Abstract
Trillions of bacteria, fungi, viruses, archaea and small arthropods colonize the skin surface, collectively comprising the skin microbiome. Generations of researchers have classified these microbes as transient versus resident, beneficial versus pathogenic, collaborators versus adversaries. Culturing and direct sequencing of microbial inhabitants identified distinct populations present at skin surface sites. Herein, we explore the history of this field, describe findings from the current molecular sequencing era, and consider the future of investigating how microbes and antimicrobial therapy contribute to human health.
Introduction
Microbiology and dermatology are intimately related. Both fields have long recognized that the cutaneous surface is inhabited by myriad bacteria, fungi, and viruses and that these microbial communities are intricately linked to human health and disease (Chiller et al., 2001; Fredricks, 2001; Grice and Segre, 2011; Kong, 2011; Leyden et al., 1987). While many oft-studied organisms are pathogens, commensal microbes also play a significant, if perhaps often unappreciated, role in human health. Still open to question is the extent to which commensals provide direct benefit or simply prevent fellow harmful microbes from establishing residence.
Three major questions that have been addressed with different methods over the decades are: (1) What microbes are present on the skin surface? (2) How does microbial diversity contribute to health and disease states? and (3) How do dermatologic practices alter microbial diversity?. Extensive reviews on the current state of skin microbiome research have been recently published (Grice and Segre, 2011; Kong, 2011). This review represents a non-comprehensive historical overview of the >5 decade long exploration into the relationships between resident and transient as well as commensal and pathogenic skin microbes. Finally, we discuss anticipated progress in the future and potential impact of external factors including antimicrobials on the healthy skin microbiome.
Cool, acidic, desiccated healthy skin is a rather inhospitable environment for microbial growth. Epidermis represents a formidable physical barrier, resisting penetration by microorganisms while retaining moisture and nutrients inside the body (Madison, 2003). Moreover, skin is a continuously self-renewing organ, with squames (and adherent bacteria) constantly shed from the skin surface as a result of terminal differentiation (Elias, 2005). Although these obstacles would seemingly resist establishment of microbial inhabitants of the skin and skin appendages, we estimate that ~1 billion bacteria inhabit a typical square centimeter of skin – covering the surface and extending down into the appendages and glands (Grice et al., 2008).
The microbiota is generally conceived of as two groups: (1) Resident microbes belong to a relatively fixed group of microorganisms that is routinely found in the skin, and that reestablishes itself after perturbation. Resident microbes are often considered to be commensal, meaning that the microbes are not harmful and may provide benefit to the host. (2) Transient microbes do not establish themselves permanently on the surface, but rather arise from the environment and persist for hours to days. Resident and transient microbes are not pathogenic under normal conditions if proper hygiene is maintained and if the normal resident flora, immune responses, and skin barrier function are intact. However, after perturbation, resident and/or the transient bacterial populations can colonize, proliferate and produce disease. For example, Staphylococcus epidermidis is a skin commensal but can be an opportunistic pathogen in immunocompromised hosts (Otto, 2009). In addition, Staphylococcus aureus can be a resident microbe in asymptomatic carriers, yet is also an important pathogen.
