications interact with each other to modulate AMPA receptors. Other AMPA receptor posttranslational modifications, including glycosylation and proteolysis, which have previously been shown to be involved in receptor assembly, trafficking and degradation, have recently been reviewed [1], and thus are not included in the current review.
Phosphorylation
Phosphorylation is a ubiquitous mechanism critical for the rapid regulation of trafficking and function of ion channels. Indeed, AMPA receptor phosphorylation is an important regulatory mechanism that controls many aspects of receptor function [16,17]. Several protein kinases including both the serine/threonine kinases, PKA, PKC and CaMKII, and tyrosine kinases, such as the Src family tyrosine kinases, phosphorylate AMPA receptor subunits and have been implicated in the modulation of receptor biophysical properties and activity-dependent receptor trafficking [16,17]. While all four subunits of the AMPA receptor are phosphorylated and their roles in receptor trafficking and function have been previously reviewed [16-18], recent studies on AMPA receptor phosphorylation have focused primarily on GluA1 and GluA2, the two most abundant subunits in hippocampal neurons [10,11]. Therefore, we will restrict our focus in the current review to the latest progress on GluA1 and GluA2 phosphorylation.
GluA1
Previous studies have shown that phosphorylation of the GluA1 C-tail directly modulates the receptor’s biophysical properties. For example, phosphorylation at S831, a PKC and CaMKII site [19-22], leads to increased single channel conductance, and phosphorylation at S845, a PKA site [20], enhances mean open probability of homomeric GluA1 receptors [23,24]. It was subsequently shown that heteromerization of GluA1 with GluA2 abolished the S831 phosphorylation-mediated increase in single channel conductance [25]; however, this effect can be restored by coexpression of GluA1/A2 heteromers with transmembrane AMPA receptor regulatory proteins (TARPs) [26], which are auxiliary subunits of neuronal AMPA receptors [7,27]. This suggests that the effect of S831 phosphorylation on AMPA receptor channel conductance depends on stoichiometry of the AMPA receptor complex, providing an additional layer of regulation of AMPA receptor function by phosphorylation. Mechanistically, phosphorylation at S831 increases the likelihood of AMPA receptor opening at larger conductance states, probably through promoting simultaneous activation of multiple subunits during gating [26]. As the majority of neuronal AMPA receptors contain TARPs [7], an increase in conductance of the AMPA receptor-TARP complex phosphorylated at GluA1 S831 may contribute to LTP-associated enhancement of AMPA receptor conductance at synapses [24,28-31].
In addition to modulating AMPA receptor channel biophysics, phosphorylation of GluA1 functions in synaptic plasticity through the regulation of receptor trafficking. The role of S831 phosphorylation in AMPA receptor trafficking remains unclear. It has been shown that GluA1 lacking phosphorylation on S831 can be delivered to synapses in a CaMKII-dependent manner [32]. In addition, hippocampal LTP is intact in knockin mice lacking phosphorylation at S831 [33], suggesting that S831 phosphorylation is unlikely to play a significant role in the regulation of activity-dependent AMPA receptor insertion into synapses. These data also indicate that enhanced channel conductance of GluA1-containing AMPA receptors phosphorylated on S831, as discussed above, unlikely represents a major mechanism underlying LTP expression. Similarly, knockin mice lacking phosphorylation at S845 also express normal LTP [33]. This is interesting, considering that LTP is impaired in double knockin mice lacking phosphorylation at both S831 and S845 sites [34]. It is plausible that while neither S831 nor S845 is absolutely required for LTP, these two sites can substitute for each other, and thus either site is sufficient to mediate LTP. Phosphorylation on S831 and S845 may function in LTP through lowering the threshold for AMPA receptor trafficking to synapses [35,36], likely through enhancing AMPA receptor delivery to extrasynaptic membranes (Figure 2a) [37]. Indeed, it has been shown that the probability to successfully induce LTP is enhanced in GluA1 S831D and S845D phosphomimetic double knock-in mice [36].
