Abstract
In Alzheimer’s disease, histochemically visualized cholinesterases with altered pH optimum for activity and inhibitable by indoleamines and the protease inhibitor bacitracin emerge in association with plaques and tangles. It has been suggested that these cholinesterases may participate in the pathologic process. However, it is not known whether the properties of cholinesterases observed in Alzheimer’s disease are due to requirements of histochemical procedures or actual biochemical properties of these enzymes. Using biochemical assays of acetylcholinesterase and butyrylcholinesterase activities, we demonstrate here that serotonin and bacitracin result in a significantly greater and dose-dependent inhibition of cholinesterases in Alzheimer’s disease cortex when compared with age-matched controls. In contrast, variations in pH did not distinguish cholinesterases in Alzheimer’s disease and control cortex. We also confirmed significant reduction of acetylcholinesterase activity in Alzheimer’s disease cortex and increased butyrylcholinesterase activity that only approached significance. We conclude that inhibition by indoleamines and bacitracin is a biochemical characteristic of a proportion of cholinesterases in Alzheimer’s disease that most likely represents the pool associated with plaques and tangles. Most of the available cholinesterase inhibitors are relatively incapable of inhibiting cholinesterases associated with plaques and tangles. The findings of the present investigation open the way for attempts to isolate cholinesterases associated with plaques and tangles and design or discovery of inhibitors specifically targeted to cholinesterases in these lesions.
Keywords: Acetylcholinesterase, Alzheimer’s Disease, Butyrylcholinesterase, Cholinesterase
Introduction
One of the major neurochemical abnormalities in the brains of patients who suffer from Alzheimer’s disease (AD) is a severe depletion of cortical cholinergic markers (see [1] for review). The cortically projecting cholinergic system displays substantial loss in AD, both at the level of neurons located within the basal forebrain [1–3] and at the level of axons and terminals located within the cerebral cortex [1, 4]. Abnormalities in basal forebrain cholinergic neurons and cortical cholinergic axons, and depletion of cortical cholinergic innervation in AD occur relatively early in the course of the disease and are more substantial and widespread than loss of other cortically projecting neurotransmitter-specific systems [1, 5, 6]. These observations, along with the fact that the basal forebrain cholinergic system is involved in the cognitive processing of memory and attention [1], have made this system a target of therapy in AD.
Acetylcholinesterase (AChE, EC 2.3.1.6) is a relatively selective enzyme responsible for hydrolysis of the cholinergic transmitter acetylcholine. It is present in all cholinergic structures and in a subpopulation of non-cholinergic neurons throughout the nervous system [1, 7]. Recent observations indicate that butyrylcholinesterase (BuChE, EC 3.1.1.8), which is present in a subpopulation of cortical and subcortical neurons, is also capable of hydrolysis of acetylcholine [8–10]. Both enzymes are also present in glial cells [11].
In the AD brain, cholinesterases (ChE) are not confined to normal neural elements [12]. Our observations and those of others have indicated the presence of histochemically demonstrable and intense AChE and BuChE activities in plaques and tangles (AD-ChEs) [13, 14]. This observation is a consistent findings and has been demonstrated in many cohorts and by various groups [12, 15, 16]. In addition, plaques found in the aged human and non-human primate and tangles in the aged human brain contain AChE and BuChE activities [17–19].
In an earlier series of experiments, we demonstrated a number of properties which distinguish ChEs in plaques, tangles and glial cells in histochemical systems [12]. First, ChEs in normal neurons and axons were visualized best at a pH of 8.0 whereas AD-ChEs in plaques, tangles and glial cells required a much lower pH (6.8) for optimum visualization [13]. Furthermore, ChEs in plaques and tangles but not those in neurons and axons displayed similarities to aryl acylamidase activity (AAA) [20]. A proportion of both AChE and BuChE in the CNS possess an AAA activity that catalyses the hydrolysis of acylamides of aromatic amines [21]. The AAA activity of ChEs is inhibited by indoleamines [22, 23]. We found that indoleamines, such as serotonin (5-HT) and its precursor 5-hydroxytryptophan (5-HTP), were potent inhibitors of AD-ChE activity in plaques and tangles but did not affect ChEs in neurons and axons [20]. Of great interest, unlike ChEs in neural structures, AD-ChEs were also inhibited by the general protease inhibitor bacitracin and by carboxypeptidase inhibitor [20]. These findings suggest that AD-ChEs may possess novel targets and may interact with substrates other than acetylcholine [12].
