Abstract
The mouse cortical collecting duct (CCD) M-1 cells were grown to confluency on coverslips to assess the interaction between TRPV4 and Ca2+-activated K+ channels. Immunocytochemistry demonstrated strong expression of TRPV4, along with the CCD marker, aquaporin-2, and the Ca2+-activated K+ channels, the small conductance SK3 (KCa2.3) channel and large conductance BKα channel (KCa1.1). TRPV4 overexpression studies demonstrated little physical dependency of the K+ channels on TRPV4. However, activation of TRPV4 by hypotonic swelling (or GSK1016790A, a selective agonist) or inhibition by the selective antagonist, HC-067047, demonstrated a strong dependency of SK3 and BK-α activation on TRPV4-mediated Ca2+ influx. Selective inhibition of BK-α channel (Iberiotoxin) or SK3 channel (apamin), thereby depolarizing the cells, further revealed a significant dependency of TRPV4-mediated Ca2+ influx on activation of both K+ channels. It is concluded that a synergistic cross-talk exists between the TRPV4 channel and SK3 and BK-α channels to provide a tight functional regulation between the channel groups. This cross-talk may be progressive in nature where the initial TRPV4-mediated Ca2+ influx would first activate the highly Ca2+-sensitive SK3 channel which, in turn, would lead to enhanced Ca2+ influx and activation of the less Ca2+-sensitive BK channel.
Keywords: TRPV4 channel, Ca2+-activated K+ channel, calcium signaling, hyperpolarization, cross-talk, mechanosensitive
1. Introduction
TRP channels are a ubiquitous superfamily of cationic channels that, with a few exceptions, are calcium-permeable and gated by a diverse range of stimuli. TRPV4 is a particularly notable example as it has been shown to be modulated not only by mechanical stimuli, including shear stress and hypotonic cell swelling, but also by polyunsaturated fatty acids, some phorbol esters, and moderate heat [1–4]. In the renal collecting duct TRPV4 is strongly expressed and appears to play a role in mechanical control of Ca2+ signaling dynamics [5–6]. However, once activated, many factors can come into play in modulating the activity of the TRP channels, including TRPV4, from phosphorylation status [7–9] to membrane trafficking [13–15]. Indeed, alterations in membrane trafficking are known to strongly influence TRPV4 activity [15] where we have recently shown that in overexpression systems alterations in membrane trafficking from the plasma membrane appears to underlie a major component of TRPV4 desensitization following activation [13]. Since TRPV4 is known to associate with the actin cytoskeleton [16], it may be that insertion and retrieval cycles of TRPV4 from the plasma membrane may be a central component modulating TRPV4-mediated intracellular Ca2+, [Ca2+]i, dynamics in collecting duct cells.
The TRPV4 channel is now known to be expressed in renal collecting duct cells where it appears to function as a flow sensor [6, 9, 17]. We have previously shown expression of TRPV4 in mouse renal collecting duct cells [6] and, most recently, that TRPV4 is most strongly expressed in the aquaporin-2 positive cells (principal cells) of the cortical collecting duct (CCD) [5]. This segment of the collecting duct system is an important site of flow-sensitive K+ secretion where the Ca2+-dependent maxi-K channel, BK channel, appears to underlie the K+ secretion in a Ca2+-dependent manner [18–20]. Whether other Ca2+-dependent K+ channels participate in this phenomenon is not known although our current study shows expression of the highly Ca2+-sensitive SK3 channel in the collecting duct cell line, M-1 cells. Regardless, a dynamic interplay may exist between the TRPV4 and Ca2+-dependent K+ channels in renal collecting duct cells where Ca2+ influx leads to activation of the K+ channel which, in turn, would hyperpolarize the cell membrane and increase the driving force for Ca2+ influx. Hence, the TRPV4 channel may display a synergistic cross-talk with the calcium-activated K+ channel to control calcium influx, membrane potential, and K+ secretion.
Recent studies have now shown that some TRP channels may associate with Ca2+-dependent K+ channels and tightly control the K+ channel activity [21–22]. Indeed, it has been shown for TRPC1 that it may associate with the Ca2+-dependent BK channel in vascular smooth muscle cells to control membrane potential [23] while a similar association of TRPA1 with small and intermediate Ca2+-activated K+ channels in vascular endothelial cells may serve a similar function [24]. Other studies have shown that TRPV4 may also play a key role in modulating Ca2+-activated K+ channels, particularly the BK channel, in a variety of cell types including vascular smooth muscle cells, endothelial cells and epithelial cells [25–30]. In most studies this interaction between TRPV4 and BK (or other K+ channels) appear to be an indirect, but functional, interplay. Regardless, TRPV4 and other TRP channels may be important modulators of Ca2+-dependent K+ channels which, in turn, may modulate Ca2+ influx via the TRP channels.
The purpose of the present study was to elucidate the underlying potential pathways controlling TRPV4-mediated [Ca2+]i dynamics following hypotonic-induced stimulation of TRPV4 in mouse renal collecting duct cells, the M-1 cells. We demonstrated that these cells express TRPV4 and the aquaporin-2 water channel (CCD marker) along with two Ca2+-dependent K+ channels, the SK3 and BKα. Somewhat surprisingly, hypotonic stimulation was not associated with alterations in plasma membrane expression of TRPV4 despite the biphasic nature of [Ca2+]i changes. Further, TRPV4 did not appear to be closely associated with BK or SK3 expression. However, a dynamic functional cross-talk between TRPV4 and the K+ channels was demonstrated with the K+ channel activation generating a maximal stimulation of TRPV4-mediated Ca2+ influx. As such, these studies may have direct implications relating to the mechanisms of modulation of Ca2+ signaling and flow-dependent K+ secretion in the CCD.