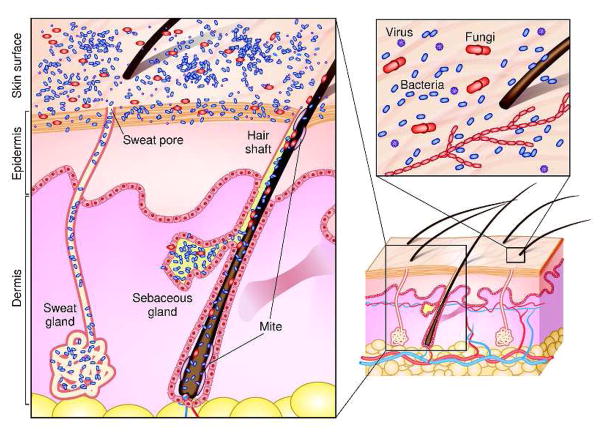
Microbial diversity varies across the niches comprising the average adult human’s 1.8m2 of skin. For example, hairy, moist underarms lie a short distance from smooth dry forearms, but these two niches are ecologically distinct as are their resident microbial communities (Grice et al., 2009). Intrinsic factors including age, genetic makeup, and immune reactivity also influence the composition of skin microorganism communities. Environmental factors such as climate and extrinsic factors like hygiene may also have profound effects on microbial communities. The topography of human skin varies at a microscopic as well as a macroscopic level. Distinct habitats are characterized by differences in skin thickness and folds and densities of hair follicles and glands. Cutaneous invaginations and appendages, including sweat glands (eccrine and apocrine), sebaceous glands, and hair follicles, are likely to be associated with their own unique microbiota (Figure 1). For example, sebaceous glands secrete lipid rich sebum, a hydrophobic coating that protects and lubricates hair and skin. While sebum generally serves as an antibacterial coating, Propionibacterium acnes hydrolyses triglycerides present in sebum, releases free fatty acids that promote bacterial adherence, and then colonizes sebaceous units (Marples et al., 1971). This example shows how humans and their commensal microbial communities have evolved together to provide mutual benefit.
Figure 1. Schematic of skin histology viewed in cross-section with microorganisms and skin appendages.
Microorganisms (virus, bacteria and fungi, mites) cover the surface of the skin and reside deep within the hair and glands.
Early culture-based studies to define the microbial skin residents
Historically, culture-based approaches have been the standard for characterizing microbial diversity. However, some bacteria (including Treponema pallidum, for example) require fastidious growth conditions and are notoriously difficult to isolate. Other bacterial species, e.g. Staphylococcus aureus, grow readily under standard culture conditions and subsequently overcrowd more fastidious bacteria. Comprehensive skin microbial surveys performed decades ago were limited by the ability to provide the appropriate growth conditions required to culture and isolate fastidious microbes. Despite these limitations, pioneering studies used cultivation techniques to identify members of skin microbial communities, including S. epidermidis and other coagulase-negative staphylococci. Other microorganisms that are generally regarded as skin colonizers include those of the phylum Actinobacteria (the genera Corynebacterium, Propionibacterium, and Brevibacterium) and the genus Micrococcus. The most commonly isolated fungal species are Malassezia spp., that are especially prevalent in sebaceous areas (Fredricks, 2001). Demodex mites are microscopic arthropods, which feed on sebum and colonize sebaceous areas of the face (Figure 1). The role of commensal viruses has not been studied, and investigations are currently limited by the available molecular and microbiological means to identify and characterize viruses.
Research characterizing skin microflora was international, including early luminaries such as Mary J. Marples of New Zealand, Albert Kligman and James J. Leyden at the University of Pennsylvania, David Taplin at the University of Miami, and William C. Noble of the University of London. Kligman published one of the earliest comprehensive reviews on the subject in 1954, launching the field with the idea that ‘a great deal more remains to be learned about the forces which control the bacterial ecology of the surface of the skin’ (Pillsbury and Kligman, 1954). We had the opportunity to interview Noble and Leyden about their perspectives on the history of this field.
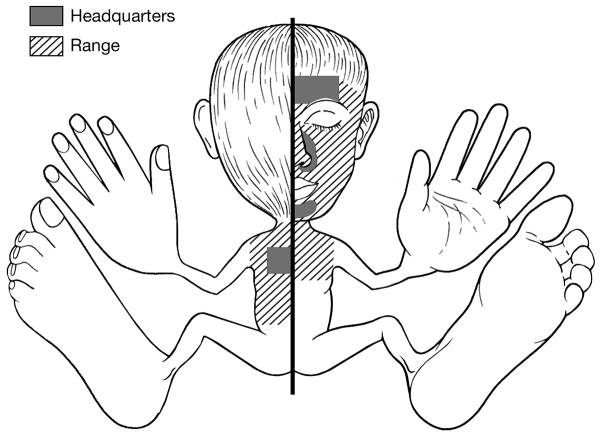
Mary Marples brought ecological theory to the study of skin microflora making the analogy: ‘In the soil the densest populations of microorganisms are in the rhizosphere, the region that surrounds plant roots. The comparable region in the skin is the hair follicle’’(Marples, 1969). In Marples’ one thousand-page ‘The Ecology of the Human Skin’, numerous subjects affecting skin bacterial load and diversity are discussed and depicted with homunculi, scaled models of the human body illustrating the total volume of sweat produced, density and types of hair, regional distribution of eccrine sweat glands. Figure 2 is an adaptation of one of these homunculi, highlighting the body regions are that considered to be the main reservoirs (termed “headquarters”) and the distribution (termed “range”) of Propionibacterium acnes (Figure 2).