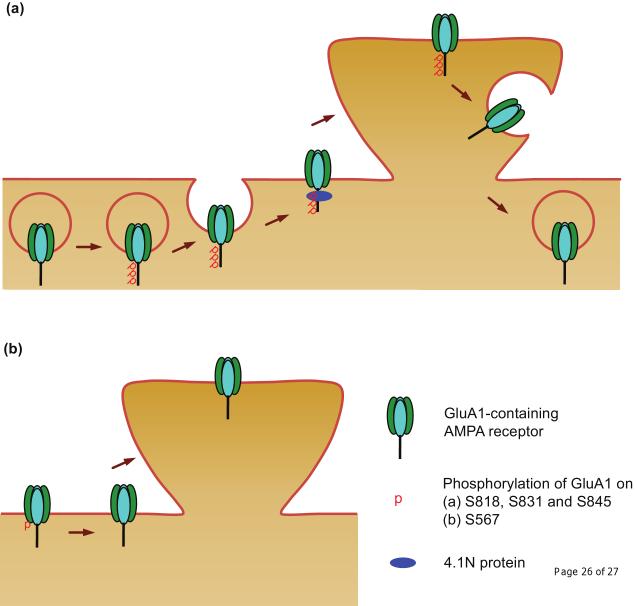
Figure 2.
Phosphorylation of GluA1 regulates AMPA receptor trafficking.
(a) Phosphorylation on S818, S831 and S845 of the GluA1 C-tail enhances delivery of the receptor to the neuronal surface. Among these phosphorylation events, phosphorylation at S818 facilitates the GluA1 interaction with 4.1N protein, which stabilizes the receptor on the surface. Conversely, dephosphorylation, especially at S845, appears to be important for receptor endocytosis. (b) Phosphorylation on S567 in the GluA1 Loop1 domain negatively regulates AMPA receptor trafficking to synapses and thus dephosphorylation at this site is a prerequisite for synaptic insertion of the receptor. The arrows (brown) represent specific steps of AMPA receptor trafficking.
In contrast to LTP, LTD is critically dependent on S845, but not on S831 phosphorylation, as knockin mice lacking phosphorylation at S845, but not at S831, have deficits in hippocampal LTD [33]. It has previously been shown that LTD is associated with a persistent dephosphorylation at S845 (Figure 2a) [38-40], which regulates AMPA receptor endocytosis and degradation [34,41,42]. Thus it is likely that S845 phosphorylation is involved in LTD through the regulation of AMPA receptor endocytosis. GluA1 S845 phosphorylation also contributes to stabilization of GluA2-lacking AMPA receptors at perisynaptic membranes, although the role of such regulation by GluA1 S845 phosphorylation in hippocampal synaptic plasticity remains unclear [43].
More recently, two additional phosphorylation sites in the GluA1 C-tail, S818 and T840, have been identified [44-46]. Phosphorylation of S818 by PKC promotes GluA1 synaptic insertion and preventing S818 phosphorylation diminishes LTP [45]. Interestingly, S818 phosphorylation may work in concert with phosphorylation at S831 and S845 to trigger stable incorporation of GluA1 into synapses, which underlies LTP expression (Figure 2a) [45], consistent with a synergistic role for the multiple phosphorylation sites in the GluA1 C-tail in controlling AMPA receptor trafficking. In contrast, although T840 is phosphorylated by PKC and p70S6 kinase in vitro, phosphorylation at this site does not appear to play a significant role in LTP [44,46]. Instead, phosphorylation on T840 may function in LTD [44,46], suggesting distinct functional consequences for phosphorylation of the GluA1 C-tail sites in different forms of synaptic plasticity.
