Abstract
Xeroderma pigmentosum (XP) is a rare, autosomal recessive disorder of DNA repair characterized by sun sensitivity and ultraviolet (UV) induced skin and mucous membrane cancers. Described in 1874 by Moriz Kaposi in Vienna, nearly 100 years later James Cleaver in San Francisco reported defective DNA repair in XP cells. This eventually provided the basis for a mechanistic link between sun exposure, DNA damage, somatic mutations and skin cancer. XP cells were found to have defects in 7 of the proteins of the nucleotide excision repair pathway and in DNA polymerase eta. XP cells are hypersensitive to killing by UV and XP cancers have characteristic “UV signature” mutations. Clinical studies at NIH found a nearly 10,000-fold increase in skin cancer in XP patients under age 20 years demonstrating the substantial importance of DNA repair in cancer prevention in the general population. About 25 % of XP patients have progressive neurological degeneration with progressive loss of neurons, probably from DNA damage induced by oxidative metabolism which kills non-dividing cells in the nervous system. Interestingly, patients with another disorder, trichothiodystrophy have defects in some of the same genes as XP but they have primary developmental abnormalities without an increase in skin cancer.
PROLOGUE FABLE
Imagine it’s the late 1800’s. An infant is taken outside on the first sunny day of spring. After a short time, the child becomes irritable and starts crying. By the next morning her skin becomes very red and blistered with eyes swollen shut. Parents wonder what sort of malady this could be? Slowly the skin and eyes heal. But it happens again and again. Strong sunlight seems to bother her eyes which are often red and watering. Just past her first birthday, her skin starts to develop freckle-like spots, mostly in areas not covered by clothing. In older childhood growths develop and turn into tumors that seem to eat through her skin. She doesn’t always respond when called as if she were becoming deaf, she is having more difficulty walking and her thinking has become quite slow.
We now know xeroderma pigmentosum (XP) as a rare autosomal recessive disorder of DNA repair, which manifests clinically as photosensitivity, actinic damage to the skin, cancer of UV exposed areas of the skin and mucous membranes of the eyes and mouth, and in some patients, progressive neurologic degeneration (Table 1). The skin is normal at birth and the disorder may present in two different ways. Some patients have an exaggerated response to UV exposure with pronounced burning and blistering on minimal exposure to sunlight (Figure 1A). Others have a normal acute response to sun exposure. However, they all develop freckle-like pigmentary changes in sun exposed areas which eventually appear as poikiloderma (hyperpigmentation, hypopigmentation, atrophy and telangiectasias). Unlike children in the general population, this freckling (lentiginous hyperpigmentation) typically appears before the age of 2 years (Figure 1B). The average age of first skin cancer is less than 10 years (Figure 2A).
Table 1.
COMPARISON OF FEATURES OF XERODERMA PIGMENTOSUM, TRICHOTHIODYSTROPHY AND COCKAYNE SYNDROME
| FEATURE | XERODERMA PIGMENTOSUM (XP) |
XP WITH NEUROLGIC ABNORMALITIES |
TRICHOTHIO- DYSTROPHY (TTD) |
COCKAYNE SYNDROME (CS) |
XP/CS COMPLEX |
|---|---|---|---|---|---|
| SKIN | |||||
| Skin sun sensitivity | yes | severe | yes / no | yes | yes |
| Lentiginous skin pigmentation | yes | yes | no | no | yes |
| Sunlight - induced skin cancer | yes | yes | no | no | yes |
| EYES | |||||
| Photophobia | yes | yes | yes/ no | yes | yes |
| Conjunctival growths | yes | yes | no | no | yes |
| Cancer (anterior eye / lids) | yes | yes | no | no | not reported |
| Congenital cataracts | no | no | yes | yes | no |
| Pigmentary retinal degeneration | no | no | no | yes | yes |
| SOMATIC | |||||
| Short stature | no | no / yes | yes | yes | yes |
| Immature sexual development | no | no | no / yes | yes | yes |
| NERVOUS SYSTEM | |||||
| Progressive sensorineural deafness | no | yes | no | yes | yes |
| Developmental delay | no | yes | yes | yes | yes |
| Progressive neurological degeneration | no | yes | unknown | yes | yes |
| Primary neuronal degeneration | no | yes | no | no | no |
| Dysmyelination of brain | no | no | yes | yes | yes |
| Cerebral atrophy | no | yes | no/ yes | yes | yes |
| Cerebellar atrophy | no | yes | no | yes | yes |
| Calcifiation (basal ganglia) | no | no | no/ yes | yes | yes |
| DISEASE MECHANISM | |||||
| Reaction to exogenous or endogenous DNA damaging agents | yes - severe | yes - severe | no | yes | yes |
| Developmental defect | no | yes | yes - severe | yes - severe | yes - severe |
| Nucleotide excision repair defect | yes | yes | yes | yes | yes |
| Molecular defects | XPA, XPC, XPD, XPE, XPF, XPG, XP VARIANT (POLH) | XPA, XPB, XPD, XPF, XPG | XPB, XPD, TTDA, TTDN1 | CSA, CSB | XPB, XPD, XPG |
Figure 1.
XP and TTD patients studied. a) Patient XP420BE complementation group XP-D at 9 months of age with severe blistering erythema of the malar area following minimal sun exposure. Note sparing of her forehead and eyes that were protected by a hat. b) Patient XP358BE (XP-C) at age 2 years did not sunburn easily but developed multiple hyperpigmented macules on her face. A rapidly growing SCC or keratoacanthoma grew on her upper lip and a pre-cancerous lesion appeared on her forehead. c) Northern African patient XP393BE (XP-C) (Mahindra et al. 2008) at age 23 years with numerous hyperpigmented macules on his face. Nodular basal cell cancer is present on his left nasal root. Pigmented basal cell cancer is present on his left cheek. His eyes show cornea scarring from unprotected sun exposure. d) Patient XP19BE (XP-A) (Robbins et al. 1991) at age 35 years with neurological degeneration. He has numerous hyperpigmented macules on sun exposed areas of his face and neck. Progressive sensorineural deafness requires use of a hearing aid. Images a–d from (Bradford et al. 2011) e. Corneal clouding, pterygium, contact lens, loss of lashes on lower eyelid. f. Sharp demarcation between the poikilodermatous changes seen in sun exposed skin compared to double covered area of the buttocks of 35 year old XP patient. g. Loss of vermillion border of the lips with prominent telangiectasias and scaring of the lips and anterior tongue. h: squamous cell carcinoma of the anterior tongue in African man (from (Mahindra et al. 2008)). i–j Clinical appearance of TTD. i : 3 yo girl with short brittle hair which is sparse and broken off at different lengths. She rarely has haircuts except to trim uneven areas. She has a smiling, outgoing personality typical of TTD. j. Tiger tail banding under polarizing microscopy (Original magnification × 10.) (Images i–j from (Liang et al. 2005).
Figure 2.