2. Materials and Methods
2.1. Cell Culture and Transfection
Mouse kidney M-1 (cortical collecting duct) cells from American Type Culture Collection were grown in standard DMEM/Ham’s F12 media with growth supplements (Sigma, D8437) and 10% FBS at 37 °C, pH 7.4 [9]. Cells were seeded onto coverslips and grown to confluency at 37 °C [6]. In some studies a recombinant mouse TRPV4 construct, tagged with the fluorophore mVenus at the C-terminus, was used to transiently transfect M-1 cells (TRPV4-mVenus) using the Effectene Kit (Qiagene) as before [13]. Transfected cells were used within 24–48 hours of transfection.
2.2. Immunobloting and Immunocytochemistry
M-1 cells were grown on 10 cm tissue culture plates, rinsed with ice-cold PBS, harvested (scraped), lysed and subjected to separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previouasly described [6, 13]. Whereupon, the separated protein were transferred from the gels to PVDF membranes and immunobloted. Primary antibodies used for Western blotting included antibodies against TRPV4 (anti-TRPV4 at 1:500 dilution, Alomone Labs), BK-α (anti-BK-α at 1:200 dilution, Alomone Labs), and SK3 (anti-SK3 at 1:200 dilution, Alomone Labs). Binding of primary antibodies was detected with secondary antibody conjugated to HRP using an ECL detection reagent (Amersham).
In initial studies of TRPV4 expression and trafficking to/from the plasma membrane, a highly enriched plasma membrane fraction from M-1 cells was used for immunoblotting. The enriched plasma membrane fraction of M-1 cells grown on 10 cm tissue culture plates was achieved with a sucrose gradient-based membrane protein extraction kit (Biovision, Inc.). An intermediate preparation of a whole cell lysate was applied to the sucrose gradient, subjected to centrifugation, and the resultant pellet (plasma membrane fraction) solubilized in a modified RIPA buffer (0.1% Na deoxycholate, 0.01% SDS, 1% NP-40, and 20 mM Mg acetate in PBS) containing protease inhibitors (Sigma-Aldrich). The BCA assay (Thermo Scientific) was used to measure protein concentrations. Membrane aliquots were subjected to SDS-PAGE separation and detected on PVDF membranes. Lack of contamination by cytosolic components (anti-α-tubulin, Sigma-Aldrich) was verified for each preparation. TRPV4 band intensities were quantified with Image J (version 1.42q, NIH) for differing treatments. TRPV4 channel intensities were normalized to the plasma membrane marker, pan cadherin (anti-pan cadherin, AbCam), as a loading control. All experiments were repeated 3–4 times.
A Nikon A1 confocal microscope was used for immunofluorescent imaging to assess cellular localization of the channel proteins as described before [6, 13, 31]. Cells on coverslips were washed, fixed in 4% paraformaldehyde (RT, 20 min), and stained, as above, with primary antibodies against TRPV4, BK-α, SK3, along with aquaporin-2 (anti-AQP-2 tagged with ATTO 550, 1:200 dilution, Alomone Labs) and plasma membrane marker pan cadherin (anti-pan cadherin, 1:125 dilution, Sigma-Aldrich). Cells were subsequently stained with secondary antibody labeled with FITC (anti-AQP-2-ATTO-550 directly labeled), mounted with VectraShield mounting media (Vector Laboratories) and imaged with a Nikon A1 confocal microscope. In some studies phalloidin (Alex Fluor 647 phalloidin) was used for staining of F-actin.
2.3. Measurement of Intracellular Calcium
The fura 2 fluorescence ratiometric method was used to measure intracellular calcium levels, [Ca2+]i, in M-1 cells grown to confluency on coverslips as done extensively before [6, 9]. Cells on coverslips were loaded with fura-2/AM (2–10 μM) for 45 – 60 minutes at room temperature, washed, attached to the bottom of a perfusion chamber and imaged with InCa Imaging Workstation (Intracellular Imaging, Inc.) at 37 °C. Typically 10–30 cells (ROIs) were simultaneously monitored on a coverslip and the results averaged for each experiment. Intracellular calcium was estimated from the fura-2 fluorescence by excitation at 340 nm and 380 nm and calculating the ratio of the emission intensities at 511 nm in the usual manner every 1–3 seconds. Results are presented as the emission ratios and denoted F340/F380. In some studies, cells were subjected to intracellular fura 2 calibration and the ratios converted to intracellular calcium activity, [Ca2+]i, as described by Grynkiewicz et al. [32] using methods previously outlined [6, 9].
2.4. Chemicals and Solutions
M-1 cells were typically studied in a control isotonic (Ctl) media containing (in mM): 145 NaCl, 5.4 KCl, 0.5 MgCl2, 0.4 MgSO4, 3.3 NaHCO3, 2.0 CaCl2, 10 HEPES, 5.5 glucose, pH 7.4, having an osmolarity of 310 mOsm/L (Ctl or ISO media). The hypotonic media (HYPO) was identical to the Ctl media except 45 mM NaCl was removed to provide an osmolality of 220 mOsm/L. In some studies, the Ca2+ was removed from the standard solutions and 0.5 mM EGTA added, pH 7.4, to chelate any residual Ca2+ (0 Ca2+) as typically done [6].
The following drugs and chemicals were used in the study: GSK1016790A (GSK101, Santa Cruz Biotechnology; stock solution, 10 μM in DMSO), HC-067047 (Tocris Bioscience; stock solution 100 μM in DMSO), iberiotoxin (IbTX, Alomone Labs: 50 mM stock in PBS), apamin (Alomone Labs; 100 mM stock in PBS), and EGTA, ethyleneglycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid (Sigma Chemical).