Figure 2. Homonculus showing major sites and range of Propionibacterium acnes colonization.
Classic microbiologists swabbed and cultured diverse sites to characterize the predominant sites of colonization (“headquarters”) and general distribution (“range”). Adapted from Marples’ “The Ecology of the Human Skin”.
The research of William C. Noble, editor of ‘The Skin Microflora and Microbial Skin Disease’, contributed to our understanding of how skin and nasal microbes play important roles in the epidemiology of bacterial and fungal infections, particularly in the hospital setting. When Noble completed his PhD in 1964, the field of microbiology in dermatologic conditions was largely confined to atopic dermatitis associated with Staphylococcus aureus and acne with Propionibacterium acnes. “It soon became clear from our own studies and that of others that skin was a microbial habitat in its own right’ (Noble, 1993). In describing his research interests, he stated, ‘It should be evident that the research which I and my… students followed was not always in a straight line. If it was plotted out it would resemble a plant, a stem rooted in dermatology but with many side shoots.’ Noble’s work moved from microbiology to dermatology to public health. Noble described early baseline studies, obtaining samples from representative individuals in Dutch villages to calculate S. aureus nasal carriage. When transporting the thousand bacterial cultures back to England, Noble simply explained the public health relevance to the customs officer, who replied ‘Very good, Sir’. Many things were easier at that time – local nurses went into schools to look for fungus in children’s hair and no one questioned the nurses’ authority with their white coats and a swab in their hand. As an example of how the understanding of skin microbiology impacted public health needs, Noble described frequent outbreaks of erythrasma in a mental health hospital where patients in this overrun facility only had water to bathe once a week – ‘Build a new water tower’ was the simple scientific solution (Somerville et al., 1970). When a senior microbiologist learned that Noble would move to an Institute of Dermatology he remarked ‘You’re a jolly lucky fellow, Noble. There’s so much to do’. And in fact, one of Noble’s salient comments during our interview was on the extent to which he enjoyed tremendous intellectual freedom.
Taplin performed seminal work on skin infections of soldiers serving in the Vietnam War. Not only were these young men exposed to completely novel microbial organisms, they were also exposed to harsh battle conditions. Taplin described foot lesions associated with Pseudomonas cepacia in the toewebs of troops training in swamp water (Taplin et al., 1971). Taplin expanded these findings to characterize and distinguish tropical-immersionfoot versus warm-water-immersion-foot syndrome. Both of these immersion disorders caused a large number of foot casualties among combat forces in Vietnam, but had different clinical presentations, histopathology, and preventative measures (Allen and Taplin, 1973). Taplin recapitulated warm-water-immersion-foot disorder with five volunteers who stood in Florida swamp water for 36 to 72 hours. While recovery generally required 4 to 5 days in hospital, Taplin and colleagues were instrumental in introducing topical applications of silicone grease as an effective prophylaxis against warm-water-immersion-foot.