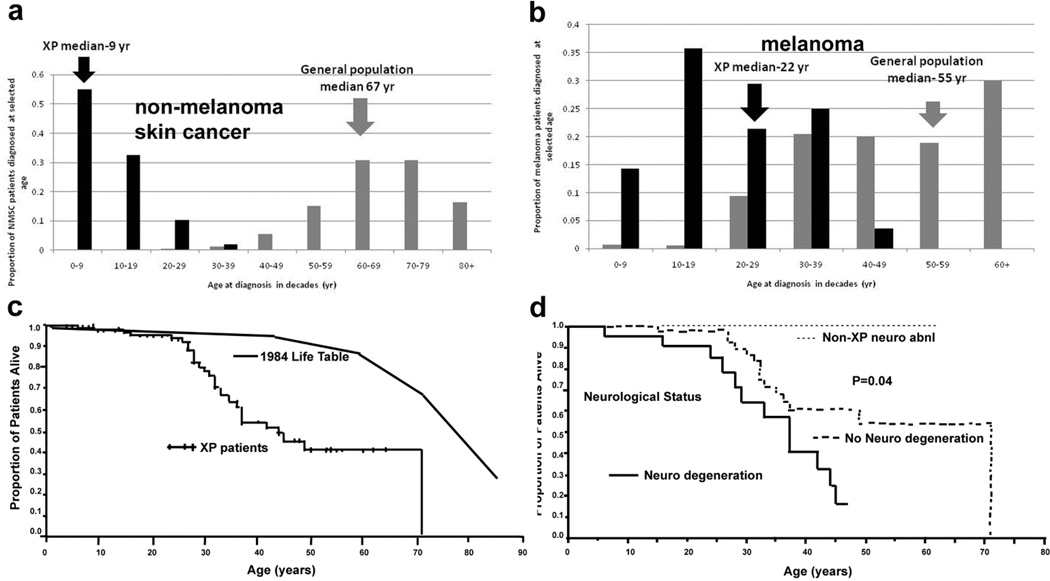
XP skin cancer by age at first skin cancer diagnosis and skin cancer type and mortality compared to U.S. general population. a. Proportion of NMSC patients diagnosed at selected ages. b. Proportion of melanoma patients diagnosed at selected ages. Individuals with both NMSC and melanoma were used for both analyses. General population data taken from (Glass and Hoover 1989). c. Kaplan Meier curve of xeroderma pigmentosum patient survival compared to US general population: 30% of XP patients had died by age 32. The survival of the XP patients was significantly less than the general population (p<0.001). d. Kaplan Meier curve of xeroderma pigmentosum patient survival stratified by neurologic phenotype. Patients with neurologic degeneration had poorer survival rates than those without neurologic degeneration (p=0.04). (Graphs from (Bradford et al. 2011)).
Our evolving understanding of the puzzling clinical findings of XP have raised many questions related to cell biology, photobiology, photo-carcinogenesis, neurodegeneration, and genome stability. Answering the questions has illuminated several areas of biology but there are still many yet to be clarified.
HISTORY OF XERODERMA PIGMENTOSUM
The road to our current understanding of XP starts in the late 19th century with Moriz Kaposi, the Hungarian-born professor of dermatology in Vienna. In 1874, Kaposi described four patients with xeroderma or “parchment skin” in the early textbook of dermatology (Hebra and Kaposi 1874) which he wrote with Professor Ferdinand Hebra, his father-in-law (Kraemer et al. 1987). “ In addition to the parchment-like dryness, thinness, and wrinkling of the epidermis, the checkered pigmentation, and the small dilatations of the vessels, the most remarkable symptoms were the contraction and, at the same time, thinning of the skin.”, features designating poikiloderma. His description distinguishes the pigmented lesions from normal as “dark brown, pigmented spots resembling those of freckles”, recognizing the clinical distinction between freckles and clinically atypical pigmented lesions (e.g., lentigines) (Figure 1B,C,D and F). He notes that the “condition of the skin ceased with an abrupt line of demarcation” (Figure 1F) at the upper third of the arm”, but does not postulate why. He mentions the occurrence of a pear-shaped, red, granulating, fissured tumor that had developed within one year, and states “We recognised the growth to be an epithelioma, and destroyed it in great part.” However, his puzzlement was evidenced by his statement “ I have no more to say respecting this peculiar disease…”
In 1883, Albert Neisser of Breslau, Germany reported XP with neurologic abnormalities in two siblings who had XP with progressive neurologic degeneration beginning in the second decade (Neisser 1883). Today it is recognized that about 25% of XP patients in the US develop progressive neurologic degeneration (Bradford et al. 2011) and Table 1 and Figure 1D.
In 1878, R. W. Taylor, MD of New York reported the first XP patients in the US at the inaugural meeting of the American Dermatological Association. In1888 he reviewed the world literature and reported a total of 40 cases (Taylor 1888).
The state of understanding of the disease was proposed in a 1926 report as follows (Per 1926).
Xeroderma pigmentosum is due to an extreme sensitization of the skin to ultra-violet rays of the sun.
This congenital insufficient resistance of the skin to the actinic rays seems to be dependent on the consanguinity of the parents.
Photodynamic substances in the organism (haematoporphyrin) do not play any part in the pathogenesis of this disease.
The actinic rays of the sun produce an unquestionably unfavourable influence on the course of xeroderma pigmentosum.
Medical preventive measures have a high value in this disease. However, notwithstanding detailed pathological studies of the disease … no one has succeeded in making clear the obscure pathogenesis of this disease.”
In 1932, de Sanctis and Cacchione described 3 brothers in the same family with features of XP, mental deficiency, dwarfism and gonadal hypoplasia with progressive neurologic degeneration beginning at 2 years of age (de Sanctis and Cacchione 1932). This severe phenotype is not commonly observed. XP was described in a black African in 1938 (Loewenthal and Trowell 1938) (Figure 1C) and in an American black in 1940 (King and Hamilton 1940).
Gartler reported ultraviolet hypersensitivity of XP cells in 1964 (Gartler 1964) but the importance of this report was not recognized for years. DNA repair abnormalities in XP were brought to the attention of the general scientific community by Cleaver’s report in 1968 describing deficient excision repair in cultured skin fibroblasts (Cleaver 1968). Stable DNA photoproducts were identified by Setlow (Setlow and Setlow 1962). These photoproducts were not removed by XP cells (Setlow et al. 1969; Cleaver and Trosko 1970). XP cells were reported to be defective in the excision repair pathway by which UV damage to DNA is repaired in vitro (Reed et al. 1969) and also in vivo (Epstein et al. 1970). The excision repair proficient form of XP was described in 1971(Burk et al. 1971) and subsequently named “XP Variant” (Cleaver 1972). Cell fusions studies (1972) demonstrated heterogeneity of the XP molecular defects (De Weerd-Kastelein et al. 1972). Fusion of fibroblasts from different XP patients to form heterokaryons (a cell with nuclei from different patients) was found to exhibit correction (complementation) of the defective repair: The different nuclei supplied what the other was lacking to correct the defect. This implied that different cells had different defects and led to the characterization of different complementation groups A through G. (Kraemer et al. 1975a; Kraemer et al. 1975b; Arase et al. 1979; Keijzer et al. 1979) (Figures 3 and 4). Two decades of research and advances in molecular biology, including generation of rodent cell complementation groups and yeast mutants, finally yielded the genes responsible for the different complementation groups : XPA (Tanaka et al. 1990), XPB/ERCC3 (Weeda et al. 1990a; Weeda et al. 1990b), XPC (Legerski and Peterson 1992), XPD/ERCC2 (Flejter et al. 1992a; Flejter et al. 1992b), XPE/DDB2 (Dualan et al. 1995), XPF/ERCC4 (Sijbers et al. 1996), XPG/ERCC5 (Mudgett and MacInnes 1990), and XP VARIANT (polymerase eta) (Johnson et al. 1999; Masutani et al. 1999) (Figure 3 and 4 and Table).
Figure 3.
Nucleotide excision repair (NER) pathway. Transcription coupled repair (TCR) removes damage from actively transcribing genes while global genome repair (GGR) removes damage from the remainder of the genome. In GGR damage such as ultraviolet induced cyclobutane pyrimidine dimers (CPD) or 6-4 photoproducts (6-4 PP) are recognized by proteins including the XPE (DDB2) and XPC gene products. In TCR, the lesion appears to block the progress of RNA polymerase II in a process involving the CSA and CSB gene products. Following initial damage recognition the pathways converge. The XPB (ERCC3) and XPD (ERCC2) helicases unwind the region surrounding the lesion along with the XPA and XPG (ERCC5) gene products, and replication protein A (RPA). The XPF and XPG (ERCC5) endonucleases perform incisions to remove the lesion in a fragment of about 30 nucleotides. The resulting gap is filled in by de novo DNA synthesis. This system is coordinated so that if one part of the pathway is mutated the entire pathway fails to function normally. Mutations in the genes in rectangles have been associated with clinical disease. This diagram is modified from (Van Steeg and Kraemer 1999; Kraemer et al. 2007).