In addition to the multiple phosphorylation sites on the GluA1 C-tail, recently the GluA1 Loop1 domain was shown to be phosphorylated on S567. Biochemical studies demonstrated that CaMKII phosphorylates S567, although it is a relatively weak substrate compared to S831 in the C-tail [12], raising the possibility that this residue may be a target for additional regulation by other kinases. Interestingly, although GluA2 Loop1 contains a homologous residue T574, it is not as efficiently phosphorylated by CaMKII [12], indicating that other sequence determinants in GluA1 Loop1 likely play a role in the preferential phosphorylation by CaMKII. GluA1 Loop1 itself facilitates AMPA receptor targeting to synapses, and phosphorylation at S567 negatively modulates Loop1-dependent synaptic delivery of AMPA receptors (Figure 2b) [12]. Such a dual mechanism mediated by GluA1 Loop1 and S567 phosphorylation controls synaptic targeting of GluA1-containing receptors [12]. Any role for S567 phosphorylation in synaptic plasticity remains to be determined.
It largely remains unclear how phosphorylation events on GluA1 engage AMPA receptor trafficking machinery to regulate receptor targeting and synaptic plasticity. One possibility is that phosphorylation may modulate GluA1-mediated protein-protein interactions that function in AMPA receptor trafficking. Indeed, one elegant study recently showed that PKC phosphorylation at GluA1 S818 enhances the GluA1 interaction with a neuronal specific actin-binding protein 4.1N, which leads to increased exocytosis of AMPA receptors at extrasynaptic membranes and underlies synaptic delivery of AMPA receptors during LTP induction (Figure 2a) [47].
The regulation of AMPA receptor trafficking and synaptic plasticity by GluA1 phosphorylation has important implications for animal cognition. For example, in double knockin mice lacking phosphorylation at the S831 and S845 sites, both the retention of newly acquired spatial memory and norepinephrine-mediated enhancement of contextual learning are impaired [34,35]. In addition, a recent study from Huganir’s group uncovers an imperative role for GluA1 S845 phosphorylation in fear memory erasure [48], demonstrating how altered AMPA receptor trafficking induced by GluA1 phosphorylation modulates animal behavior. Taken together, these studies reveal the behavioral significance of GluA1 phosphorylation and highlight the importance of GluA1 phosphorylation-mediated modulation of AMPA receptor trafficking in brain function.
GluA2
The vast majority of AMPA receptors in the forebrain contain GluA2 [10,11,14], which controls calcium permeability of AMPA receptors and confers characteristic biophysical properties (reviewed in [49]). There are two serine phosphorylation sites in the GluA2 C-tail (S863 and S880) [50-52]. Of these, S880 is a well-characterized site that has been suggested to play an important role in receptor trafficking. S880 is located within the GluA2 C tail PDZ ligand (-S880VKI) that binds to two different PDZ proteins, GRIP/ABP (glutamate receptor interacting protein/AMPA receptor binding protein) and PICK1 (protein interacting with C-kinase 1) [53-56]. Phosphorylation at S880, mediated by PKC, differentially regulates the GluA2 interaction with GRIP/ABP and PICK1 [50,51]. While phosphorylation of GluA2 at S880 disrupts the interaction with GRIP/ABP, its binding to PICK1 remains intact [50,51]. As GRIP/ABP has been suggested to anchor AMPA receptors at both synaptic and intracellular compartments and PICK1 has been shown to promote receptor trafficking, such differential regulation of GluA2 interactions with GRIP/ABP or PICK1 by S880 phosphorylation provides a dynamic modulation of AMPA receptor trafficking and synaptic plasticity (reviewed in [3-5,49]).
In addition to S880, recent studies implicate a role for GluA2 tyrosine phosphorylation in AMPA receptor trafficking and plasticity. An early study demonstrated that GluA2 is tyrosine phosphorylated at tyrosine 876 (Y876) both in vitro and in vivo by the Src family tyrosine kinases [57]. Interestingly, like S880, Y876 phosphorylation causes a reduction of GluA2 binding to GRIP but does not affect receptor interactions with PICK1 [57]. Importantly, interfering with GluA2 tyrosine phosphorylation impairs AMPA-, NMDA- and insulin-induced endocytosis of the GluA2 subunit and LTD, suggesting a critical role for the regulation of GluA2 tyrosine phosphorylation in activity-dependent AMPA receptor trafficking [57-62]. GluA2 tyrosine phosphorylation is also involved in Homer1a-dependent homeostatic scaling [63]. A recent study shows that GluA2 tyrosine phosphorylation at Y876 controls BRAG2 activity, which is a guanine-nucleotide exchange factor (GEF) for the coat-recruitment GTPase Arf6, to initiate the internalization of AMPA receptors during LTD induction [64]. In this scenario, upon binding to GluA2, BRAG2 is activated by the tyrosine-rich motif in the GluA2 C-tail, triggering Arf6 activation, which in turn contributes directly to the formation of clathrin-coated vesicles for receptor endocytosis [64]. Tyrosine phosphorylation on Y876 in the GluA2 C-tail negatively regulates BRAG2 activity, thus preventing the receptor from endocytosis [64].