Data are presented as mean values ± SEM. Student’s t-test or ANOVA were used to test for statistical significance, as appropriate, with P < 0.05 considered significant.
3. Results
3.1. Expression of TRPV4 in aquaporin-2-positive M-1 Cells
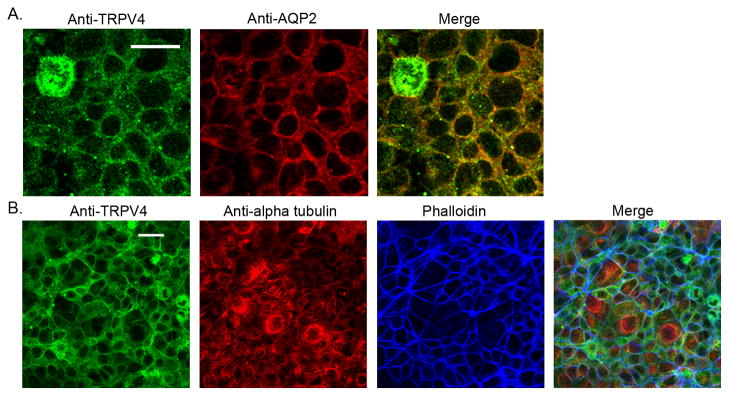
We have recently shown that TRPV4 is expressed in the mouse cortical collecting duct [5–6] with strong expression in the aquaporin-2 (AQP2)-expressing cells, indicative of the principal cell (PC). We now show similar staining patterns in M-1 cells (Fig. 1A). Immunostaining of confluent M-1 monolayers shows strong expression of both AQP-2 and TRPV4 in M-1 cells with significant co-localization at, or near, the plasma membrane (Fig. 1A, top panel, Merged). These results demonstrate CCD PC-like properties of the M-1 cells.
Figure 1.
Immunofluorescent imaging and immunostaining of M-1 cells. A. Immunofluorescent staining of M-1 cells for TRPV4 (Anti-TRPV4, green) and AQP-2 (Anti-aquaporin 2, red). Merged images (right panel) show significant co-localization of TRPV4 and AQP-2 proteins within the cytoplasm and at, or near, the plasma membrane. Scale bar = 20 μm. B. Immunofluorescent staining of M-1 cells for TRPV4 (Anti-TRPV4, green), α-tubulin (Anti-alpha tubulin, red), and F-actin (Phalloidin, blue). F-actin staining shows well-organized actin cortical bands near the periphery of the cell while α-tubule shows little defined organization. Co-localization of TRPV4 with actin is apparent near the cell membrane as apparent in the Merged image. Scale bar = 20 μm.
TRPV4 can also associate with the actin cytoskeleton which may promote trafficking between the cytosol and plasma membrane [16]. Staining of M-1 cells for α-tubulin (microtubules) and F-actin shows a highly organized actin cortical band around the periphery of the cells, as evidenced by the phalloidin staining patterns (Fig. 1B), typical of epithelial cells [33–34]. In contrast, the microtubule staining was not highly organized in these cells. TRPV4 expression and the actin cortical band demonstrate significant co-localization indicative for a membrane protein with associations to actin and membrane trafficking.
3.2. Activation of TRPV4 by hypotonic swelling (HYPO) with minimal TRPV4 trafficking
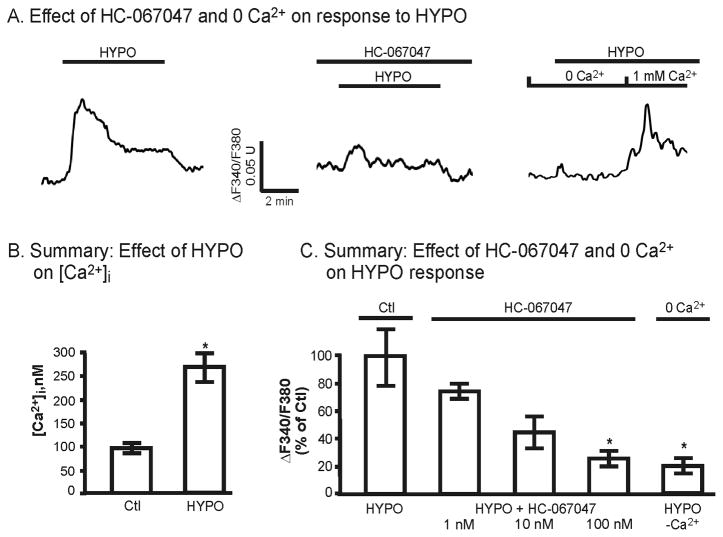
It is well documented that TRPV4 is a mechanically-sensitive channel that can be activated by hypotonic cell swelling and mechanical stresses [see 35]. We have previously demonstrated that such mechanical stress can rapidly lead to activation of TRPV4 in TRPV4-transfected HeLa or HEK cells and in M-1 cells [6, 9]. In the current study we took advantage of this mode of TRPV4 activation to evaluate the affect of channel activation on [Ca2+]i dynamics and membrane trafficking. As shown in Figure 2A, exposure of M-1 cells to hypotonic media (reducing the osmolality from 310 to 220 mOsm/kg) lead to an influx of Ca2+ with biphasic changes in [Ca2+]i [6]. Typically [Ca2+]i reached a peak increase within 1–3 minutes of stimulation followed by relaxation to a pseudo-plateau level over 5–10 minutes. This response is primarily related to hypotonicity-induced activation of TRPV4 as application of the selective TRPV4 antagonist, HC-067047 [36], reduced the HYPO-induced changes in [Ca2+]i in a dose-dependent fashion (Fig. 2A and 2C, pre-incubated with HC-067047 for 5 minutes). Addition of 100 nM HC-067047, a dose sufficient to completely abolish GSK101-induced activation of TRPV4 (see Fig. 4D), reduced the peak HYPO-induced [Ca2+]i change to 17 ± 4% of control HYPO responses observed in the absence of the blocker (Fig. 2C). Similarly, upon removal of extracellular Ca2+ (0 Ca2+), the HYPO-induced increase in [Ca2+]i was reduced to 20 ± 5% of control values, demonstrating that most of the increase in [Ca2+]i in the presence of extracellular Ca2+ was due to Ca2+ influx. In addition, the small increase in [Ca2+]i under 0 Ca2+ conditions, as for the case with 100 nM HC-067047, likely reflects release of Ca2+ from internal stores upon HYPO stimulation (Also see [6]).