Figure 4.
DNA repair diseases - relationship of clinical disorders (red rectangles) to molecular defects (gray ovals) in DNA repair diseases. Ten clinical diseases and 13 molecular defects are represented. One disease may be caused by mutations in several different genes. Conversely, different mutations in one gene may result in several different clinical diseases. Modified from (Kraemer 2004; Kraemer et al. 2007).
WHAT HAVE WE LEARNED ABOUT XP AND WHAT HAS XP TAUGHT US ABOUT BASIC BIOLOGY?
Relationship of Sun Exposure to Skin Cancer Epidemiologic studies in the normal population have been used as evidence to support a role for sunlight as a cause of skin cancer. For example, a higher frequency of skin cancer reported in a) Caucasians with light colored skin and eyes and frequent sunburns, b) individuals with large outdoor exposures such as sunbathers and outdoor laborers, c) association with latitudes closer to the equator, and d) exposed areas of the body compared to covered areas. However, this relationship is most clearly and powerfully demonstrated in XP patients, where UV damage leads to an early onset and increased frequency of both non-melanoma skin cancer (NMSC) and melanoma. In XP patients, the median age of first NMSC was 9 years (Bradford et al. 2011) compared to 67 years in the general population (Figure 2A). Similarly, the median age of first XP melanoma was 22 years, compared to 55 yr in the general population (Figure 2B). This highlights the profound role of an intact DNA repair system in providing protection against skin cancer, in effect, giving the average Caucasian individual more than half a century delay in the onset of skin cancer.
XP patients can develop hundreds of skin cancers. Compared to the general population, XP patients under age 20 years have a 10,000- fold increase in the frequency of NMSC, 2000-fold increase in melanomas, a 1000-fold increase in cancer of the sun exposed tissues of the eye and 100,000-fold increase tongue cancers (Kraemer et al. 1994; Bradford et al. 2011). The anatomic distribution of NMSC in XP patients is similar to that in the general population with over 80% occurring on the face, head and neck (Kraemer et al. 1994). The distribution of melanomas is different from that of NMSC in XP patients and in the general population. Melanoma occurs more commonly on the extremities and in both groups more than 45% of melanomas were found on the extremities. This suggests that there are different mechanisms involved in the generation of melanoma versus NMSC. The similarity of the distribution of both melanomas and NMSC in both groups suggests that the mechanism of carcinogenesis in XP patients mirrors that in the general population. However, the reversal in median age of onset of NMSC and melanomas in XP patients (9 years and 22 years) in comparison to the general population (67 years and 55 years) (Figure 2A and B) points to a greater role of sun exposure/ DNA repair in induction of NMSC.
The UV exposed areas of the skin, tongue and eye have a high cancer risk (Figure 1C, E, G and H). While there is an increased risk to the anterior portion of the eye (Ramkumar et al. 2011), the lens is a barrier to UV penetration and acts as protection to deeper eye structures. Similarly, the UV exposed areas of the lips and tongue have an increased cancer risk compared to deeper, more shielded mucous membrane surfaces. The observation that covered areas of the skin and other tissues are highly protected in XP patients (Figure 1F), with lower cancer frequencies, demonstrates the benefit of UV protection in the prevention of sun induced malignancy.
XP patients under age 20 years have an approximately 50-fold increase in cancers of the brain and other central nervous system (Kraemer et al. 1994). These include brain medulloblastoma (Giannelli et al. 1981), glioblastoma, spinal cord astrocytoma (DiGiovanna et al. 1998) and Schwannoma. These are not sunlight exposed tissues and the relationship of these cancers to DNA damage is not known. On the other hand, carcinogens in cigarette smoke bind to DNA and cause the type of damage that would be repaired by the NER system in normal cells (Maher et al. 1977; Maher et al. 1987). Thus XP patients are at greater risk of smoking induced cancer. A 34 year old smoker with XP died of lung cancer (Kraemer et al. 1994). In a study of 106 XP patients followed at the NIH for almost 4 decades, the median age at death of the XP patients was 32 years, a significant reduction compared to the general population (Figure 2C) (Bradford et al. 2011). The median age at death of XP patients with neurologic degeneration (29 years) was younger than those patients who had no neurological degeneration (37 years) (Figure 2D). Neurological degeneration was second only to cancer in cause of death in XP patients.
Role of Sun Burning in the Development of UV Induced Skin Damage and Skin Cancer
Studies in the general population have dissociated the role of acute burning versus chronic lower exposures in the causation of skin cancer. The chronic exposure of light skinned, outdoor workers has been associated with the development of multiple basal cell carcinomas and squamous cell carcinomas. In contrast, acute blistering burns in childhood have been implicated as a cause of melanoma. However, experience with XP demonstrates that this relationship is more complex. XP typically presents as either of two diverse clinical scenarios. A young child of 1–2 years of age outdoors in a relatively shady environment can develop an alarming blistering or oozing eruption (Figure 1A). The onset of the eruption may be delayed for a day or so and may be misdiagnosed as impetigo. After repeated occurrences the parents learn to rigorously protect the child. Other children with XP do not burn after minimal sun exposure (Figure 1B). However, most XP patients do develop early onset freckling before the age of 2 years. XP children who do not burn but only freckle, may not utilize rigorous sun avoidance and paradoxically may accumulate more sun exposure and often develop skin cancers in early childhood (Bradford et al. 2011). It is somewhat surprising that in the general population, blistering burns are associated with earlier onset of melanoma, while in XP, this is reversed. XP patients who never burned on minimal sun exposure were found to be significantly more likely to develop skin cancer at an earlier age than those who always or sometimes burned on minimal sun exposure (Bradford et al. 2011).
XP patients with defects in complementation groups A, B, D and G tend to have blistering burns on minimal sun exposure; while those in groups C, E and variant do not (Figures 3 and 4 and Table 1). However, all are at high risk to develop early onset freckling, lentigines and skin cancers. These observations dissociate the acute burning from the mechanism of UV carcinogenesis raise important unanswered questions about how the different abnormalities of DNA repair lead to increased cancer risk in all, but acute photosensitivity only in some. Clearly, the inflammatory reaction of acute burning is not necessary for the development of skin cancer in XP patients.
A Model of Photoaging?
Photoaging, or dermatoheliosis, describes changes to the skin from chronic exposure to the sun or UV radiation. This includes pigmentary changes and alterations in texture and color. Early damage to melanocytes appears as freckling (tan, symmetrical, round macules) and later as lentigines (colored variable intensity of brown with irregular shapes, sizes and borders). In contrast, solar elastosis gives the skin a bumpy, yellowed appearance and is thought to be secondary to damage to structural components of the dermis including elastic and collagen fibers. Telangiectasias are the vascular components of UV damage and atrophy becomes noticeable when the full spectrum of poikilodermatous changes are present. These changes are common in the sun exposed areas of light skinned Caucasians in the general population who have sustained excessive, chronic UV damage, where skin laxity, sagging, and wrinkles are prominent. In contrast, while patients with XP develop freckling at an early age followed by the development of large numbers of lentigos and telangiectasias, they do not develop the skin laxity, sagging, wrinkles or cutis rhomboidalis of the posterior neck. As described by Kaposi in 1874 (Hebra and Kaposi 1874), they actually develop skin tightening-the opposite of wrinkling. This contrast dissociates the mechanisms causing the pigmentary and vascular changes (DNA damage in the epidermis and upper dermis) from the causes of damage to structural components of dermal elastic and collagen fibers. This may be explained in part by the absorption of shorter wavelength, DNA damaging, UVB by the epidermis and greater penetration of UVA into the deeper dermis with a direct damaging effect on protein. The degree of elastosis may serve as a “dosimeter” of the amount of UV reaching the dermis (Robbins et al. 1974). Thus XP patients demonstrate severe epidermal changes with minimal UV exposure to the proteins in the dermis because of their defective repair of epidermal DNA damage.