Palmitoylation
Protein S-palmitoylation is a posttranslational modification whereby palmitic acid is covalently attached to intracellular cysteine residues of the protein substrates by palmitoyl acyltransferase (PAT), primarily mediated by a large DHHC (Asp-His-His-Cys) protein family [65,66]. Palmitoylation is a labile and reversible process with de-palmitoylation regulated by palmitoyl thioesterase (PTE) [65,66]. Such bidirectional lipid modifications play important roles in localization, trafficking and function of proteins and are widely involved in the regulation of neuronal development and synaptic transmission. Dysfunction of protein palmitoylation has been implicated in a number of neurological and psychiatric disorders, including Huntington’s disease, mental retardation and schizophrenia [66].
All four AMPA receptor subunits are palmitoylated at two conserved sites, one within the pore domain (C585 in GluA1), and the other one in the C-tail juxtamembrane region (C811 in GluA1) [15]. Palmitoylation of AMPA receptor subunits in the M2 hairpin structure, mediated by the palmitoyl acyltransferase DHHC3, occurs in the early secretory pathway within the endoplasmic reticulum (ER), where it regulates the stability of AMPA receptors and protects the receptors from degradation (Figure 3) [15,67]. Once the receptors are transported to the Golgi apparatus, palmitoylation acts to suppress AMPA receptor surface trafficking by accumulating receptors in the Golgi apparatus (Figure 3) [15]. Thus, depalmitoylation of AMPA receptors in the M2 hairpin structure in the Golgi apparatus acts as a triggering signal for receptor forward trafficking. On the other hand, palmitoylation at the GluA1 C-tail C811 inhibits the GluA1-4.1N protein interaction, an interaction playing an important role in stabilizing AMPA receptors on the surface, thus enhances stimulation-induced endocytosis of AMPA receptors (Figure 3) [15,47].
Figure 3.
Palmitoylation of the AMPA receptor plays versatile roles in receptor trafficking.
Palmitoylation at C585 in the GluA1 M2 hairpin structure takes place at the endoplasmic reticulum (ER) and functions in stabilizing the receptor in the ER. Once the receptor is trafficked to the Golgi apparatus, depalmitoylation at C585 is required for forward trafficking of the receptor. Palmitoylation of GluA1 at C811 in the C-tail inhibits nearby PKC phosphorylation on S818. Phosphorylation of S818 enhances the GluA1 interaction with 4.1N protein, thus stabilizing the receptor on the surface. Therefore, palmitoylation of C811 negatively regulates the GluA1-4.1N interaction, facilitating stimulation-induced endocytosis of the AMPA receptor. The arrows (brown) represent specific steps of AMPA receptor trafficking.
AMPA receptor palmitoylation is highly regulated by neuronal activity. Glutamate stimulation rapidly induces depalmitoylation of AMPA receptors without affecting total receptor levels in neuronal cultures [15,67]. This is similar to the regulation of palmitoylation of PSD-95, a major postsynaptic density protein critical for AMPA receptor trafficking, which also undergoes activity-dependent depalmitoylation [68]. Conversely, blockade of neuronal activity by tetrodotoxin (TTX) increases palmitoylation of AMPA receptors [67]. As palmitoylation of the AMPA receptor plays an important role in the regulation of receptor trafficking [15,47,67], such activity-dependent modulation of palmitoylation/depalmitoylation of the AMPA receptor provides an important mechanism for synaptic plasticity.