A number of investigations have indicated that ChEs may be involved in the pathology of the amyloid-β peptide (Aβ), a major constituent of plaques. Administration of ChE inhibitors has been shown to interfere with Aβ production, most likely by reducing the levels of the amyloid precursor protein from which Aβ is cleaved [24–26]; however at least in some instances this effect may be due to other properties of these agents separate from enzymatic inhibition [25]. Both AChE and BuChE appear to influence the aggregation of Aβ [27, 28], a major event in the process of plaque formation. They also increase the toxicity of Aβ [28, 29]. Our preliminary observations indicate that inhibition of both AChE and BuChE activities reduces the toxic effects of Aβ in the aged primate brain (Maloof et al., Soc. for Neurosci. Abst., Session # 830.12, 2004). BuChE expression increases as plaques go through the process of maturation from the diffuse type to the pathologic compact variety [30]. The proposed influence of BuChE in the pathology of plaques appears to be greater than that of AChE [31]. Importantly, while AChE activity is decreased in the AD brain, BuChE activity either remains unchanged or is increased [12, 32, 33].
Given the possible involvement of AD-ChEs in disease pathology, it would be highly desirable to fully characterize these enzymes. A major question in this regard is whether the characteristics of AD-ChEs described earlier are true biochemical properties of these enzymes or are a by-product of the requirements for the histochemical procedures used, such as tissue fixation. In the present set of experiments, we used homogenized fresh frozen cortical tissue from Alzheimer’s disease and non-demented brains in a biochemical assay of cholinesterase activity. We found that, similar to our histochemical observations, indoleamines and bacitracin inhibited a considerably greater proportion of biochemically determined AChE and BuChE activities in AD cortex when compared with non-demented controls.
Materials and Methods
Materials
The following chemicals were obtained from Sigma-Aldrich Inc. Acetylthiocholine iodide, butyrylthiocholine iodide, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), AChE inhibitor BW284C51 (1,5-bis (4-allyl dimethylammonium phenyl) pentan-3-one dibromide), the BuChE inhibitor Iso-OMPA (tetraisopropyl pyrophosphoramide), 5-HT (serotonin), 5-HTP, bacitracin, Tween 20, Triton X-100, disodium EDTA and sodium phosphate (monobasic and dibasic).
Cases
Cortical tissue from nine clinically and pathologically confirmed cases of AD and eight age- and sex-matched control cases with no clinical or pathologic evidence of neurological or psychiatric disorders were used in these experiments (Table). Age (control 78.4 ± 3.7; AD 75.5 ± 4.6) and postmortem interval (control 15.2 ± 0.9; AD 17 ± 2.7) were not different in the two groups of brains (p>0.05). Following autopsy, brain tissue blocks were rapidly frozen on dry ice and stored at −80° C until used. Identical cortical regions from the middle temporal gyrus (Brodmann area 20) and prefrontal cortex (Brodmann area 9), areas that show abundant accumulation of plaques and tangles in AD brains [34], were used for analysis. Tissue from inferior parietal lobule (Brodmann area 39–40), which was available from a few cases, was used in trial experiments to confirm the pattern of changes detected in the other cortical areas.
Table.