Leyden had a prolific career working closely with Kligman for many years where “we were simultaneously doing a hundred things.” Underlying their productive research was intellectual freedom, financial support, and, perhaps as importantly, pure enjoyment. They described ecologic shifts in the cutaneous microflora related to long-term antibiotic usage as well as age-related changes. They analyzed a full range of skin changes associated with human life: skin microflora in diaper dermatitis, atopic dermatitis, acne vulgaris, dandruff. Their skin microbiology research was wide-ranging from gram-negative folliculitis as a complication of antibiotic therapy in acne to axillary odor. Leyden and Kligman were prodigious writers, as prominently reviewed in a JID article in 1976 (Kligman et al., 1976). During the interview, Leyden paid homage to other influential investigators in the field of skin microbiology including Peter Williamson, Richard Marples (son of Mary Marples), Gerd Plewig, Bill Noble, Sydney Selwyn, Sam Shuster, Keith Holland, Howard Maibach, and Raza Aly. Leyden recounted to us that at one point an 88-year-old Kligman turned to him and said, “We had a lot of fun, didn’t we?” Leyden responded, “We had more fun than any two people would ever dare to dream. We had a lot of fun. (Everyday was)…like the time of the gold rush. (Any study we) did was a nugget.”
Entering the molecular microbiology era
As these luminaries recognized, microbes flourish in the context of a large community and only a minority of bacteria thrive in isolation. Culture-based techniques essentially select for laboratory ‘weeds’ – species that flourish under the typical nutritional and physiological conditions used by diagnostic microbiology laboratories. These ‘weeds’ may not be the most abundant or influential organisms in the community. For example, hair follicles and sebaceous glands represent anoxic environments, harboring anaerobic microorganisms that are often slow growing and that require special conditions for growth and during sample transport and processing.
The development of molecular techniques to identify and quantify microbial organisms has revolutionized our view of the microbial world and ushered in another “gold rush” in studying skin microbes. Genomic characterization of bacterial diversity relies on sequence analysis of the 16S ribosomal RNA gene, which is present in all bacteria and archaea but not in eukaryotes. The 16S rRNA gene contains variable regions, enabling taxonomic classification, and conserved regions, serving as binding sites for PCR primers. Importantly, an organism does not need to be cultured to determine its type by 16S rRNA sequencing (Dethlefsen et al., 2007; Turnbaugh et al., 2007).