Ubiquitination
Ubiquitination is an evolutionally conserved posttranslational process that attaches a single ubiquitin or polymeric ubiquitin chains to lysine residues of a substrate protein (reviewed in [69]). Protein ubiquitination is catalyzed by a sequential action of three enzymes: the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and the ubiquitin ligase (E3) [69]. The E3 ligase transfers ubiquitin to its targets at lysine residue(s) and determines substrate specificity [69]. In contrast to the relatively small numbers of E1 and E2 enzymes, there are hundreds of E3 ligases, allowing for enormous diversity and specificity. Ubiquitination is a reversible modification in that various deubiquitinases can cleave or trim polyubiquitin chains and terminate the ubiquitination-induced signaling [69]. Modification of proteins with ubiquitin generally serves as a targeting signal for protein degradation that is mediated by the proteasome or lysosomal machinery and plays an important role in intracellular trafficking of membrane proteins [69]. Many key synaptic proteins are direct targets for activity-dependent, ubiquitination-mediated degradation, including Shank, PSD-95 and NMDA receptors [70].
Early studies have indicated a crucial role for ubiquitination-mediated signaling in regulating AMPA receptor trafficking and turnover [71-73]. In addition, AMPA receptors in C. elegans were shown to be ubiquitinated nearly a decade ago [74], which regulates the density of the receptors at synapses [75]. Recently, both GluA1 and GluA2 subunits of mammalian AMPA receptors were shown to be direct targets of ubiquitination in cultured neurons [76-78]. Ubiquitination of GluA1 in neurons primarily occurs on the cell surface, mainly on residue K868 in the C-tail, and can be rapidly induced by treatment of an AMPA receptor agonist, AMPA [76,78]. Importantly, GluA1 ubiquitination precedes and is required for AMPA-induced endocytosis of the receptors [76,78]. Supporting this notion, blockade of polyubiquitination or proteasome activity prevents AMPA-induced internalization of GluA1 [71]. Upon ubiquitination, a portion of internalized GluA1 is degraded by the lysosome, which controls the total level of GluA1 in neurons [78]. For GluA2, its ubiquitination can be induced by blocking GABAergic inhibitory transmission with bicuculline and also depends on AMPA receptor activation [77]. Interestingly, bicuculline-induced GluA2 ubiquitination can be rapidly reversed by restoring normal activity in the cultured neurons, indicating that ubiquitination of AMPA receptors is a highly regulated process sensitive to ongoing neuronal activity [77]. In contrast to GluA1 ubiquitination, GluA2 ubiquitination occurs after receptor endocytosis [77], suggesting a subunit-specific regulation of AMPA receptor trafficking by ubiquitination. Notably, NMDA application, although capable of inducing AMPA receptor internalization, does not trigger ubiquitination on either GluA1 or GluA2 [76-78]. Instead, NMDA-induced AMPA receptor internalization depends on ubiquitination of PSD-95 [73], demonstrating a pathway specific recruitment of the ubiquitin-proteasome system for AMPA receptor trafficking.
Which E3 ligase(s) mediates AMPA receptor subunit ubiquitination? Recently, Nedd4 (neural precursor cell expressed, developmentally down-regulated) has been identified as an E3 ligase that ubiquitinates GluA1 [76,78]. Nedd4 was originally isolated from a screen from embryonic mouse brain [79]. Recent studies show that Nedd4 is enriched at synapses, physically interacts with GluA1 and mediates ubiquitination of GluA1 in heterologous cells and neurons [76,78]. Overexpression of Nedd4 in neurons increases GluA1 ubiquitination, reduces surface levels of GluA1, diminishes AMPAR-mediated synaptic transmission, and facilitates targeting of AMPA receptors to late endosomal/lysosomal compartments for degradation [76,78]. Conversely, knockdown of Nedd4 through RNA interference decreases GluA1 ubiquitination and inhibits AMPA-induced endocytosis of GluA1-containing AMPA receptors [76,78]. Another recent report demonstrates the ubiquitin E3 ligase, anaphase-promoting complex (APC), also promotes GluA1 ubiquitination [80]. The APC complex interacts with and ubiquitinates GluA1, and has been reported to control downregulation of synaptic GluA1 induced by EphA4 activation during homeostatic plasticity [80]. Neither Nedd4 nor the Apc complex, however, appears to interact with GluA2 [76,78,80], suggesting that distinct E3 ligases may exist in neurons to catalyze ubiquitination of the GluA2 subunit. Currently, it is unclear how neuronal activity regulates E3 ligases for AMPA receptor ubiquitination. It is plausible that recruitment and activation of E3 ligases for AMPA receptors depends on calcium, as both GluA1 and GluA2 ubiquitination require local increase of calcium concentration [76,77].