Characteristics of Cases
| Case Number | Age (Years) | Sex | Postmortem Interval (Hours) | Diagnosis |
|---|---|---|---|---|
| 1 | 87 | F | 13 | Normal |
| 2 | 73 | M | 16 | Normal |
| 3 | 77 | F | 15 | Normal |
| 4 | 64 | F | 14 | Normal |
| 5 | 93 | M | 14 | Normal |
| 6 | 88 | M | 20 | Normal |
| 7 | 89 | M | 12 | Normal |
| 8 | 70 | F | 13 | Normal |
| 9 | 65 | M | 29.3 | Alzheimer’s Disease |
| 10 | 91 | F | 27.8 | Alzheimer’s Disease |
| 11 | 66 | M | 5 | Alzheimer’s Disease |
| 12 | 91 | F | 22 | Alzheimer’s Disease |
| 13 | 51 | F | 10 | Alzheimer’s Disease |
| 14 | 69 | M | 12 | Alzheimer’s Disease |
| 15 | 87 | M | 13 | Alzheimer’s Disease |
| 16 | 80 | M | 13 | Alzheimer’s Disease |
| 17 | 80 | F | 22 | Alzheimer’s Disease |
F, Female; M, Male.
Biochemical Determination of Cholinesterase Activity
AChE and BuChE enzyme activities were assayed according to the method of Ellman et al. [35] with slight modifications. It has been reported that use of the detergent Triton-X100 may inhibit BuChE activity [36]. For this reason, the effects of Triton-X100 and Tween were compared and the detergent that resulted in the highest BuChE activity was used in the remainder of the experiments. Tissue samples were thawed on ice and sonicated at medium power in 50 mM phosphate buffer containing 10mM EDTA and 0.5% Tween or Triton-X100 to yield 5–10 mg of tissue per ml of the sonicate. Five μl of this tissue preparation was added to 445 μl of a solution containing 50 mM sodium phosphate, 0.75 mM specific BuChE inhibitor iso-OMPA or the specific AchE inhibitor BW284C51 (for determination of AchE and BuChE activity, respectively), and DTNB. This mixture was incubated at 37° C for 30 minutes to inhibit BuChE and AchE activities, respectively. Blanks contained the sonication medium without tissue. Then 50 μl of a 5 mM solution of acetylthiocholine iodide (for determination of AchE activity) or butyrylthiocholine iodide (for determination of BuChE activity) were added to this solution and the optical density (OD) of 200 μl of this mixture was determined at 412 nm in a spectrophotometer. The rest of the solution was allowed to incubate at 37° C for 30 minutes and the OD determined in 200 μl of this solution at 412 nm. The net OD after the last incubation of the sample minus the blank was used to calculate the level of ChE activity in tissue. ChE activity was determined in duplicate or triplicate and expressed as mmol/hr/g protein. Protein was measured using the Bio-Rad colorimetric assay.
Investigation of the effects of pH, Indoleamines and Bacitracin
To investigate the effects of pH on ChE activities in control and AD cortex, phosphate buffer with five different pHs varying at 0.5 increments between 6.3 and 7.8 was used in the biochemical reaction. In a second set of experiments, we tested the pH used for usual enzymatic reactions (7.4) and the pHs at which optimum ChE activity is seen histochemically in neurons/axons (8.0) and plaques/tangles (6.8) [12, 13].
Inhibition of ChEs by the indoleamines 5-HT and 5-HTP and by the general protease inhibitor bacitracin in control and AD cortex were investigated through determination of ChE activity in the absence or presence of 10−3, 10−4, 10−5 and 10−6 M concentration of each compound.
Statistical Analysis
Statistical analysis was carried out using the GraphPad Instat software (version 3.0, GraphPad Software, Inc.). All data were normally distributed, therefore analysis of variance followed by Tukey’s post hoc tests were used to determine significant effects. The probability for accepting a significant effect was set at p<0.05.
To determine differential inhibition by indoleamines and bacitracin in control compared with AD cortex, in each case the ChE activity in the presence of each concentration of inhibitor was expressed as percentage of ChE activity in the absence of inhibitor and the percentage data was used for statistical analysis.