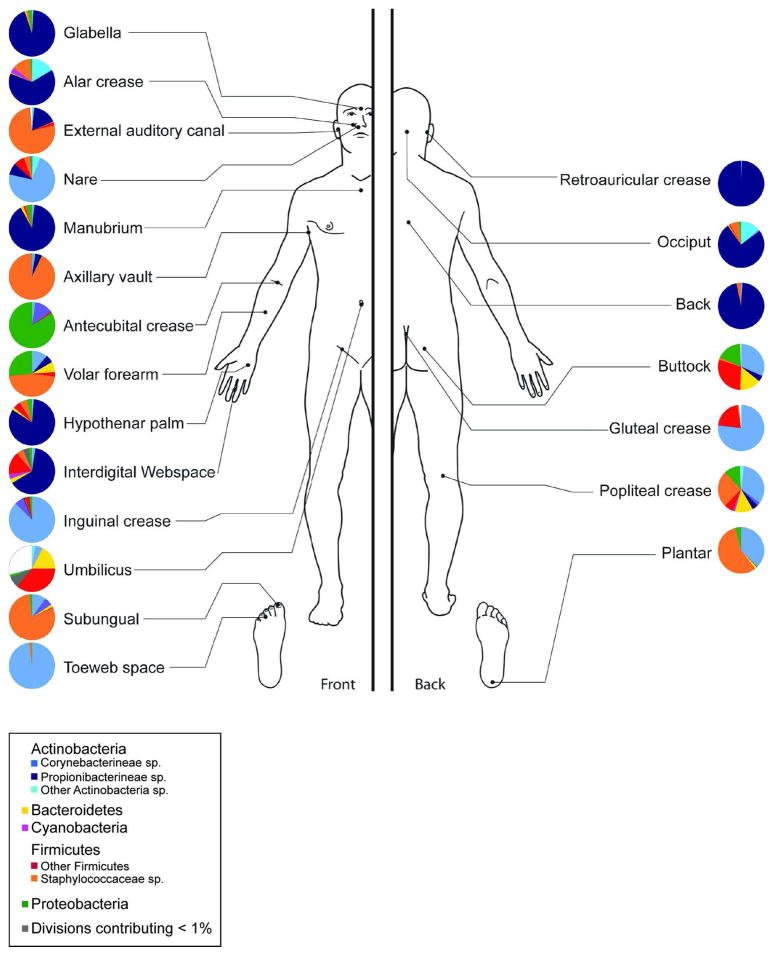
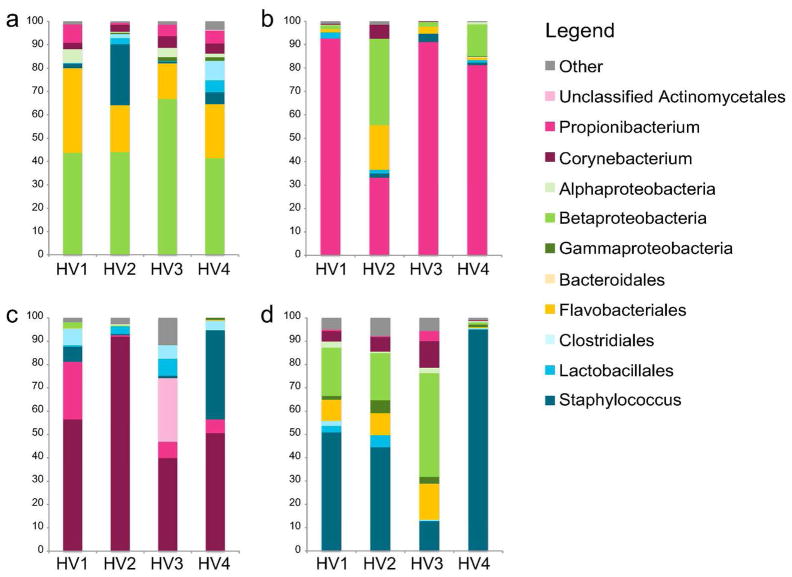
Genomic approaches to characterize skin bacteria have revealed a much greater diversity of organisms than that revealed by culture-based methods, predominantly from four different phyla: Actinobacteria, Firmicutes, Bacteroidetes and Proteobacteria. Our group and others have demonstrated that the proportion of these bacterial phyla is dependent on the physiology of the skin site, with specific bacteria being associated with moist, dry and sebaceous microenvironments (Figure 3) (Costello et al., 2009; Gao et al., 2007; Grice et al., 2009). Propionibacterium spp. are the dominant organisms in sebaceous areas, which confirms classical microbiological studies. Staphylococcus and Corynebacterium spp. are the most abundant organisms colonizing moist areas. The most diverse skin sites are the dry areas, with mixed representation from all four phyla. A surprising feature of the microbiota of dry sites, as captured by molecular analysis, is the abundance of gram-negative organisms, which were previously thought to colonize the skin only rarely, as contaminants from the gastrointestinal tract. As shown in Figure 4, the microbiome of the antecubital fossa, back, nare, and plantar heel are more similar to the same site on another individual than to a different site on the same individual. In this sense, the ecological niche, or skin site, is a greater determinant of the microbiota composition than the individual genetic variation among healthy volunteers. Some sites on an individual are similar, such as different sebaceous regions that share common ecological features. Molecular analysis of skin microbiota has also revealed that the temporal variability of the skin microbiome is dependent on the site sampled. In healthy adults, skin niches including the nares, glabella, and external auditory canal demonstrate relative stability as compared to dry regions such as the inner forearm and the heel. In general, contralateral sites on the same individual are more similar to each other than to a corresponding site on another individual.
Figure 3. Topographical distribution of bacteria on skin sites.
The skin microbiome is highly dependent on the microenvironment of the sampled site. Sebaceous sites are labeled in blue, moist sites are labeled in green and dry surfaces are labeled in red. Family-level classification of bacteria colonizing an individual subject is shown. Data is from Grice et al, 2009.