A Model for Clinical Research: Chemoprevention of Skin Cancers
Because of the high frequency of skin cancers in XP patients and the associated morbidity, effective chemoprevention approaches would convey enormous benefit. In fact, XP has been used as a model for skin cancer chemoprevention studies. Since each patient may develop large numbers of new skin cancers significant differences may be observed with small numbers of patients. A trial of oral isotretinon conducted with only 7 XP patients demonstrated a statistically significant (63%) reduction in new skin cancers compared to the 2-year interval before treatment (Kraemer et al. 1988). This controlled clinical trial was one of the first to conclusively demonstrate effective chemoprevention of any cancer in humans. Today isotretinoin and the related retinoid acitretin are widely used in patients at high risk of developing new skin cancers who have other predisposing conditions including post-transplantation and the nevoid basal cell carcinoma syndrome. T4 endonuclease V, a bacterial DNA repair enzyme, was also tested in a double-blind study of 20 XP patients and found to lower the rate of actinic keratoses and basal cell carcinoma (Yarosh et al. 2001).
Specificity in Sensitivity to Damaging Agents
One of the lessons learned from XP is that patient hypersensitivity to damaging agents is specific. While XP cells are hypersensitive to killing by UV they have normal killing after x-rays. In normal cells the bulky DNA damage caused by UV is repaired by the NER system which is defective in XP patients (Figure 3). Most of the X-ray damage is different and the X-ray repair systems are normal in XP cells. In fact, patients with XP who develop inoperable eye or internal tumors such as brain or spinal cord tumors have been treated with high dose x-irradiation as therapy and tolerated the treatment well (Grier 1919; Giannelli et al. 1981; DiGiovanna et al. 1998). This is in contrast to patients who are hypersensitive to x-irradiation, such as patients with the nevoid basal cell carcinoma syndrome (NBCC). NBCC patients have a germline mutation in the PATCH gene, and their cells retain only one of the two normally present functional alleles. Basal cell carcinomas result when NBCC cells sustain a second hit, which can be the result of x-irradiation. NBCC patients who develop neuroblastoma at a young age and receive treatment with radiation therapy frequently develop large number of basal cell carcinomas in the radiation port, where they may also be at risk for development of additional central nervous system tumors (Kleinerman 2009). These observations clearly highlight the differences in mechanisms of repair of UV induced versus x-irradiation induced DNA damage.
DNA Repair - Molecular Mechanisms of Carcinogenesis
The skin of XP patients is hypersensitive to sun exposure and this is reflected in a hypersensitivity of cultured skin fibroblasts following exposure to UV radiation (Ruenger et al. 2008; Kraemer and Ruenger 2008). Thus examination of cultured cells from XP patients provides an opportunity to obtain insights into detailed mechanism of the relationship of UV damage to carcinogenesis. For example, cells from XP patients are hypersensitive to killing by UV and by UV-mimetic chemical compounds such as benzo-a-pyrene in cigarette smoke (Maher et al. 1977; Maher et al. 1987; Kraemer and Ruenger 2008). In addition, XP cells are hypermutable following UV exposure thereby linking sun exposure to somatic mutations.
UV exposure of DNA produces several types of stable dipyrimidine nucleotide photoproducts (Kraemer and Ruenger 2008). The major photoproduct is the cyclobutane pyrimidine dimer (CPD) of adjacent thymines (T), cytosines (C) or mixed T and C. Also formed are 6-4 pyrimidine-pyrimodone TC photoproducts (6-4PP). These DNA lesions serve as substrates for the nucleotide excision repair (NER) pathway (Figure 3). In normal cells the DNA distorting 6-4PP are repaired more rapidly (within 6 h) than the CPD (about 50% removed by 12 h). Neither photoproduct is repaired by XP cells. Unrepaired photoproducts are pre-mutagenic lesions. During replication, a DNA polymerase meeting an unrepaired photoproduct can stop replication – leading to cell death. Since the photoproduct distorts the nucleotides they do not code properly. Polymerases that bypass the photoproducts frequently incorporate the incorrect nucleotide (for example incorporating a T in place of a C that is involved in a TC photoproduct) (Lange et al. 2011). This leads to a C to T mutation which is characteristic of UV mutagenesis. In cultured XP cells and UV treated plasmids grown in XP cells these C to T or CC to TT “UV signature mutations” are frequently found after UV exposure (Bredberg et al. 1986; Gozukara et al. 1994).
If purified plasmid DNA is exposed to UV and transfected (introduced) into cells, the plasmid DNA is subject to repair by the DNA repair processes of the cell. The efficiency of repair can be assessed by use of a plasmid that codes for a marker gene. The DNA damage in the plasmid can be measured or modified before introduction into the cells and then the effect measured (Emmert et al. 2002). We used this assay to demonstrate that one UV photoproduct in the coding strand of the marker gene was sufficient to block its transcription in sensitive XP cells (Protic-Sabljic and Kraemer 1985) thereby demonstrating the importance of DNA repair in removal of DNA damage that blocks transcription. Similarly, replicating plasmid coding for a suppressor tRNA marker that is assessed in bacteria revealed that the XP cells introduce a high frequency of mutations into the plasmids. Sequence analysis of the recovered plasmids showed that the mutations following UV exposure of the plasmid are frequently at sites of dipyrimidine photoproducts and lead to C to T mutations (Bredberg et al. 1986). This is strong direct evidence of the role of UV in mutagenesis. These cellular studies have provided a molecular foundation for demonstration the UV induced origin of mutations found in non-melanoma skin cancer (Giglia et al. 1998; Couve-Privat et al. 2004) and melanomas (Daya-Grosjean and Sarasin 2005; Wang et al. 2009) in cancer suppressing genes [p53, PTCH and PTEN] in XP patients. This CC to TT UV “signature” has been used to link sun exposure to mutations in many other cancer related genes in melanomas and other skin cancers in the general population (Prickett et al. 2009; Pleasance et al. 2010; Wei et al. 2011).
XP cells are defective in NER (Figure 3) (Van Steeg and Kraemer 1999). This system serves to recognize DNA damage, excise the damage and replace the damaged region with undamaged DNA. Global genome repair (GGR) serves to identify DNA damage in the 99% of the DNA that is not involved in transcription. Transcription coupled repair (TCR) is triggered by a stalled RNA polymerase that contacts DNA damage in actively transcribed genes comprising the remaining 1% of the DNA. DNA photoproducts in the global genome are recognized by several proteins acting in tandem including double strand DNA binding protein 2 (DDB2) and XPC. TCR related proteins include Cockayne syndrome A and B. After recognition the DNA is unwound by XPB and XPD helicases which are part of the 10 subunit basal transcription factor IIH (TFIIH). These proteins are thus involved in both DNA repair and transcription of many other genes. The XPA protein maintains the open DNA region containing the damage which is then cut out by XPF /ERCC1 and XPG endonucleases as part of an approximately 30 nucleotide single stranded fragment. The resulting gap is filled in by DNA polymerase and ligase. The TCR pathway acts more rapidly than the GGR pathway and in fact shows strand specific repair with preferential repair of the transcribed strand. The NER pathway is closely coordinated so that if one of the proteins is defective then the entire pathway does not function correctly. Thus mutations in any of the above proteins lead to clinical diseases (Figures 3 and 4). The “XP variant” form of XP has normal NER. These patients have clinical XP with increased skin cancer susceptibility. Their cells are deficient in an error-prone DNA polymerase, polymerase eta, which normally serves to permit DNA replication past unrepaired photoproducts. Identification of this class of bypass polymerases provides insights into the varied mechanisms that organisms have developed to cope with DNA damage (Lange et al. 2011).