Results
Tween VS Triton X-100
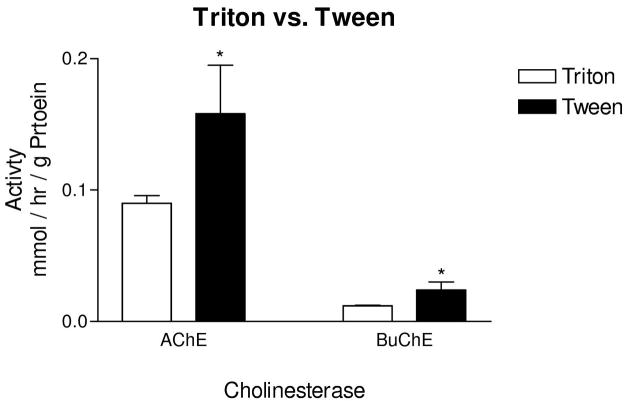
AchE and BuChE activities measured in the presence of 0.5% of the detergent Tween 20 were consistently and significantly greater in both cortical areas and in both control and AD cortex when compared with the same concentration of Triton X-100 (p<0.05, Fig. 1). Therefore, in the remainder of the experiments, Tween 20 was used as detergent.
Figure 1.
Effects of detergent on AChE and BuChE activities. Use of Tween 20 in the sonication medium resulted in significantly greater AChE and BuChE activity when compared with the use of Triton X-100. In the ChE assay system used, Triton X-100 appeared to result in inhibition of activity. Bars represent mean activity ± standard error of the mean. AChE and BuChE activity in temporal and frontal cortex were averaged and used as data points for each case. * p<0.05.
Cholinesterase Activity in Control and Alzheimer Cortex
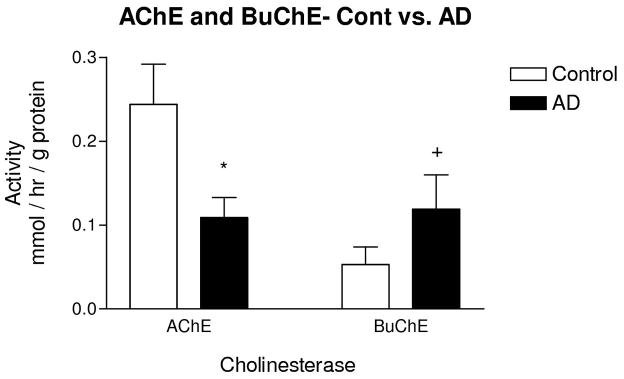
Averaged AchE activity in all cortical areas was significantly lower in AD cortex when compared with controls (50%, p<0.009, Fig. 2). Averaged BuChE activity was higher in AD cortex (50%, Fig. 2), but this difference only approached statistical significance due to high variability (p=0.086).
Figure 2.
Activity of ChEs in AD cortex. AChE activity was significantly lower in AD cases when compared with controls. BuChE activity was higher in AD when compared with controls, but the difference only approached significance. Bars represent mean activity ± standard error of the mean. AChE and BuChE activity in temporal and frontal cortex were averaged and used as data points for each case. * p<0.009, + p=0.086.
Effect of pH on Cholinesterase Activity
In virtually all cases, both AchE and BuChE activity showed an increase with increasing pH. This increase was statistically significant for AchE activity when the lowest pHs (6.0–6.8), were compared with the highest pHs (7.8–8.0, p<0.05). The increases in BuChE activity were not statistically significant (p>0.05). At all pHs, there was less AchE activity in AD cortex when compared with controls. Additionally, the non-significant increase in BuChE in AD cortex was seen at all pHs (data not shown).