Figure 4. Interpersonal variation of the skin microbiome.
Characterization of the skin microbiota, as determined by 16S rRNA sequencing, of four sites on four healthy volunteers (HV1, HV2, HV3, HV4) is depicted. Skin microbial variation is more similar dependent on the site than the individual. A. Antecubital crease; B. Back; C. Nare; D. Plantar heel. Data from Grice et al, 2009.
Several studies have assessed skin microbial communities with molecular typing of 16S rRNA genes with interesting results. An early genomics-based skin microbial study demonstrated that the microbial community composition of the palmar surface of the hand was significantly affected by handedness, time since last hand washing, and an individual’s sex (Fierer et al., 2008). The Knight group later examined whether the residual skin bacteria left on objects could be matched to the individual who touched the object (Fierer et al., 2010). The authors showed that skin-associated bacteria identified on inanimate objects could be linked to specific individuals. Investigators have compared the skin microbiome in infants delivered vaginally and by C-section just after birth. While vaginally delivered infants acquired bacterial communities resembling their own mother’s vaginal microbiota, C-section infants harbored bacterial communities more similar to those found on the skin surface (Dominguez-Bello et al., 2010). A recent study of the evolution of the infant skin microbiome demonstrated that cutaneous microbial communities become more diverse with increasing age (Capone et al., 2011).
In 2008, the NIH Common Fund launched an integrated Human Microbiome Project to characterize the microbial diversity of 5 body sites (skin, nares, oral cavity, gut and vagina from women) of 250 healthy volunteers, the largest multi-body site study of the human microbiome to date. This project aims to take advantage of new, high-throughput DNA sequencing technologies to establish a baseline to empower future clinical studies examining associations between changes in the microbiome and health/disease (Peterson et al., 2009). The project has already begun to serve as a tremendous resource for human microbiome investigators with regards to sequence data, technology and analytical tool development, and discussions on the social, legal, and ethical implications of this type of research. Analyses of this large NIH-funded project are expected to be published soon.
Skin disorders associated with microbiota
DNA sequencing studies enable researchers to concurrently search for a microbial contribution toa clinical disorder in the important context of the microbial community. Atopic dermatitis (AD), a chronic relapsing disorder that affects ~15% of US children, is a classic example of a disorder commonly associated with S. aureus microbial colonization and infection. The prevalence of AD has doubled or tripled in industrialized countries over the past three decades with no clear cause. This observation raises the intriguing possibility that environmental differences in industrialized countries, e.g. reduction in parasitic infections, reduction in common childhood diseases, increase in hygiene practices, increase in antibiotic use, etc., modulate the gene–environment interaction on the skin surface, resulting in AD’s increased prevalence. Classic AD manifests at stereotypical sites, including the antecubital fossa and the popliteal fossa, sites that harbor similar organisms when compared to other body sites. Common effective treatments for AD include topical or systemic antibiotics, and steroids as well as dilute bleach baths to lower the bacterial load. Our group and others are currently investigating the interaction among different species of Staphylococcus spp., the microbial communities en toto, and disease course (Hanifin, 2009). Future studies may provide insights into the potential role of S. aureus in the onset of AD and the therapeutic potential of selectively modifying the skin microbiome.
Genomic approaches to studying skin microbes have also been utilized in acne vulgaris and plaque psoriasis. Inflammatory acne vulgaris has been classically associated with Propionibacterium acnes. 16S-based sequencing has been used to study acne vulgaris, indicating that healthy follicles harbor P. acnes exclusively and acne lesions harbor a combination of S. epidermidis, Corynebacterium spp., and predominant P. acnes. Future skin microbiome studies of acne vulgaris may expand our understanding of how P. acnes behaves within communities of skin bacteria. In light of the common use of topical and systemic antibiotics in the treatment of acne, investigations in the acne skin microbiome may provide a deeper understanding of the mechanisms of antibiotic-resistance in cutaneous microorganisms. Psoriasis has also been studied using 16S-based sequencing. Lesional skin from plaque psoriasis patients exhibited increased bacterial diversity, including increased Streptococcus and less P. acnes, than skin from healthy controls and nonlesional skin in psoriasis patients (Gao et al., 2008).