XP Neurologic Degeneration
About 25% of the XP patients have progressive neurological degeneration (Bradford et al. 2011). These patients often have defects in the XPA, XPB, XPD or XPG gene (Table 1 and Figure 4). They are usually born with normal size and weight. The earliest clinical abnormalities are frequently absent deep tendon reflexes and high frequency hearing loss and these can act as screening tests. Affected individuals may have delayed developmental milestones. The age of onset and rate of progression of the neurological abnormalities is variable among patients. Typical involvement includes sensorineural hearing loss, progressive intellectual impairment which may progress in severe cases to slurred speech, loss of ability to walk, difficulty swallowing and requirement for use of a feeding gastrostomy. Imaging studies show thinning of the cortex of the brain with concomitant dilation of the ventricles, and thickening of the skull bones. The pathology is a primary neuronal degeneration without evidence of inflammation or infiltration by other cells. XP patients with neurological degeneration have a high mortality (Figure 2D) (Bradford et al. 2011).
Relationships Within the Family of DNA Repair Disorders
There are three related, clinically defined disorders of DNA repair that can be used as archetypes to understand the spectrum of genotype/phenotype relationships within this group (Table 1 and Figure 4)(Kraemer et al. 2007). Photosensitivity, neurologic/developmental abnormalities and skin cancer are important pathological features which can be used to distinguish between these three archetypes: XP, trichothiodystrophy (TTD) and Cockayne syndrome (CS). In addition, there are several related or overlapping disorders with similar features that form a family of syndromes involving neural, oncologic, cutaneous, developmental and other abnormalities. Table 1 lists detailed clinical features which may be useful in distinguishing between XP, XP with neurologic disease, TTD, CS and XP/CS complex. While photophobia and skin sun sensitivity may be seen in all of these conditions, lentiginous hyperpigmentation is seen in XP but not TTD nor CS. Pigmentary retinal degeneration is seen in CS, but not in XP or TTD. More precise clinical delineation has permitted the identification of subtle overlap syndromes in patients with features of two of these disorders. Figure 4 diagrams the current state of the evolving genotype/phenotype relationships within this group. Phenotypes representing the clinical disorders are shown in red and molecular defects are shown in grey with the overlapping patterns showing the underlying molecular defect identified in patients within each phenotypic group. XP can be diagnosed on clinical criteria based on the presence of acute burning on minimal sun exposure, early onset freckling before the age of 2 years and skin cancer. While XP patients usually do not have developmental abnormalities, about 25% of XP patients develop progressive neurologic degeneration. TTD is a disorder characterized by short, brittle hair, and multisystem abnormalities (Figure 1I–L). TTD developmental abnormalities may be evident in the pregnant mother carrying a TTD affected fetus. These pregnancy abnormalities may include abnormal triple screen test results, preterm delivery, preeclampsia, placental abnormalities or HELLP syndrome (Moslehi et al. 2010; Tamura et al. 2011). The newborn may present with a collodion membrane, short stature, micrognathia, and have increased risk of infections, growth and developmental delay, congenital cataracts and other abnormalities. While patients frequently have photosensitivity, they do not develop skin cancer or the freckle-like pigmentary abnormalities of XP. In contrast to XP, the neurologic involvement in TTD patients is usually not one of progressive decline. CS has features of both disorders with photosensitivity and both developmental delay and progressive growth and neurologic decline, but not skin cancer. Cerebro-oculo-facial-skeletal syndrome is a severe variant of CS with abnormalities beginning in utero. Infants are born with contractures (arthrogryposis), thought to be due to decreased fetal movement, extreme microcephaly, congenital cataracts and facial dysmorphism (Laugel et al. 2008; Laugel et al. 2010). Careful and precise assessment of clinical features of each disorder has led to the identification of patients with several overlap syndromes. Patients with XP/TTD have features of both diseases, albeit mildly attenuated. They have tiger tail banding and hair shafts defects, but less prominent than occurs in TTD leading to longer hair. They are at risk for skin and possibly internal malignancies, but at a lower frequency than seen in XP. Similarly, overlap syndromes of XP/CS, CS/TTD and COSF/TTD have been described (Figure 4). Different mutations in the XPD gene have led to the greatest heterogeneity in clinical phenotype. Patients with the rare UV-sensitive syndrome have mild photosensitivity without pigmentary abnormalities or apparent CNS defects (Itoh et al. 1996). Their cells have the same transcription defects as CS cells and have been reported to have defects in the CS-A or CS-B genes (Horibata et al. 2004; Nardo et al. 2009) (Figure 4). This suggests that the CNS defect in CS patients may be related to an additional property of the CS proteins.
HOW HAS XP HELPED US UNDERSTAND THE BASIC BIOLOGY AND MECHANISMS UNDERLYING OTHER DISEASES ?
When the genes that are defective in XP patients were identified, the homologous genes were soon identified in mice. Knockout mice with many of these defects have been generated [see Mouse Mutation Database v5 at http://pathcuric1.swmed.edu/Research/research.htm] and serve as models for probing the role of these genes in carcinogenesis. For example mice with defects in XPA, and XPC have increased susceptibility to UV induced skin cancer (deVries A. et al. 1995; Sands et al. 1995; Cheo et al. 2000; Tanaka et al. 2001). XP heterozygous mice also have an increase in cancers both of the skin and of internal organs (Cheo et al. 2000). This suggests that humans who are heterozygous for XP disease causing mutations – such as 1 million Japanese people who are carriers of an XPA founder mutation (Hirai et al. 2006) - may be at increased cancer risk. However, mice are not perfect models for these diseases since TTD and CS patients do not have increased skin cancer susceptibility but mice with TTD and CS mutations do have increased post-UV cancer frequency (van der Horst et al. 2002).
Single nucleotide polymorphisms (SNP’s) are variants in the DNA sequence that occur with a frequency of at least 1% in the general population. SNP’s may affect gene function, or more commonly, act as markers of genetic differences to which they are linked elsewhere in the genome. A polyA-T polymorphism (PAT) in intron 9 of the XPC DNA repair gene, was found to be linked to an A to C SNP in exon 15 that changed amino acid 939 from Lysine (AAA) to Glutamine (CAA) but did not alter the XPC function (Khan et al. 2000) The PAT polymorphism was also linked to an XPC intron 11 -5C/A SNP that altered the frequency of alternatively spliced XPC mRNA which was shown to have reduced DNA repair ability (Khan et al. 2002). This XPC PAT polymorphism was associated with increased susceptibility to head and neck squamous cell carcinoma (Shen et al. 2001) and to cutaneous melanoma (Blankenburg et al. 2005). The use of polymorphisms in the XPD (ERCC2) DNA repair gene as an indication of cancer susceptibility has been questioned (Clarkson and Wood 2005). However, recent meta-analysis of 13 case control studies of bladder cancer (Stern et al. 2009) and 56 case control studies of several types of cancer (Wang et al. 2008) found a weak but consistent association with several polymorphisms in the XPD gene.