Figure 2.
Effect of HYPO and the TRPV4 antagonist, HC-067047, on M-1 [Ca2+]i levels. A. [Ca2+]i response to HYPO (220 mOsm) in the presence and absence of HC-067047 (100 nM) and extracellular Ca2+ (0 Ca2+). In the absence of blocker (A), HYPO induces a biphasic time course with a peak increase in [Ca2+]i within 1–3 minutes followed by relaxation to pseudoplateau levels. Under 0 Ca2+ conditions, the increase in [Ca2+]i is markedly reduced except for a small transient increase in [Ca2+]i, indicative of Ca2+ release from internal stores. B. Effect of HYPO on peak [Ca2+]i levels (n=10). C. Summary effect of TRPV4 inhibition by HC-067047 and 0 Ca2+ on the peak change in [Ca2+]i given as a % of the control HYPO response. Data are presented for the HYPO response in control (HYPO, n = 5, 100% response), 1 nM (n = 4), 10 nM (n = 3), 100 nM (n = 3) HC-067047 and for 0 Ca2+ (n = 2). *P<0.05.
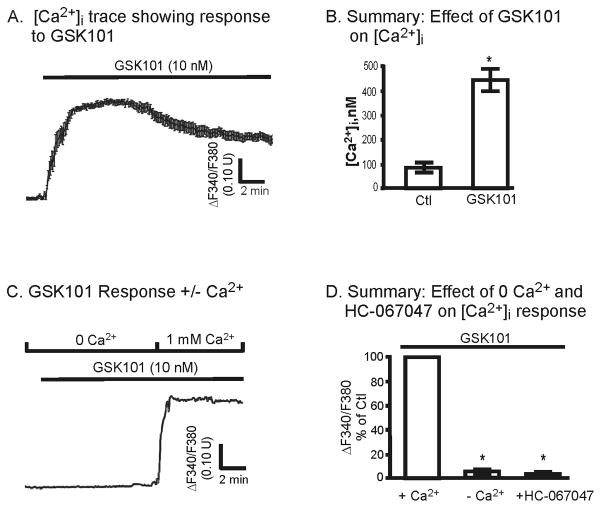
Figure 4.
Effect of the selective TRPV4 agonist, GSK101, on M-1 cell [Ca2+]i levels. A. Typical [Ca2+]i trace from one coverslip (average of 10 cells) showing effect of TRPV4 activation following addition of 10 nM GSK101. The [Ca2+]i response is slightly biphasic reaching a peak within 1–6 minutes. B. Summary of GSK101 affects on [Ca2+]i (n=7). C. Representative time course showing the effect of GSK101 on [Ca2+]i in the presence and absence of extracellular Ca2+ (0 Ca2+). Note the abrupt increase in [Ca2+]i following re-introduction of Ca2+ with prior treatment by GSK101. D. Summary of the affect of GSK101 on the peak change in the F340/F380 ratio in the presence and absence of 0 Ca2+ (-Ca2+, n = 2) and HC-067047 (100 nM, n = 4). *P<0.05.
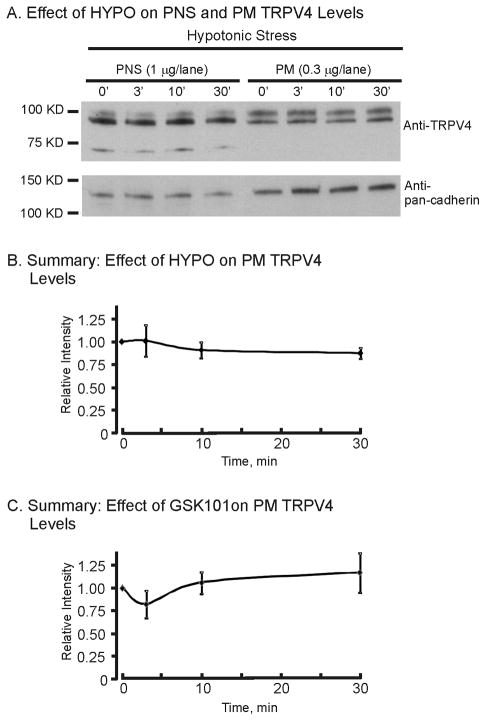
The reason for this transient nature of the TRPV4-mediated response to HYPO is not known. We have shown that TRPV4 activation in TRPV4-transfected HeLa cells leads to a rapid desensitization of the TRPV4 currents that appears to largely reflect retrieval of TRPV4 from the plasma membrane [13]. However, in the M-1 cells which endogenously express TRPV4 this does not appear to be the case. As shown in Figure 3, expression of TRPV4 in the post-nuclear supernatant (PNS, cell lysate) or in a highly purified plasma membrane fraction (PM) did not significantly change following HYPO exposure (normalized to pan-cadherin, a plasma membrane marker). A summary of the TRPV4 band intensities (normalized to relative intensity of 1 at 0′) did not significantly change for up to 30 minutes of HYPO exposure (Fig. 3B) demonstrating a fundamental difference from that observed in heterologous expression systems [13; unpublished data]. Hence, it appears that the biphasic nature of the TRPV4-mediated [Ca2+]i changes are not related to changes in TRPV4 trafficking to, or from, the plasma membrane in M-1 cells. These results, however, cannot rule out parallel changes in TRPV4 insertion and retrieval rates, leaving the abundance of TRPV4 at the plasma membrane relatively constant.