Chronic wounds, affecting diabetic, elderly, and immobile individuals, are an example where commensal skin organisms invade and become pathogenic after breaching the skin barrier. Although bacteria do not cause the initial wounding event, they are thought to contribute to the lack of healing and persistent inflammation that is associated with chronic wounds. However, molecular studies have not yet identified a unique organism that colonizes wounds of the same etiology (for example, in diabetic foot ulcers or venous leg ulcers). This is in contrast to burn wounds, in which a particular microbial species, such as Streptococcus pyogenes, Enterococcus spp. or Pseudomonas aeruginosa is usually readily identifiable (Grice et al., 2010; Martin et al., 2010).
There is a general agreement that microorganisms likely contribute to various skin disorders. Much remains to be learned about the possible role of microbes in skin diseases and how microbial communities interact with underlying genetic and environmental variations to potentially lead to human diseases. Many common skin disorders are associated with certain age groups, a specific body distribution, and/or particular microorganisms. Whether this specificity is driven by the endogenous microbial community structures remains to be determined.
Symbionts, commensals or neutral bystanders
Another question is whether indigenous skin microorganisms provide some benefit to the host, and whether these microbes are truly symbiotic, or commensal. Skin microbes contribute to the skin innate defense by producing antimicrobial peptides. In a recent example of host and microorganism cooperating to combat invasion by pathogens, the commensal skin bacteria S. epidermidis was shown to inhibit nare colonization and biofilm formation by S. aureus. In combination, a subset of S. epidermidis expressing an endopeptidase and an antimicrobial peptide synergistically interfered with S. aureus colonization (Iwase et al., 2010). This example raises several important points for consideration, including complex microbial interactions and potential co-evolution of the host and resident microorganisms. In this 75th anniversary series, Richard Gallo reviews the relationship between the commensal microbes, potential pathogens, and the host response including immune cells and expression of innate defense proteins [REF needed]. His laboratory has elegantly demonstrated that commensal S. epidermidis affects inflammation and enhances keratinocytes’ expression of antimicrobial peptides to augment skin defense against infection (Lai et al., 2010; Lai et al., 2009). As our understanding of the roles of skin microbes expands, we may identify functions of skin microorganisms beyond contributing as protectors against pathogens. Skin microbes may educate our skin immune system analogous to that observed with effects of gut microbes on the development of the gut-associated lymphoid tissue in germ-free mice.
Further challenges: defining the functional potential of the skin microbiome
Although molecular approaches to surveying bacterial diversity provide a less biased representation of skin microbiota than culture-based assays, they are not without their limitations. First and foremost, DNA sequencing does not distinguish living from dead organisms. Thus, molecular studies are technically surveying the history of the skin microbiota. Since skin is an organ that is exposed to the environment, it is difficult to determine which of the identified species are transient and which species are members of the resident community. Moreover, 16S rRNA gene sequencing cannot distinguish between methicillin-sensitive and methicillin-resistant Staphylococcus spp. isolates – a critical piece of clinical information. Future genomic studies will move from 16S rRNA surveys of skin bacteria to 18S rRNA surveys of fungi and then on to full metagenomic sequencing, simultaneously assessing the full genetic material of the microbial community. The current limitations of metagenomics are both the limited DNA starting material and the development of computational methods to analyze the complex results.
Antimicrobial therapies are a mainstay of dermatologic practice, but they have associated risks that are not fully characterized. Recent studies of the gut microbial community have demonstrated that treatment with ciprofloxacin resulted in profound and rapid loss of microbial diversity. Some but not all members of the microbial community began to recover within a week after completion of treatment. However, even after 10 months, the gut never returned completely to its baseline composition, suggesting that antibiotic perturbation may shift the community to an alternative state (Dethlefsen and Relman, 2011). Expanding on this idea, vancomycin-resistant enterococci (VRE) were able to displace the normal recovering commensal organisms if mice were pre-treated with antibiotics. Similarly in human guts, colonization by VRE preceded bloodstream infection in patients undergoing transplantation (Ubeda et al., 2010). These findings raise additional questions for skin biology, including exploring the impact of topical, repeated, or continuous use of antibiotics on microbial community stability and on beneficial microbes.