Interestingly wild type mice appear to have a defect in NER since assays DNA repair indicate reduced repair, yet the rodent cells have normal post-UV survival. These cells have a defect in the DDB2 NER gene in the dermis with defective GGR but normal TCR. A “humanized” mouse was developed that had addition of the DDB2 gene shows increased DNA repair (Alekseev et al. 2005). Interestingly, mouse keratinocytes have a higher level of DDB2 and greater repair than fibroblasts (Pines et al. 2009)
While patients with defects in some of the NER genes show profound, progressive neurologic abnormalities, mice with defects in XPA or CS do not. However, crossing of Xpa or Xpc mice with Csb mice does result in mice with severe neurological abnormalities (Murai et al. 2001; Laposa et al. 2007). The XPG mutant mouse does show neurological abnormalities. These mouse model systems have been used to mimic some features of neurological degeneration in the general population. A major theory of neuro-degeneration involves generation of free radicals by oxidative processes involving the mitochondria. These have been studied in mice with NER defects. Treatment with anti-oxidants are assessed in these mice.
XP cells have been used as reagents to determine the mechanism of action of chemotherapeutic agents. For example, XP cells with defective TCR are hypersensitive to killing by cis-platinum but XPC cells with a GGR defect have a normal response (Furuta et al. 2002). This indicates that the TCR pathway plays a role in the action of platinum and suggests that tumors that are resistant to platinum may have alterations in their TCR related genes. Interestingly, use of XP cells determined that a toxin from the sea squirt ET743, was activated by this TCR machinery to induce lethal DNA strand breaks (Takebayashi et al. 2001). This agent is currently in clinical trials for cancer therapy.
Immune diversity is generated by hypermutability of immunoglobulin variable genes in maturing B lymphocytes. The spectrum of mutations in B cells from XP variant patients with defects in the DNA polymerase eta were found to show a deficiency in the frequency of A-T mutations and a concomitant rise in G-C mutations. This data indicates that polymerase eta in involved in generating mutations in immunoglobulin variable genes (Zeng et al. 2001).
EPILOGUE
We are in the second decade of the 21st century. A young child is taken out on the first sunny day of spring in a modern umbrella stroller, but the child becomes irritable. By the next morning her skin becomes red and blistered, necessitating a trip to the emergency room. The pediatrician thinks this is a burn from the sun but is concerned about how parents could have let this happen. After a second episode, an astute dermatologist recognizes acute burning on minimal sun exposure, begins a work up for photosensitivity disorders, and instructs the parents to immediately start measures for aggressive sun protection. The child will not burn again. We have learned a great deal in the approximately 140 years since Kaposi’s description in 1874 (Hebra and Kaposi 1874). We now know that the skin changes of XP, are the result of UV exposure. Once a diagnosis is made, the family can be guided to sources of skin and eye protection (e.g., sun blocks, protective clothing, window tinting), an easy to use UV meter, and instructional materials for the school, and all of this may be facilitated through contact with patient support groups that can be help in identifying resources for the family (Tamura et al. 2010). We know many, but probably not all of the disease causing genes. Genetic testing may be available for confirmation of diagnosis not only in patients but also in utero and for future pregnancy planning. Vitamin D supplementation will prevent deficiency. Monitoring for possible neurologic involvement will permit early detection and management (for example hearing aids). The Americans with Disabilities Act (ADA) requires a safe, UV protected school environment and mandated individualized educational plans (IEP) assist in education.
This child may develop freckles and lentigines, but the family should have the knowledge and tools to avoid most of the damage that UV can cause in XP. If a skin cancer develops, advanced topical and surgical management can provide a cure with minimal discomfort and cosmetic alteration. Patients now can live healthy fulfilling lives well into adulthood despite having many hundreds of skin cancers (Oh et al. 2011). While we have far, we still have a long way to go. Many patients are unable or unwilling to utilize extreme sun protection and better methods are needed. Genetic testing is not readily available. We do not have effective intervention for neurologic decline. And, we often have too long a delay in diagnosis. Individuals who do not have extreme sun sensitivity may not be diagnosed early, leading to substantial sun damage at a young age. While all XP patients are at high risk for the development of skin cancer, we do not understand why some patients burn and others just freckle. We do not have a method to reverse the damage. And why don’t patients with TTD who have photosensitivity and abnormal NER develop skin cancer? We can diagnose, treat, prevent damage and understand some of the pathophysiologic mechanisms, but for all of these, still not as well as we would like.
ACKNOWLEDGMENT
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank the patients for participating in our studies and the XP patient support groups for helping people with XP and their families.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alekseev S, Kool H, Rebel H, Fousteri M, Moser J, Backendorf C, et al. Enhanced DDB2 expression protects mice from carcinogenic effects of chronic UV-B irradiation. Cancer Res. 2005;65(22):10298–10306. doi: 10.1158/0008-5472.CAN-05-2295. [DOI] [PubMed] [Google Scholar]
- Arase S, Kozuka T, Tanaka K, Ikenaga M, Takebe H. A sixth complementation group in xeroderma pigmentosum. Mutat.Res. 1979;59:143–146. doi: 10.1016/0027-5107(79)90202-1. [DOI] [PubMed] [Google Scholar]
- Blankenburg S, Konig IR, Moessner R, Laspe P, Thoms KM, Krueger U, et al. Assessment of 3 xeroderma pigmentosum group C gene polymorphisms and risk of cutaneous melanoma: a case-control study. Carcinogenesis. 2005 doi: 10.1093/carcin/bgi055. [DOI] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J.Med.Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A, Kraemer KH, Seidman MM. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc.Natl.Acad.Sci.U.S.A. 1986;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk PG, Lutzner MA, Clarke DD, Robbins JH. Ultraviolet-stimulated thymidine incorporation in xeroderma pigmentosum lymphocytes. J.Lab Clin.Med. 1971;77(5):759–767. [PubMed] [Google Scholar]
- Cheo DL, Meira LB, Burns DK, Reis AM, Issac T, Friedberg EC. Ultraviolet B radiation-induced skin cancer in mice defective in the Xpc, Trp53, and Apex (HAP1) genes: genotype-specific effects on cancer predisposition and pathology of tumors. Cancer Res. 2000;60(6):1580–1584. [PubMed] [Google Scholar]
- Clarkson SG, Wood RD. Polymorphisms in the human XPD (ERCC2) gene, DNA repair capacity and cancer susceptibility: an appraisal. DNA Repair (Amst) 2005;4(10):1068–1074. doi: 10.1016/j.dnarep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver JE. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Cleaver JE, Trosko JE. Absence of excision of ultraviolet-induced cyclobutane dimers in xeroderma pigmentosum. Photochem.Photobiol. 1970;11(6):547–550. doi: 10.1111/j.1751-1097.1970.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Couve-Privat S, Le Bret M, Traiffort E, Queille S, Coulombe J, Bouadjar B, et al. Functional analysis of novel sonic hedgehog gene mutations identified in basal cell carcinomas from xeroderma pigmentosum patients. Cancer Res. 2004;64(10):3559–3565. doi: 10.1158/0008-5472.CAN-03-4040. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L, Sarasin A. The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat.Res. 2005;571(1–2):43–56. doi: 10.1016/j.mrfmmm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- de Sanctis C, Cacchione A. L'idiozia xerodermica. Rivista Sperimentale di Freniatria e Medicina Legale delle Alienazioni Mentali. 1932;56:269–292. [Google Scholar]