Inhibition of Cholinesterase Activity by Indoleamines and Bacitracin
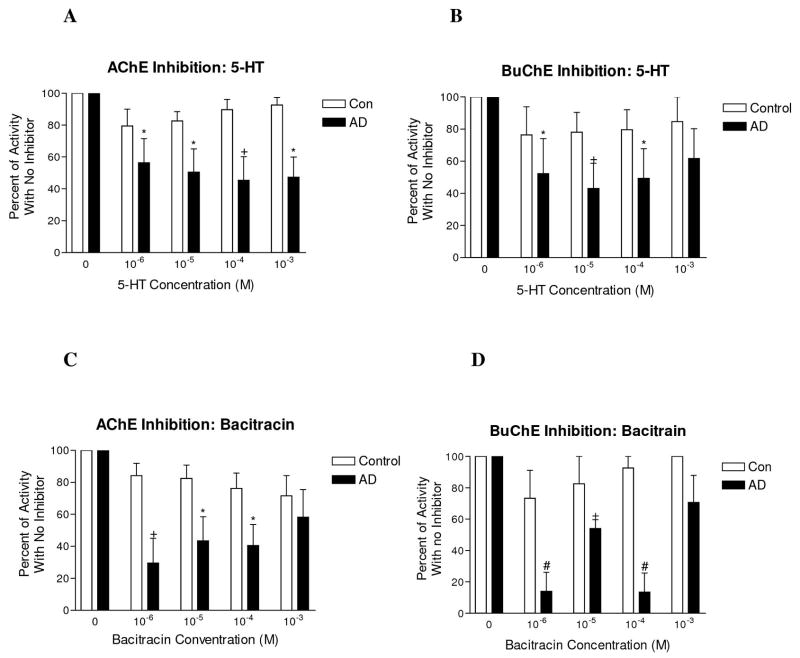
5-HT resulted in minimal inhibition of AchE and BuChE activity in control cerebral cortex (p>0.05 for all concentrations when compared with no inhibitor condition, Fig. 3A and B). However, in AD cortex, substantial dose-dependent inhibition was obtained at several concentrations. The proportion of total AchE that was inhibited in AD was significantly greater when compared with the condition with no inhibitor. (p<0.05–0.01, Fig 3A). Similarly, the proportion of BuChE inhibited in AD cortex was significantly greater when compared with no inhibitor condition (p<0.05–0.01, Fig 3B). An inverted U-shaped dose-response curve was obtained for 5-HT dose and extent of AchE and BuChE inhibition. Identical results were obtained in the temporal and frontal cortices. Trial experiments using 5-HTP or parietal cortex tissue showed identical pattern of inhibition (data not shown).
Figure 3.
Inhibition of AChE and BuChE activity in control and AD cortex by 5-HT and the protease inhibitor bacitracin. (A) In control temporal cortex, 5-HT resulted in inhibition of a negligible proportion of AChE when compared with no inhibitor condition. In contrast, 5-HT resulted in inhibition of a substantial proportion of AChE in AD temporal cortex. Inhibition in AD was significantly greater than the no inhibitor condition at every concentration of 5-HT used. (B) Similar to AChE, 5-HT resulted in inhibition of a negligible proportion of BuChE in control temporal cortex and a substantial proportion of BuChE in AD cortex. BuChE inhibition by 5-HT was significantly greater in AD when compared with no inhibitor condition for the three lower concentrations of 5-HT. (C) In control temporal cortex, bacitracin resulted in inhibition of a small proportion of AChE when compared with the no inhibitor condition. In AD, the proportion of AChE inhibited was substantially higher and was significantly greater than the no inhibitor condition for the three lowest concentrations used. (D) Similar to AChE, bacitracin inhibited a small proportion of BuChE in control frontal cortex and a substantially larger proportion in AD cortex which was significantly different than the no inhibitor condition for the three lowest concentrations used. Similar results were obtained in the frontal and temporal cortex. Trial experiments in the inferior parietal lobule (Brodmann area 40–41) displayed a similar pattern of inhibition as did trials using 5-HTP as inhibitor. * p<0.05, + p<001, # p<0.001.
Similar to indoleamines, bacitracin resulted in minimal inhibition of AchE and BuChE activity in control cortex (p>0.05 for all concentrations when compared with no inhibitor condition, Fig 3C and D). In contrast, in AD cortex, bacitracin resulted in substantial dose-dependent inhibition of AchE. The proportion of AchE in the presence of the three lower concentrations of bacitracin was significantly lower in AD cortex when compared with the no inhibitor condition (p<0.05–0.01, Fig 3C). Additionally, the proportion of BuChE in the presence of the three lower concentrations of bacitracin was significantly lower when compared with no inhibitor condition (p<0.05–0.001, Fig 3D). As was the case with indoleamines, an inverted U-shaped dose-response curve was obtained for bacitracin dose and extent of AchE and BuChE inhibition and identical results were obtained in frontal and temporal cortex. Trial experiments using parietal cortex tissue displayed an identical pattern of inhibition.