Figure 3.
Effect of HYPO and GSK101 on plasma membrane (PM) expression of TRPV4 in M-1 cells. A. Western blot of TRPV4 expression in the post-nuclear fraction (PNS, whole cell) and plasma membrane fraction (PM) following HYPO exposure over a 30-minute time period. B. Time course of the relative TRPV4 expression abundance (TRPV4/pan cadherin) following HYPO exposure. Values are normalized to the isotonic, control (ISO), condition (0 min) (n = 3). HYPO stimulation of TRPV4 did not lead to alterations in net TRPV4 expression levels at the plasma membrane. C. Time course of the relative TRPV4 expression levels (TRPV4/pan cadherin) following GSK101 (10 nM) exposure. Values are normalized to the control condition (0 min) (n = 3). GSK101 stimulation of TRPV4 did not lead to alterations in net TRPV4 expression levels at the plasma membrane.
3.3. Selective activation of TRPV4 by the agonist GSK1016790A with minimal TRPV4 trafficking
The GSK1016790A (GSK101) compound was recently identified as a selective agonist of TRPV4 [37–38]. We have demonstrated selective activation of TRPV4 by GSK101 in TRPV4-expressing HeLa cells [13] as also shown by others [39–40]. In the current studies GSK101 was used to selectively activate TRPV4 in confluent monolayers of M-1 cells as an alternative approach to activate TRPV4. GSK101 was shown to potently activate Ca2+ influx in M-1 cells, as evident from the rapid increase in the F340/F380 ratio (Fig. 4A), corresponding to an increase in [Ca2+]i levels from 95±28 to 455±55 nM (n = 7, Fig. 4B). A modest biphasic response was still apparent, but with an elevated plateau phase compared to HYPO stimulation. This is similar to that shown before in TRPV4-transfected HeLa cells [13]. However, GSK101 had no affect on [Ca2+]i levels when extracellular Ca2+ was removed (Fig. 4C). Indeed, on average GSK101 did not significantly increase [Ca2+]i levels in the absence of extracellular Ca2+ (Fig. 4D) demonstrating its affect only on Ca2+ influx pathways. To further assess the specificity of GSK101 on TRPV4, the effect of prior incubation (5 min) with HC-067047 (100 nM) was evaluated. As shown in Figure 4C, HC-067047 completely abolished activation of TRPV4 by GSK101 as evidence by the abolition of an increase in [Ca2+]i. Hence, GSK101 appears to solely activate Ca2+ influx pathways, i.e., TRPV4, with little or no influence on Ca2+ stores.
TRPV4 expression at the plasma membrane was likewise analyzed via immunoblots in a similar manner as done for HYPO exposure, described above. When TRPV4 intensities were normalized to the plasma membrane marker, pan cadherin, it was apparent that plasma membrane levels of TRPV4 likewise were not significantly altered for up to 30 minutes of GSK101 exposure (see Fig. 3C). Again, this differs from that observed with GSK101 stimulation in the heterologous expression systems where TRPV4 activation was followed by rapid net retrieval from the plasma membrane [13].
3.4. Expression of BK and SK3 Channels and Their Dependency on TRPV4 Expression
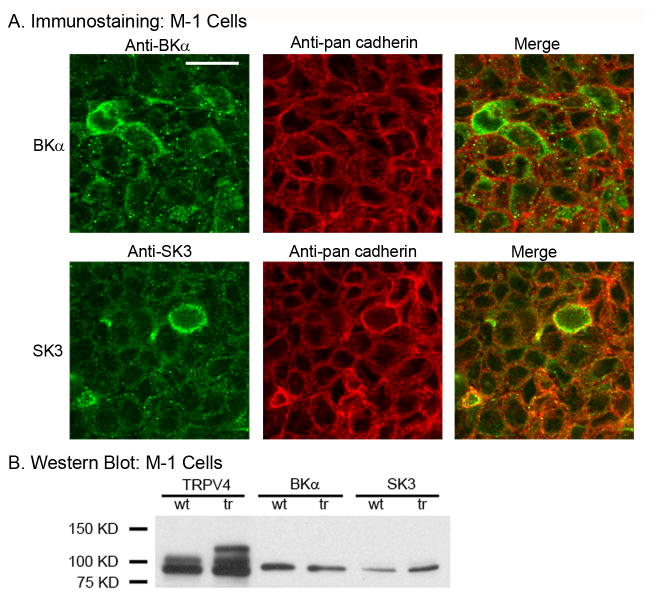
A preliminary screen of M-1 cells identified expression of two Ca2+-dependent K+ channels in confluent monolayers of M-1 cells, the BKα and SK3 channels. As shown in the immunofluorescent images of Figure 5A, expression of both BKα and SK3 were apparent in M-1 cells. Modest colocalization with the plasma membrane marker, pan cadherin, demonstrates both cytoplasmic and plasma membrane, or near plasma membrane, expression for both K+ channels.
Figure 5.