As the current arsenal of effective antimicrobial weapons against potential pathogens is gradually diminished by antibiotic resistance, it is vital to explore not only antimicrobial chemicals derived from microorganisms, but also the possibility that prebiotics and probiotic microbial organisms themselves may offer promise as viable alternatives. Our current society paradoxically strives to colonize our guts with probiotic yogurts, but sterilize our skin with hand sanitizers. Rather than direct skin application of live bacterial cultures, we may need to identify emollients or other bacterial products to promote the growth of commensals. With continued expansion of our knowledge of the skin microbiota, these questions will guide future research efforts directed towards understanding the complex interactions governing the host–microorganism relationship.
References
- Allen AM, Taplin D. Tropical immersion foot. Lancet. 1973;2:1185–9. doi: 10.1016/s0140-6736(73)92949-8. [DOI] [PubMed] [Google Scholar]
- Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the human skin microbiome early in life. J Invest Dermatol. 2011;131:2026–32. doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001;6:170–4. doi: 10.1046/j.0022-202x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–8. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105:17994–9. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N, Lauber CL, Zhou N, McDonald D, Costello EK, Knight R. Forensic identification using skin bacterial communities. Proc Natl Acad Sci U S A. 2010;107:6477–81. doi: 10.1073/pnas.1000162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks DN. Microbial ecology of human skin in health and disease. J Investig Dermatol Symp Proc. 2001;6:167–9. doi: 10.1046/j.0022-202x.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104:2927–32. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, et al. A diversity profile of the human skin microbiota. Genome Res. 2008 doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–53. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Liechty KW, Segre JA. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A. 2010;107:14799–804. doi: 10.1073/pnas.1004204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320–2. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–9. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Leyden JJ, McGinley KJ. Bacteriology. J Invest Dermatol. 1976;67:160–8. doi: 10.1111/1523-1747.ep12513007. [DOI] [PubMed] [Google Scholar]
- Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med. 2011;17:320–8. doi: 10.1016/j.molmed.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–21. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, McGinley KJ, Nordstrom KM, Webster GF. Skin microflora. J Invest Dermatol. 1987;88:65s–72s. doi: 10.1111/1523-1747.ep12468965. [DOI] [PubMed] [Google Scholar]
- Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol. 2003;121:231–41. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- Marples MJ. Life on the human skin. Sci Am. 1969;220:108–15. doi: 10.1038/scientificamerican0169-108. [DOI] [PubMed] [Google Scholar]
- Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol. 1971;56:127–31. doi: 10.1111/1523-1747.ep12260695. [DOI] [PubMed] [Google Scholar]
- Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol. 2010;130:38–48. doi: 10.1038/jid.2009.221. [DOI] [PubMed] [Google Scholar]
- Noble WC. Skin Microblora and Microbial Skin Disease. Cambridge University Press; 1993. [Google Scholar]
- Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555–67. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH Human Microbiome Project. Genome research. 2009;19:2317–23. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillsbury DM, Kligman AM. Modern Trends in Dermatology, 2nd series. London: Butterworth; 1954. Some current problems in cutaneous bacteriology; pp. 187–213. [Google Scholar]
- Somerville DA, Seville RH, Cunningham RC, Noble WC, Savin JA. Erythrasma in a hospital for the mentally subnormal. Br J Dermatol. 1970;82:355–60. doi: 10.1111/j.1365-2133.1970.tb06832.x. [DOI] [PubMed] [Google Scholar]
- Taplin D, Bassett DC, Mertz PM. Foot lesions associated with Pseudomonas cepacia. Lancet. 1971;2:568–71. doi: 10.1016/s0140-6736(71)92150-7. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycinresistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–41. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]