Discussion
Consistent with previous reports [12, 32, 33], we observed a significant loss of AchE activity in AD cortex when compared with non-demented controls. The literature reports increased or no change in BuChE activity in AD cortex [12, 32, 33]. We found increased BuChE activity in AD cortex that only approached significance. Thus, our findings support the well- established loss of AchE activity in AD cortex and stable or possible increase of BuChE activity.
Our histochemical findings had indicated pH dependency of AchE and BuChE activities in normal neural structures and plaques/tangles [12, 13]. ChE activity in the former was visualized best at pH 8.0, whereas that in the latter required a lower pH of 6.8. If this dependence of activity on pH is true biochemical property of ChEs in the two compartments, then it would be expected that at pH 6.8, the ChEs that emerge in plaques and tangles would result in higher biochemically measured AchE and BuChE activities in AD cortex when compared with controls. However, we did not observe such a pattern. AchE and BuChE activity displayed a consistent elevation with increased pH. Furthermore, AchE activity was always lower in AD cortex regardless of pH. In addition, BuChE activity showed the same pattern in AD when compared with controls at all pHs tested. Therefore, it appears that the differences in pH optimum for visualizing ChE activities in plaques and tangles in tissue sections is due to fixation or other properties of histochemical systems and not true differential biochemical property of ChEs in AD and control cortex.
In contrast to pH, inhibition patterns clearly distinguished biochemically determined ChE activity in AD cortex from those in normal brains. At the range of concentrations used, indoleamines and bacitracin resulted in virtually no inhibition of AchE or BuChE in cortical tissue from normal control subjects when compared with no inhibitor condition. On the other hand, these agents resulted in a dose-dependent inhibition of a proportion of AchE and BuChE in AD. Thus, inhibition by indoleamines and bacitracin appears to be an intrinsic biochemical property of a proportion of ChEs in AD cortex. Given that indoleamines and bacitracin selectively inhibit ChEs in plaques and tangles in histochemical systems [20], it is very likely that the biochemically inhibitable ChEs we observed in AD cortex belong to the pool of enzyme associated with these pathological lesions.
The biochemical observations reported here support our earlier suggestion that a distinct pool of AchE and BuChE emerge in AD cortex, associated with plaques and tangles [12]. We have found that ChEs with identical histochemical properties to those found in plaques and tangles are also found in glial cells [11]. Thus the biochemically different pool of ChEs is AD may originate in glia which display significant proliferation and activation in the course of the disease process [37, 38].
At present, amelioration of the cholinergic deficit in AD is accomplished through administration of ChE inhibitors [8]. The rationale for the use of these agents is to inhibit the hydrolytic enzyme AchE and thereby increase the available pool of the neurotransmitter acetylcholine. Importantly, recent evidence has confirmed that butyrylcholinesterase (BuChE), another major ChE in the brain, is also capable of hydrolyzing ACh [8–10], and therefore its inhibition may further enhance cholinergic transmission in AD.
As reviewed earlier, ChEs are most likely involved in the pathology of plaques. AChE and BuChE influence Aβ production [24–26], its aggregation [27,28] and its toxicity [28,29], and BuChE appears to influence the maturation of plaques [30]. Since AD-ChEs are associated with the pathology of AD and emerge in the course of the disease, it is likely that it is this pool of ChEs that is involved in the pathology of Aβ summarized above. These considerations indicate that it may be desirable to inhibit the biochemically distinct pool of AD-ChEs that are associated with plaques, tangles and glial cells and that such a strategy may provide a means of retarding the disease process in AD. However, we have shown using histochemical systems that with few exceptions, commonly used ChE inhibitors, are considerably less effective in inhibiting ChEs in plaques and tangles when compared with ChEs in neurons and axons [12].