Expression of BKα and SK3 K+ channels in M-1 cells. A. Immunofluorescent staining of M-1 cells for BKα (Anti-BKα, green) and pan cadherin (Anti-pan cadherin, red) (Upper panel) and for SK3 (Anti-SK3, green) and pan cadherin (Anti-pan cadherin, red) (Lower panel). Merged images show modest collocation of BKα and SK3 with pan cadherin at the plasma membrane. Scale bar = 20 μm. B. Western blot analysis of TRPV4, BKα, and SK3 expression levels in wild type (wt) M-1 cells and in TRPV4-transfected (tr) M-1 cells. Note, the TRPV4 construct used for transfection (TRPV4 tagged with mVenus) runs higher on immunoblots and, therefore, leads to multiple bands on the immunoblot. TRPV4 expression levels were, as expected, greatly elevated with TRPV4 overexpression (tr) whereas BKα and SK3 showed little or no dependence on TRPV4 overexpression levels.
Western blot analysis of confluent M-1 cells also demonstrated strong expression of both BKα and SK3 (Fig. 5B). Both K+ channels also appeared to functionally interact with TRPV4 (see below). We, therefore, assessed whether expression of the K+ channels may be dependent upon TRPV4 expression since TRPV4 appears to be an initiating component. To begin to assess for a potential dependence of BKα and SK3 on TRPV4, M-1 cells were transiently transfected (2 days) with a mouse TRPV4 construct (mVenus-tagged TRPV4 cDNA) to yield TRPV4-overexpressing M-1 cells. Immnuoblots of wild type M-1 cells (wt) and TRPV4-transfected M-1 cells (tr) were evaluated. As shown in Figure 5B, TRPV4 was strongly expressed in wt M-1 cells (also see Fig. 1B, TRPV4) displaying the expected double band near 100 kD. In TRPV4-mVenus transfected M-1 cells (tr), the additional bands of the TRPV4-mVenus construct were also apparent at higher molecular weights as expected (reflecting the mVenus tag). TRPV4 transfection enhanced TRPV4 expression levels as expected. However, BKα expression levels appeared to decline modestly (BKα, wt versus trs) while SK3 expression levels tended to increase modestly (SK3, wt versus trs). A strong dependence of BKα and SK3 expression on TRPV4 was not readily apparent from this analysis. Immunoprecipitation analysis was not performed since our primary anti-TRPV4 antibody did not immunoprecipitate well. Hence, a dependence of BK or SK3 expression on TRPV4 expression did not appear to be present in the M-1 cells.
3.5. Functional coupling between TRPV4 and both BK and SK3 in M-1 cells
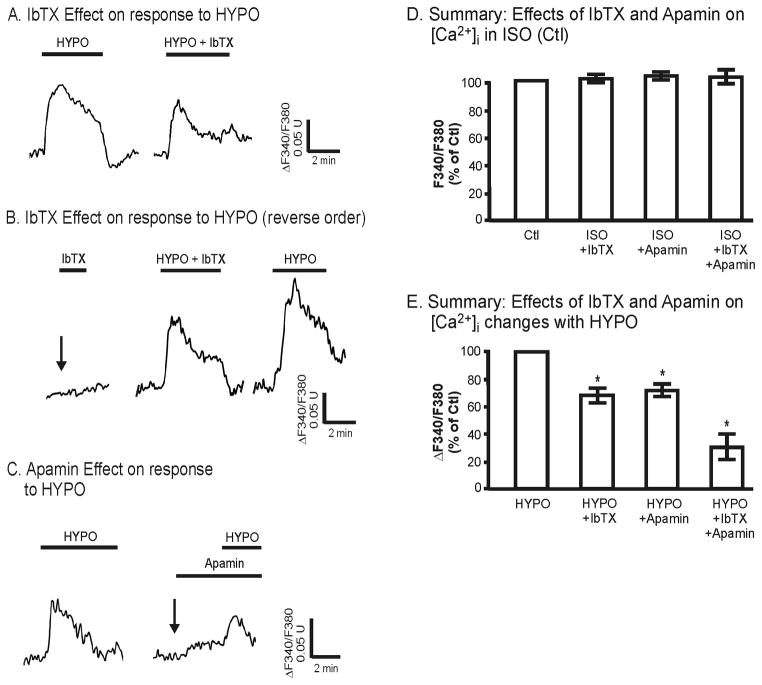
Does hypotonicity-induced activation of TRPV4 and the associated Ca2+ influx depend upon the Ca2+-dependent K+ channels? We were particularly interested in assessing whether activation of BK or SK3 would, in and of itself, also modulate intracellular calcium levels since this would, in part, control K+ secretion by collecting duct cells. As shown in Figure 6A and 6B, HYPO leads to the expected biphasic elevation in [Ca2+]i due to TRPV4-mediated Ca2+ influx (and Ca2+ release). Prior to HYPO treatment, addition of the selective BK antagonist, iberiotoxin (IbTX, 100 nM), in the isotonic state (Fig. 6B, arrow) had no effect on [Ca2+]i, results consistent with an inactive BK channel (Fig 6D). In contrast, in the presence of IbTX the HYPO-induced changes in [Ca2+]i were markedly blunted as shown in representative double test protocols (Fig. 6A versus 6B). In these studies, cells were first exposed to HYPO conditions, returned to control isotonic media (Ctl) for 5–10 minutes, then followed by a second HYPO exposure in the presence of IbTX (Fig. 6A). IbTX led to a significant inhibition of the Ca2+ response (Fig. 6A). Reversing the order with IbTX added during the first exposure to hypotonic media produced a similar level of inhibition (Fig. 6B). On average IbTX treatment reduced the peak change in the HYPO-induced [Ca2+]i by 32 ± 6% (n = 6, Fig. 6D). Hence, the HYPO-induced [Ca2+]i is enhanced with BK activation (no IbTX), but depressed in the presence of BK inhibition (IbTX).