- De Weerd-Kastelein EA, Keijzer W, Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat.New Biol. 1972;238(81):80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- deVries A, van Oostrom CT, Hofhuis FM, Dortant PM, Berg RJ, De Gruijl FR, et al. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature. 1995;377(6545):169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- DiGiovanna JJ, Patronas N, Katz D, Abangan D, Kraemer KH. Xeroderma pigmentosum: spinal cord astrocytoma with 9-year survival after radiation and isotretinoin therapy. J Cutan.Med.Surg. 1998;2(3):153–158. doi: 10.1177/120347549800200308. [DOI] [PubMed] [Google Scholar]
- Dualan R, Brody T, Keeney S, Nichols AF, Admon A, Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. 1995;29:62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- Emmert S, Slor H, Busch DB, Batko S, Albert RB, Coleman D, et al. Relationship of neurologic degeneration to genotype in three xeroderma pigmentosum group G patients. J Invest Dermatol. 2002;118(6):972–982. doi: 10.1046/j.1523-1747.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- Epstein JH, Fukuyama K, Reed WB, Epstein WL. Defect in DNA synthesis in skin of patients with xeroderma pigmentosum demonstrated in vivo. Science. 1970;168(938):1477–1478. doi: 10.1126/science.168.3938.1477. [DOI] [PubMed] [Google Scholar]
- Flejter WL, McDaniel LD, Askari M, Friedberg EC, Schultz RA. Characterization of a complex chromosomal rearrangement maps the locus for in vitro complementation of xeroderma pigmentosum group D to human chromosome band 19q13. Genes Chromosomes.Cancer. 1992a;5(4):335–342. doi: 10.1002/gcc.2870050409. [DOI] [PubMed] [Google Scholar]
- Flejter WL, McDaniel LD, Johns D, Friedberg EC, Schultz RA. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc.Natl.Acad.Sci.U.S.A. 1992b;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62(17):4899–4902. [PubMed] [Google Scholar]
- Gartler SM. Inborn erros of metabolism at the cell culture level. New York: 1964. [Google Scholar]
- Giannelli F, Avery J, Polani PE, Terrell C, Giammusso V. Xeroderma Pigmentosum and medulloblastoma: chromosomal damage to lymphocytes during radiotherapy. Radiat.Res. 1981;88:194–208. [PubMed] [Google Scholar]
- Giglia G, Dumaz N, Drougard C, Avril MF, ya-Grosjean L, Sarasin A. p53 mutations in skin and internal tumors of xeroderma pigmentosum patients belonging to the complementation group C. Cancer Res. 1998;58(19):4402–4409. [PubMed] [Google Scholar]
- Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989;262(15):2097–2100. [PubMed] [Google Scholar]
- Gozukara EM, Parris CN, Weber CA, Salazar EP, Seidman MM, Watkins JF, et al. The human DNA repair gene, ERCC2 (XPD), corrects ultraviolet hypersensitivity and ultraviolet hypermutability of a shuttle vector replicated in xeroderma pigmentosum group D cells. Cancer Res. 1994;54(14):3837–3844. [PubMed] [Google Scholar]
- Grier GW. Report of two cases of xeroderma pigmentosum with malignancy of the eyeball successfully treated by roentgen ray. American J Roentgenology. 1919;6:556–558. [Google Scholar]
- Hebra F, Kaposi M. On diseases of the skin including exanthemata, volume III. The New Sydenham Society. 1874;(61):252–258. [Google Scholar]
- Hirai Y, Kodama Y, Moriwaki S, Noda A, Cullings HM, MacPhee DG, et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutat.Res. 2006;601(1–2):171–178. doi: 10.1016/j.mrfmmm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Horibata K, Iwamoto Y, Kuraoka I, Jaspers NG, Kurimasa A, Oshimura M, et al. Complete absence of Cockayne syndrome group B gene product gives rise to UV-sensitive syndrome but not Cockayne syndrome. Proc.Natl.Acad.Sci.U.S.A. 2004;101(43):15410–15415. doi: 10.1073/pnas.0404587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Yamaizumi M, Ichihashi M, Hiro-Oka M, Matsui T, Matsuno M, et al. Clinical characteristics of three patients with UVs syndrome, a photosensitive disorder with defective DNA repair. Br.J Dermatol. 1996;134(6):1147–1150. [PubMed] [Google Scholar]
- Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285(5425):263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- Keijzer W, Jaspers NG, Abrahams PJ, Taylor AM, Arlett CF, Zelle B, et al. A seventh complementation group in excision-deficient xeroderma pigmentosum. Mutat.Res. 1979;62:183–190. doi: 10.1016/0027-5107(79)90231-8. [DOI] [PubMed] [Google Scholar]
- Khan SG, Metter EJ, Tarone RE, Bohr VA, Grossman L, Hedayati M, et al. A new xeroderma pigmentosum group C poly(AT) insertion/deletion polymorphism. Carcinogenesis. 2000;21(10):1821–1825. doi: 10.1093/carcin/21.10.1821. [DOI] [PubMed] [Google Scholar]
- Khan SG, Muniz-Medina V, Shahlavi T, Baker CC, Inui H, Ueda T, et al. The human XPC DNA repair gene: arrangement, splice site information content and influence of a single nucleotide polymorphism in a splice acceptor site on alternative splicing and function. Nucleic Acids Res. 2002;30(16):3624–3631. doi: 10.1093/nar/gkf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H, Hamilton CM. Xeroderma pigmentosum in a Negress. Arch Dermatol. 1940;42:570–575. [Google Scholar]
- Kleinerman RA. Radiation-sensitive genetically susceptible pediatric sub-populations. Pediatr.Radiol. 2009;39(Suppl 1):S27–S31. doi: 10.1007/s00247-008-1015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH. From proteomics to disease. Nat.Genet. 2004;36(7):677–678. doi: 10.1038/ng0704-677. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Coon HG, Petinga RA, Barrett SF, Rahe AE, Robbins JH. Genetic heterogeneity in xeroderma pigmentosum: complementation groups and their relationship to DNA repair rates. Proc.Natl.Acad.Sci.U.S.A. 1975a;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH, De Weerd-Kastelein EA, Robbins JH, Keijzer W, Barrett SF, Petinga RA, et al. Five complementation groups in xeroderma pigmentosum. Mutat.Res. 1975b;33(2–3):327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N.Engl.J Med. 1988;318(25):1633–1637. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch.Dermatol. 1994;130(8):1018–1021. [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience. 2007;145(4):1388–1396. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH, Ruenger TM. Genome instability, DNA repair and cancer. In: Wolff K, et al., editors. Fitzpatrick's Dermatology in General Medicine. New York: McGraw Hill; 2008. pp. 977–986. [Google Scholar]
- Lange SS, Takata K, Wood RD. DNA polymerases and cancer. Nat.Rev.Cancer. 2011;11(2):96–110. doi: 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc.Natl.Acad.Sci.U.S.A. 2007;104(4):1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent MC, et al. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum.Mutat. 2010;31(2):113–126. doi: 10.1002/humu.21154. [DOI] [PubMed] [Google Scholar]
- Laugel V, Dalloz C, Tobias ES, Tolmie JL, Martin-Coignard D, Drouin-Garraud V, et al. Cerebro-oculo-facio-skeletal syndrome: three additional cases with CSB mutations, new diagnostic criteria and an approach to investigation. J Med.Genet. 2008;45(9):564–571. doi: 10.1136/jmg.2007.057141. [DOI] [PubMed] [Google Scholar]
- Legerski R, Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992;360(6404):610. doi: 10.1038/360610b0. [DOI] [PubMed] [Google Scholar]
- Liang C, Kraemer KH, Morris A, Schiffmann R, Price VH, Menefee E, et al. Characterization of tiger-tail banding and hair shaft abnormalities in trichothiodystrophy. J Am.Acad.Dermatol. 2005;52(2):224–232. doi: 10.1016/j.jaad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Loewenthal LJA, Trowell HC. Xeroderma pigmentosum in African Negroes. Br J Dermatol. 1938;50:66–71. [Google Scholar]