The selective biochemical properties of AD-ChEs observed in our experiments justify a search for new inhibitors that can selectively target this pool of ChEs. However, such a search requires isolation of ChEs associated with plaques, tangles and glial cells for testing of the specificity of any inhibitors discovered or designed. A previous report [39] and our preliminary observations (Yan et al., Soc. Neurosci. Abst., Session # 115.10, 2007) indicate that isolation of AD-ChEs through passive release or digestion of proteins that may anchor them to cells and the extracellular matrix, may provide a mechanism for selective isolation of ChEs associated with plaques, tangles and glial cells. Future experiments are required to determine whether these strategies are specific enough and yield sufficient amounts of AD-ChEs to make them useful for development and testing of AD-ChE inhibitors.
Acknowledgments
We are grateful to Katherine Gasho and Vafa Lalezari for expert technical assistance. This work was supported in part by grants from the National Institute of Neurological Disorders and Stroke (NS057429) and Novartis Pharmaceuticals, Inc.
References
- 1.Geula C, Mesulam M-M. Cholinergic systems in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 269–292. [Google Scholar]
- 2.Mufson EJ, Bothwell M, Kordower JH. Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp Neurol. 1989;105:221–232. doi: 10.1016/0014-4886(89)90124-6. [DOI] [PubMed] [Google Scholar]
- 3.Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, et al. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- 4.Geula C, Mesulam M-M. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cerebral Cort. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- 5.Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry EK, Blessed G, Tomlinson BE, Perry TH, Crow TJ, Cross AJ, et al. Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiol Aging. 1981;2:251–256. doi: 10.1016/0197-4580(81)90032-4. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam M-M, Geula C. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 1991;306:193–220. doi: 10.1002/cne.903060202. [DOI] [PubMed] [Google Scholar]
- 8.Giacobini E. Cholinesterase Inhibitors: from Calabar bean to Alzheimer therapy. In: Giacobini E, editor. Cholinesterases and Cholinesterase Inhibitors. London: Martin Dunitz; 2000. pp. 181–226. [Google Scholar]
- 9.Mesulam M, Guillozet A, Shaw P, Quinn B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol Dis. 2002;9:88–93. doi: 10.1006/nbdi.2001.0462. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–639. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 11.Wright CI, Geula C, Mesulam M-M. Neuroglial cholinesterases in the normal brain and in Alzheimer’s disease: relationship to plaques, tangles and patterns of selective vulnerability. Ann Neurol. 1993;34:373–384. doi: 10.1002/ana.410340312. [DOI] [PubMed] [Google Scholar]
- 12.Geula C, Mesulam M-M. Cholinesterases and the pathology of Alzheimer’s disease. Alz Dis Assoc Disor. 1995;9:23–28. doi: 10.1097/00002093-199501002-00005. [DOI] [PubMed] [Google Scholar]
- 13.Geula C, Mesulam M-M. Special properties of cholinesterases in the cerebral cortex of Alzheimer’s disease. Brain Res. 1989;498:185–189. doi: 10.1016/0006-8993(89)90419-8. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam M-M, Geula C, Moran MA. Anatomy of cholinesterase inhibition in Alzheimer’s disease: effect of physostigmine and tetrahydroaminoacridine on plaques and tangles. Ann Neurol. 1987;22:683–691. doi: 10.1002/ana.410220603. [DOI] [PubMed] [Google Scholar]
- 15.Moran MA, Mufson EJ, Gomez-Ramos P. Cholinesterases colocalize with sites of neurofibrillary degeneration in aged and Alzheimer’s brains. Acta Neuropathol (Berl) 1994;87:284–292. doi: 10.1007/BF00296744. [DOI] [PubMed] [Google Scholar]
- 16.Moran MA, Mufson EJ, Gomez-Ramos P. Colocalization of cholinesterases with beta amyloid protein in aged and Alzheimer’s brains. Acta Neuropathol (Berl) 1993;85:362–369. doi: 10.1007/BF00334445. [DOI] [PubMed] [Google Scholar]