Cross-talk among posttranslational modifications
An emerging concept is that different posttranslational modifications functionally cross talk with each other to provide fine regulation of trafficking and function of the target proteins. For example, it has been demonstrated that palmitoylation of the β2-adrenergic receptor, the kainate receptor GluK2 subunit or NMDA receptor GluN2 subunits modulates their phosphorylation levels, thus altering receptor trafficking and function [81-84]. Likewise, tyrosine phosphorylation of Parkin, an E3 ligase, inhibits its autoubiquitination, thereby stabilizing the protein [85]. More recently, an intriguing posttranslational cross-talk mechanism was discovered to modulate the function of the BK channel, a voltage-gated potassium channel. In this case, the cytosolic C-tail of the BK channel interacts with the plasma membrane via palmitoylation at multiple C-tail cysteine residues. PKA phosphorylation at a serine residue close to palmitoylation sites leads to dissociation of the BK channel C-tail from the plasma membrane, and thus inactivation of the channel [86]. Indeed, these expanding lines of evidence highlight the importance of cross-modulations of proteins by different posttranslational modifications in controlling their precise cellular function [81-88]. Such interplay greatly expands the complexity and potential specificity of the biological functions of posttranslational modifications.
Recent progress also demonstrates the significance of such cross-talk among posttranslational modifications in the regulation of AMPA receptor trafficking. One elegant example is the functional interaction between C811 palmitoylation and nearby S818 phosphorylation in the GluA1 C-tail in controlling AMPA receptor trafficking (Figure 3) [47]. In this scenario, the absence of C811 palmitoylation on GluA1 facilitates phosphorylation at S818, which in turn promotes the interaction between GluA1 and 4.1N and subsequently GluA1 exocytosis [47]. It is possible that palmitoylation at C811 inhibits nearby phosphorylation by introducing allosteric restriction of PKC access to its substrates, a mechanism previously proposed for the cross-modulation of the β2-adrenergic receptor by phosphorylation and palmitoylation [82]. In addition, it has been shown that phosphorylation at the GluA1 C-tail can prevent GluA1 degradation by lysosomes [89], suggesting a potential cross-modulation between AMPA receptor phosphorylation and ubiquitination. Furthermore, the palmitoylation state of the GluA2 subunit in the M2 hairpin structure (C610) is critical for GluA2 stability in the ER [67]. A palmitoylation-deficient mutant of GluA2 (C610S) is unstable in the ER and rapidly subjected to lysosomal degradation [67], indicating that palmitoylation at C610 may suppress the ubiquitination and subsequent GluA2 degradation. As has been demonstrated for other proteins, such cross-talk by different posttranslational modifications likely represents a common mechanism for controlling various aspects of AMPA receptor trafficking and function.
In addition to phosphorylation, palmitoylation, and ubiquitination, two other posttranslational modifications, sumoylation and nitrosylation, have recently been demonstrated to play key roles in modulating synaptic protein function [90,91]. Indeed, a recent study shows that reciprocal nitrosylation and palmitoylation of PSD-95 controls its synaptic targeting [91]. However, it remains unclear if AMPA receptors can be sumoylated or nitrosylated. If AMPA receptors can be sumoylated or nitrosylated, what roles do these modifications play in the regulation of AMPA receptor trafficking, and how do these modifications interact with other posttranslational modifications discussed in this review?