Figure 6.
Effect of BK antagonist, IbTX, and SK3 antagonist, apamin, on [Ca2+]i response to HYPO in M-1 cells. A. Representative trace showing the effect of HYPO on [Ca2+]i followed by a repeat HYPO stimulation in the presence of IbTX (100 nM). B. Representative tracing showing that IbTX under isotonic control conditions had no affect on [Ca2+]i (arrow), and reversing the order of IbTX on the HYPO response showed the same inhibition by IbTX on HYPO-induced [Ca2+]i changes. C. Typical [Ca2+]i response to HYPO followed by HYPO response in the presence of apamin (300 nM). D. Summary response of resting [Ca2+]i ratio upon addition of IbTX (n = 3), apamin (n = 3), and IbTX + apamin (n = 3) under control, isotonic (ISO), conditions. The inhibitors had no affect on the resting [Ca2+]i levels. E. Summary showing the HYPO-induced changes in [Ca2+]i ratio (Δ), relative to the control HYPO response (100%), in the presence of IbTX (68 ± 6%, n = 6), apamin (72 ± 4%, n = 4), and IbTX + apamin (28 ± 10%, n = 3). Both inhibitors modestly reduced the HYPO-induced increase in [Ca2+]i ratio, but the combined response showed a much strong reduction in [Ca2+]i. *P<0.05.
In a similar manner as observed for IbTX, addition of the selective SK antagonist, apamin (300 nM), likewise had no effect on [Ca2+]i in isotonic mediate (Fig. 6C, arrow; Fig. 6D), but markedly depressed the hypotonicity-induced peak change in [Ca2+]i. On average, apamin lead to an inhibition of the peak response by 28 ± 4% (n = 4, Fig. 6E). This is slightly less than that observed with IbTX, but the overall affect is similar. However, when IbTX and apamin are added together, the inhibition of the peak response is even greater, averaging near 70% inhibition (Fig. 6E). Hence, while neither BKα or SK3 appeared to be active prior to TRPV4 stimulation (Fig. 6D), TRPV4 activation by HYPO media induced a K+ channel-sensitive Ca2+ influx where blockade of the either the BK or SK3 channels, or both, markedly reduces [Ca2+]i. This apparent cross-talk between TRPV4 and the Ca2+-dependent K+ channels displays a synergy that may modulate both cell signaling events and potentially alter net fluxes in polarized epithelial cells such as K+ secretion in the renal collecting duct (see below).
Finally, the effect of IbTX on GSK101 Ca2+ influx was also tested in a few studies. As for the HYPO situation, activation of TRPV4 by addition of GSK101 (10 nM) likewise revealed an IbTX-sensitive component of Ca2+ influx. On average IbTX lead to a 24 ± 4 % reduction in the TRPV4-mediated [Ca2+]i response to GSK101 (data not shown). Further testing with GSK101 was not performed since the high Ca2+ influx associated with GSK101 activation of TRPV4 appears to “push” the cells into a high-Ca2+ resistance state as we have previously described (see [13]).
4. Discussion
Stimulation of renal collecting duct M-1 cells by mechanical stress leads to rapid activation of TRPV4 and Ca2+ influx [6]. The present studies demonstrate that exposure of M-1 cells to hypotonic swelling conditions leads to a biphasic elevations in [Ca2+]i which is characterized by a transient peak elevation followed by relaxation to a more prolonged pseudo-plateau phase with modestly elevated [Ca2+]i level. We have previously shown for transiently transfected TRPV4-HeLa cells that following activation of TRPV4 the channel would rapidly desensitize, largely as a result of retrieval of TRPV4 from the plasma membrane into the cytosol [13]. We had anticipated a similar trafficking outcome for the M-1 cells which endogenously express TRPV4. However, we show in this study that hypotonic activation of TRPV4 in M-1 cells does not lead to net retrieval of TRPV4 from the plasma membrane (Fig. 3) despite the biphasic nature of the [Ca2+]i response (Fig. 2). TRPV4 levels at the plasma membrane remain relatively constant over many minutes of hypotonic stress. It may be that hypotonic stimulation may simultaneously activate both TRPV4 insertion and retrieval from the plasma membrane so that the net abundance remains unchanged, although this was not the case for TRPV4 in HeLa overexpression system [13]. In this regard it is interesting that Heller and coworkers [14] have recently demonstrated that PACSIN 3, an inhibitor of the endocytotic machinery [41], is highly expressed at the luminal border of TRPV4-expressing renal cells. It was shown to bind to TRPV4 and lead to TRPV4 retention in the luminal cell membrane, supposedly due to inhibition of TRPV4 endocytosis. Furthermore, it was subsequently shown that expression of PACSIN 3 can lead to inhibition of TRPV4 activation by hypotonic swelling in TRPV4-transfected HEK cells [42]. Hence, it may be that PACSIN 3 in M-1 CCD cells serves to retain TRPV4 in the plasma membrane following hypotonic swelling with subsequent inhibition, or partial inhibition, of TRPV4 activity leading to the biphasic nature of the hypotonic response. This concepts remains to be fully tested in future studies as it may reflect a critical pathway in control of TRPV4 activity in the CCD. Other pathways and modulators could also play a role in this process.