- 17.Sani S, Traul D, Klink A, Niaraki N, Gonzalo-Ruiz A, Wu CK, Geula C. Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathol (Berl) 2003;105:145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- 18.Geula C, Nagykery N, Wu CK. Amyloid-beta deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol (Berl) 2002;103:48–58. doi: 10.1007/s004010100429. [DOI] [PubMed] [Google Scholar]
- 19.Carson K, Geula C, Mesulam M-M. Electron microscopic localization of cholinesterase activity in Alzheimer brain tissue. Brain Res. 1991;540:204–208. doi: 10.1016/0006-8993(91)90508-s. [DOI] [PubMed] [Google Scholar]
- 20.Wright CI, Geula C, Mesulam M-M. Protease inhibitors and indoleamines selectively inhibit cholinesterases in the histopathologic structures of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:683–686. doi: 10.1073/pnas.90.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Checler F, Grassi J, Vincent JP. Cholinesterases display genuine arylacylamidase activity but are totally devoid of intrinsic peptidase activities. J Neurochem. 1994;62:756–763. doi: 10.1046/j.1471-4159.1994.62020756.x. [DOI] [PubMed] [Google Scholar]
- 22.George ST, Balasubramanian AS. The identity of the serotonin-sensitive aryl acylamidase with acetylcholinesterase from human erythrocytes, sheep basal ganglia and electric eel. Eur J Biochem. 1980;111:511–524. doi: 10.1111/j.1432-1033.1980.tb04967.x. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar R, George ST, Balasubramanian AS. Serotonin-sensitive aryl acylamidase activity of platelet acetylcholinesterase. Biochem Pharmacol. 1982;31:2319–2325. doi: 10.1016/0006-2952(82)90524-x. [DOI] [PubMed] [Google Scholar]
- 24.Lahiri DK, Lewis S, Farlow MR. Tacrine alters the secretion of the beta-amyloid precursor protein in cell lines. J Neurosci Res. 1994;37:777–787. doi: 10.1002/jnr.490370612. [DOI] [PubMed] [Google Scholar]
- 25.Greig NH, Utsuki T, Yu Q, Zhu X, Holloway HW, Perry T, Lee B, Ingram DK, Lahiri DK. A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17:159–165. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- 26.Shaw KT, Utsuki T, Rogers J, Yu QS, Sambamurti K, Brossi A, et al. Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci USA. 2001;98:7605–7610. doi: 10.1073/pnas.131152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-beta- peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 28.Barber KL, Mesulam M-M, Krafft GA, Klein WL. Butyrylcholinesterase (BChE) alters the aggregation state of Ab amyloid. Soc Neurosc Abst. 1996;72:1172. [Google Scholar]
- 29.Reyes AE, Chacon MA, Dinamarca MC, Cerpa W, Morgan C, Inestrosa NC. Acetylcholinesterase-Abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol. 2004;164:2163–2174. doi: 10.1016/s0002-9440(10)63774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesulam M-M, Geula C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol. 1994;36:722–727. doi: 10.1002/ana.410360506. [DOI] [PubMed] [Google Scholar]
- 31.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 32.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 33.Atack JR, Perry EK, Bonham JR, Candy JM, Perry RH. Molecular forms of acetylcholinesterase and butyrylcholinesterase in the aged human central nervous system. J Neurochem. 1986;47:263–277. doi: 10.1111/j.1471-4159.1986.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 34.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 35.Ellman GL, Courtney KV, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, et al. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J Neurochem. 2000;75:1320–1331. doi: 10.1046/j.1471-4159.2000.751320.x. [DOI] [PubMed] [Google Scholar]
- 37.Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- 38.Geula C. Pathological diagnosis of Alzheimer’s disease. In: Scinto LFM, Daffner KR, editors. Early Diagnosis of Alzheimer’s Disease. Totowa: Humana Press; 2000. pp. 65–81. [Google Scholar]
- 39.Kalaria RN, Kroon SN, Grahovac I, Perry G. Acetylcholinesterase and its association with heparan sulphate proteoglycans in cortical amyloid deposits of Alzheimer’s disease. Neuroscience. 1992;51:177–184. doi: 10.1016/0306-4522(92)90482-h. [DOI] [PubMed] [Google Scholar]