Conclusions
Reversible posttranslational modifications, including phosphorylation, palmitoylation and ubiquitination, have been shown to control various aspects of AMPA receptor trafficking and functional modulation. Recent advances highlight how posttranslational modifications regulate protein-protein interactions that mediate receptor trafficking and how different posttranslational modifications interact with each other to precisely regulate AMPA receptor-mediated synaptic transmission. It will continue to be important to identify molecular and biochemical mechanisms underlying posttranslational modifications of AMPA receptor trafficking and function, which will provide key insights into the mechanisms for synaptic plasticity. In addition, it will be equally important to determine the local signal transduction pathways that give rise to posttranslational modifications of AMPA receptors in a highly spatially and temporally controlled manner.
Highlights.
AMPA receptor phosphorylation regulates receptor trafficking and function. AMPA receptor palmitoylation plays differential roles in receptor trafficking. AMPA receptor ubiquitination functions in receptor endocytosis and degradation. Posttranslational modifications interact with each other to modulate AMPA receptors.
Acknowledgements
The authors thank Greger IH, Gray JA, Goold CP and Nicoll RA for critical comments. This work was supported by a NIH Pathway to Independence Award (K99 MH090239-01A1) from NIMH (WL) and by the NINDS Intramural Research Program (KWR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 6.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 9.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu W, Isozaki K, Roche KW, Nicoll RA. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci U S A. 2010;107:22266–22271. doi: 10.1073/pnas.1016289107. • By using a molecular replacement approach in AMPA receptor null background, the authors demonstrates that the GluA1 Loop1 domain is involved in AMPA receptor trafficking. In addition, this study identifies a novel phosphorylation site in GluA1 Loop1 that regulates Loop1-dependent AMPA receptor trafficking.
- 13.Jiang J, Parameshwaran K, Seibenhener ML, Kang MG, Suppiramaniam V, Huganir RL, Diaz-Meco MT, Wooten MW. AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus. 2009;19:392–406. doi: 10.1002/hipo.20528. • Evidence is given here that the Loop2 domain of AMPA receptor subunits plays a role in AMPA receptor trafficking through the interaction with SQSTM1/p62 protein.
- 14.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L. Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Suppiramaniam V, Wooten MW. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2006;15:266–282. doi: 10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- 19.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 20.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 21.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 22.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 23.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- 26.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca(2)/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. •• This work uncovers that the regulation of AMPA receptor channel conductance by phosphorylation at GluA1 S831 in the C-tail is dependent on the specific stoichiometry of AMPA receptors requiring the presence of TARPs. Moreover, this work reveals the mechanism for the regulation of biophysical properties of AMPA receptors by GluA1 S831 phosphorylation.
- 27.Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 28.Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 29.Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luthi A, Wikstrom MA, Palmer MJ, Matthews P, Benke TA, Isaac JT, Collingridge GL. Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci. 2004;5:44. doi: 10.1186/1471-2202-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer MJ, Isaac JT, Collingridge GL. Multiple, developmentally regulated expression mechanisms of long-term potentiation at CA1 synapses. J Neurosci. 2004;24:4903–4911. doi: 10.1523/JNEUROSCI.0170-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 33.Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 35.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. • This study shows that phosphorylation at S831 and S845 at the GluA1 C-tail is able to reduce the induction threshold for LTP.
- 36.Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc Natl Acad Sci U S A. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 38.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 39.Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- 40.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 41.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 42.Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 43.He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci. 2007;36:86–94. doi: 10.1016/j.mcn.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O’Dell TJ. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci. 2007;27:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. •• This work reveals how palmitoylation at S811 regulates nearby phosphorylation at S818 in the GluA1 C-tail, which in turn modulates the GluA1-4.1N interaction that underlies the extrasynaptic delivery of AMPA receptors.