- Maher VM, McCormick JJ, Grover PL, Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat.Res. 1977;43:117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Maher VM, Patton JD, Yang JL, Wang YY, Yang LL, Aust AE, et al. Mutations and homologous recombination induced in mammalian cells by metabolites of benzo[a]pyrene and 1-nitropyrene. Environ.Health Perspect. 1987;76:33–39. doi: 10.1289/ehp.877633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahindra P, DiGiovanna JJ, Tamura D, Brahim JS, Hornyak TJ, Stern JB, et al. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am.Acad.Dermatol. 2008;59(5):881–886. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399(6737):700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Signore C, Tamura D, Mills JL, DiGiovanna JJ, Tucker MA, et al. Adverse effects of trichothiodystrophy DNA repair and transcription gene disorder on human fetal development. Clin.Genet. 2010;77(4):365–373. doi: 10.1111/j.1399-0004.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, MacInnes MA. Isolation of the functional human excision repair gene ERCC5 by intercosmid recombination. Genomics. 1990;8:623–633. doi: 10.1016/0888-7543(90)90248-s. [DOI] [PubMed] [Google Scholar]
- Murai M, Enokido Y, Inamura N, Yoshino M, Nakatsu Y, van der Horst GT, et al. Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes. Proc.Natl.Acad.Sci.U.S.A. 2001;98(23):13379–13384. doi: 10.1073/pnas.231329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo T, Oneda R, Spivak G, Vaz B, Mortier L, Thomas P, et al. A UV-sensitive syndrome patient with a specific CSA mutation reveals separable roles for CSA in response to UV and oxidative DNA damage. Proc.Natl.Acad.Sci.U.S.A. 2009;106(15):6209–6214. doi: 10.1073/pnas.0902113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser A. Ueber das 'Xeroderma pigmentosum' (Kaposi):Lioderma essentialis cum melanosi et telangiectasia. Vierteljahrschr Dermatol Syphil. 1883:47–62. [Google Scholar]
- Oh KS, Emmert S, Tamura D, DiGiovanna JJ, Kraemer KH. Multiple skin cancers in adults with mutations in the XP-E (DDB2) DNA repair gene. J.Invest Dermatol. 2011;131(3):785–788. doi: 10.1038/jid.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Per M. Xeroderma pigmentosum (Kaposi): Report of a case, with special reference to clinical features and pathogenesis. Br J Dermatol. 1926;38(6):241–252. [Google Scholar]
- Pines A, Backendorf C, Alekseev S, Jansen JG, De Gruijl FR, Vrieling H, et al. Differential activity of UV-DDB in mouse keratinocytes and fibroblasts: impact on DNA repair and UV-induced skin cancer. DNA Repair (Amst) 2009;8(2):153–161. doi: 10.1016/j.dnarep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463(7278):191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat.Genet. 2009;41(10):1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protic-Sabljic M, Kraemer KH. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc.Natl.Acad.Sci.U.S.A. 1985;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar HL, Brooks BP, Cao X, Tamura D, DiGiovanna JJ, Kraemer KH, et al. Ophthalmic manifestations and histopathology of xeroderma pigmentosum: two clinicopathological cases and a review of the literature. Surv.Ophthalmol. 2011;56(4):348–361. doi: 10.1016/j.survophthal.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WB, Landing B, Sugarman G, Cleaver JE, Melnyk J. Xeroderma pigmentosum. Clinical and laboratory investigation of its basic defect. JAMA. 1969;207(11):2073–2079. doi: 10.1001/jama.207.11.2073. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, et al. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114:1335–1361. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Kraemer KH, Lutzner MA, Festoff BW, Coon HG. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann.Intern.Med. 1974;80:221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Ruenger TM, DiGiovanna JJ, Kraemer KH. Hereditary Diseases of genome instability and DNA repair. In: Wolff K, et al., editors. Fitzpatrick's Dermatology in General Medicine. New York: McGraw Hill; 2008. pp. 1311–1325. [Google Scholar]
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Regan JD, German J, Carrier WL. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc.Natl.Acad.Sci.U.S.A. 1969;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Setlow JK. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc.Natl.Acad.Sci.U.S.A. 1962;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sturgis EM, Khan SG, Qiao Y, Shahlavi T, Eicher SA, et al. An intronic poly (AT) polymorphism of the DNA repair gene XPC and risk of squamous cell carcinoma of the head and neck: a case-control study. Cancer Res. 2001;61(8):3321–3325. [PubMed] [Google Scholar]
- Sijbers AM, De Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, et al. Xeroderma pigmentosum group F caused by a defect in a structure- specific DNA repair endonuclease. Cell. 1996;86(5):811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Stern MC, Lin J, Figueroa JD, Kelsey KT, Kiltie AE, Yuan JM, et al. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: findings from the international consortium of bladder cancer. Cancer Res. 2009;69(17):6857–6864. doi: 10.1158/0008-5472.CAN-09-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi Y, Pourquier P, Zimonjic DB, Nakayama K, Emmert S, Ueda T, et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat.Med. 2001;(8):961–966. doi: 10.1038/91008. [DOI] [PubMed] [Google Scholar]
- Tamura D, DiGiovanna JJ, Kraemer KH. Xeroderma Pigmentosum. In: Lebwohl M, et al., editors. Treatment of Skin Disease. London: Elsevier; 2010. pp. 789–792. [Google Scholar]
- Tamura D, Merideth M, DiGiovanna JJ, Zhou X, Tucker MA, Goldstein AM, et al. High-risk pregnancy and neonatal complications in the DNA repair and transcription disorder trichothiodystrophy: report of 27 affected pregnancies. Prenat Diagn. 2011 doi: 10.1002/pd.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kamiuchi S, Ren Y, Yonemasu R, Ichikawa M, Murai H, et al. UV-induced skin carcinogenesis in xeroderma pigmentosum group A (XPA) gene-knockout mice with nucleotide excision repair-deficiency. Mutat.Res. 2001;477(1–2):31–40. doi: 10.1016/s0027-5107(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Miura N, Satokata I, Miyamoto I, Yoshida MC, Satoh Y, et al. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990;348:73–76. doi: 10.1038/348073a0. [see comments] [DOI] [PubMed] [Google Scholar]
- Taylor RW. Xeroderma pigmentosum and its relationship to malignant new-growths of the skin. The Medical Record. 1888;33(10):261–269. [Google Scholar]
- van der Horst GT, Meira L, Gorgels TG, De Wit J, Velasco-Miguel S, Richardson JA, et al. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair (Amst) 2002;1(2):143–157. doi: 10.1016/s1568-7864(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Van Steeg H, Kraemer KH. Xeroderma pigmentosum and the role of UV-induced DNA damage in skin cancer. Mol.Med.Today. 1999;5(2):86–94. doi: 10.1016/s1357-4310(98)01394-x. [DOI] [PubMed] [Google Scholar]
- Wang F, Chang D, Hu FL, Sui H, Han B, Li DD, et al. DNA repair gene XPD polymorphisms and cancer risk: a meta-analysis based on 56 case-control studies. Cancer Epidemiol.Biomarkers Prev. 2008;17(3):507–517. doi: 10.1158/1055-9965.EPI-07-2507. [DOI] [PubMed] [Google Scholar]
- Wang Y, DiGiovanna JJ, Stern JB, Hornyak TJ, Raffeld M, Khan SG, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc.Natl.Acad.Sci.U.S.A. 2009;106(15):6279–6284. doi: 10.1073/pnas.0812401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G, van Ham RC, Masurel R, Westerveld A, Odijk H, de WJ, et al. Molecular cloning and biological characterization of the human excision repair gene ERCC-3. Mol.Cell Biol. 1990a;10(6):2570–2581. doi: 10.1128/mcb.10.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G, van Ham RC, Vermeulen W, Bootsma D, van der Eb AJ, Hoeijmakers JH. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990b;62:777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat.Genet. 2011;43(5):442–446. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh D, Klein J, O'Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357(9260):926–929. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- Zeng X, Winter DB, Kasmer C, Kraemer KH, Lehmann AR, Gearhart PJ. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nat.Immunol. 2001;2(6):537–541. doi: 10.1038/88740. [DOI] [PubMed] [Google Scholar]