- 48.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- 51.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- 53.Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 55.Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox CJ, Russell K, Titterness AK, Wang YT, Christie BR. Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo. Hippocampus. 2007;17:600–605. doi: 10.1002/hipo.20302. [DOI] [PubMed] [Google Scholar]
- 60.Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnar E. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci. 2009;40:267–279. doi: 10.1016/j.mcn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang CC, Hsu KS. Sustained activation of metabotropic glutamate receptor 5 and protein tyrosine phosphatases mediate the expression of (S)-3,5-dihydroxyphenylglycine-induced long-term depression in the hippocampal CA1 region. J Neurochem. 2006;96:179–194. doi: 10.1111/j.1471-4159.2005.03527.x. [DOI] [PubMed] [Google Scholar]
- 63.Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, et al. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholz R, Berberich S, Rathgeber L, Kolleker A, Kohr G, Kornau HC. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron. 2010;66:768–780. doi: 10.1016/j.neuron.2010.05.003. ••64 This report describes an important role for GluA2 tyrosine phosphorylation in AMPA receptor endocytosis through the regulation of a guanine-nucleotide exchange factor, BRAG2 activity that controls the formation of clathrin-coated vesicles.
- 65.Shipston MJ. Ion channel regulation by protein palmitoylation. J Biol Chem. 2011;286:8709–8716. doi: 10.1074/jbc.R110.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 67.Yang G, Xiong W, Kojic L, Cynader MS. Subunit-selective palmitoylation regulates the intracellular trafficking of AMPA receptor. Eur J Neurosci. 2009;30:35–46. doi: 10.1111/j.1460-9568.2009.06788.x. [DOI] [PubMed] [Google Scholar]
- 68.El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 69.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 70.Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patrick GN, Bingol B, Weld HA, Schuman EM. Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr Biol. 2003;13:2073–2081. doi: 10.1016/j.cub.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 72.Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29:4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 75.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30:16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. •These studies demonstrate that the GluA1 and GluA2 subunits of AMPA receptors are direct substrates for ubiquitination, which is important for AMPA receptor endocytosis and degradation.
- 77.Lussier MP, Nasu-Nishimura Y, Roche KW. Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. J Neurosci. 2011;31:3077–3081. doi: 10.1523/JNEUROSCI.5944-10.2011. •These studies demonstrate that the GluA1 and GluA2 subunits of AMPA receptors are direct substrates for ubiquitination, which is important for AMPA receptor endocytosis and degradation.
- 78.Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07221.x. •These studies demonstrate that the GluA1 and GluA2 subunits of AMPA receptors are direct substrates for ubiquitination, which is important for AMPA receptor endocytosis and degradation.
- 79.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 80.Fu AK, Hung KW, Fu WY, Shen C, Chen Y, Xia J, Lai KO, Ip NY. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011;14:181–189. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- 81.Pickering DS, Taverna FA, Salter MW, Hampson DR. Palmitoylation of the GluR6 kainate receptor. Proc Natl Acad Sci U S A. 1995;92:12090–12094. doi: 10.1073/pnas.92.26.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- 83.Moffett S, Mouillac B, Bonin H, Bouvier M. Altered phosphorylation and desensitization patterns of a human beta 2-adrenergic receptor lacking the palmitoylated Cys341. EMBO J. 1993;12:349–356. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko HS, Lee Y, Shin JH, Karuppagounder SS, Gadad BS, Koleske AJ, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci U S A. 2010;107:16691–16696. doi: 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe IC, Saleem F, Chen L, Greaves J, Chamberlain LH, Knaus HG, et al. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci U S A. 2008;105:21006–21011. doi: 10.1073/pnas.0806700106. •This work reports an interesting mechanism for the regulation of BK channel properties through a cross-talk between palmitoylation and phosphorylation in the C-tail of the BK channel.
- 87.Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:5384–5389. doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valdez-Taubas J, Pelham H. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 2005;24:2524–2532. doi: 10.1038/sj.emboj.7600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kessels HW, Kopec CD, Klein ME, Malinow R. Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat Neurosci. 2009;12:888–896. doi: 10.1038/nn.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature. 2007;447:321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron. 2011;71:131–141. doi: 10.1016/j.neuron.2011.05.033. • This study uncovers a crucial mechanism controlling synaptic targeting of PSD-95, which is mediated by two competitive reciprocal posttranslational modifications, nitrosylation and palmitoylation, on the cysteine residues.