An alternative pathway that may modulate TRPV4-mediated Ca2+ influx in M-1 cells may be the Ca2+-dependent K+ channels and control of the membrane potential. It is well known that in the early collecting duct system, including the CCD, Ca2+ plays a major role in modulating K+ secretion and, hence, K+ balance [18–20]. K+ secretion in this segment has been shown to be flow sensitive arising from Ca2+-dependent K+ secretion largely through activation of BK. We have recently identified TRPV4 as a flow-sensor in the M-1 collecting duct cells [6] and shown its expression at the luminal membrane of the mouse CCD [5] while Suzuki and coworkers have demonstrated a marked reduction in flow-induced K+ secretion in CCD from mice lacking the TRPV4 gene [17]. While BK would appear to be the dominant effector pathway in this response, our present studies open up the possibility that SK3 could be a newly identified effector in this process. Both of these channels appear to be activated upon exposure of M-1 cells to hypotonic swelling conditions (or by agonist activation of TRPV4). To this end we demonstrate in these studies that there appears to be a synergistic cross-talk between TRPV4 and the K+ channels since TRPV4 activation is needed to activate BK and SK3 while at the same time activation of BK and SK3 appears to enhanced Ca2+ influx (via TRPV4) as a result of hyperpolarization of the plasma membrane (see discussion, below). While such cross-talk has not been explicitly described before for the renal collecting duct system, it is implicit from studies in cells expressing similar types of Ca2+ and K+ channels as outlined below. Hence, the TRPV4-mediated cross talk with the downstream K+ channels may underlie flow-dependent K+ secretion where the apparent synergism between channels would serve to drive this entire process and modulation of K+ balance.
Ca2+-dependent K+ channels are widely expressed in both excitable and non-excitable cells where they are thought to play a major role in modulating membrane potential [43–44]. Some cell types, such as vascular endothelial cells, express a wide range of Ca2+-dependent K+ channels, including SK, IK, and BK channels, where the channels function to control membrane hyperpolarization as an important component of the endothelial-derived hyperpolarizing factors underlying vascular dilation/blood pressure modulation [45–47]. Similarly, Ca2+-dependent K+ channels have been widely described in a diverse range of both excitable and non-excitable cells [43, 48–50] where they again play a role in modulating membrane potential. Important for the control of these channels is alterations in [Ca2+]i that can arise from Ca2+ release from internal stores or, the more likely scenarios, from stimulation of Ca2+ influx into the cells from both TRP channels and various voltage activated Ca2+ channels [25, 51]. Furthermore, functional associations of channels within a common microdomain or tethered complex can greatly alter any cross-talk although such associations are largely unknown for this channel grouping [see 44]. Hence, the interplay between Ca2+ influx channels and the range of Ca2+-dependent K+ channels can vary greatly from one setting, or cell type, to the next. In the renal collecting duct cells this appears to be dominated by TRPV4 and its cross-talk with BK and SK3.
We also hypothesize that within the collecting duct cells that the cross-talk between TRPV4 and the K+ channels likely reflects a variable synergism or progression in K+ channel activation. This would arise because of the differences in the Ca2+-sensitivity of the K+ channels being expressed: SK3 is highly Ca2+ -sensitive (mid-nanomolar range) while BK has a low Ca2+-sensitivity (low micromolar range) [see 43–44]. Hence, it may be that the initial TRPV4-mediated Ca2+ influx would first activate the SK3 channel, leading to modest membrane hyperpolarization and increased Ca2+ influx which, if [Ca2+]i were high enough, would lead to subsequent activation of BK with a further hyperpolarization and more Ca2+ influx. Other signaling pathways controlling the channel activities could, in turn, modulate the response. Hence, some diversity in the response is anticipated because of the variable Ca2+-sensitivities of the channels being expressed.
Finally, from an analysis of the driving forces for Ca2+ influx in the M-1 cells, it is apparent that cross-talk between TRPV4 and BK/SK3 can be very significant, and lead to important functional coupling. To what extent can there be a reciprocal modulation between channel types leading to increased Ca2+ influx as a consequence of membrane hyperpolarization following K+ channel activation? If it is assumed that upon TRPV4 activation the [Ca2+]i can approach at least 200 nM (likely more, see Fig. 2B and 3B), the chemical driving force for Ca2+ entry with 1 mM extracellular would be near −110 mV [ECa = 30 mV × log (1 × 10−3/200 × 10−9)]. With a resting membrane potential of near −40 mV for the M-1 cells [52], the total driving force for Ca2+ entry would be near −150 mV (−110 + −40 mV). Assuming the Vm were to hyperpolarize to −80 mV with maximal K+ channel activation, but reduced to near 0 mV with K+ channel inhibition, the driving force for Ca2+ entry would change from near −190 mV to near −110 mV for the two states, respectively. This equates to a 30–50% change in the Ca2+ driving force depending upon the state of K+ channel activation. Hence, the potential range of BK/SK3-induced modulation of the Ca2+ driving force can be very substantial when considering Vm changes only. This scenario is also consistent with the observed changes in [Ca2+]i upon inhibition of the BK and SK3 channels reported in the current study (see Fig. 6). Hence, the cross talk in M-1 cells and CCD cells may be a major factor in setting both the rate of calcium influx and the extent of membrane hyperpolarization.
In summary, the current study has demonstrated that hypotonicity-induced activation of TRPV4 in renal collecting duct cells leads to activation of Ca2+ entry without alterations in membrane trafficking to the plasma membrane. Further, this Ca2+ influx leads to activation of two Ca2+-dependent K+ channels, BK and SK3, both of which display a functional synergistic cross-talk leading to modulation of channel functions. It is likely that such regulation may underlie Ca2+ signaling events and flow-dependent K+ secretion in the TRPV4-expressing segments of the mammalian collecting duct.
Acknowledgments
This work was supported by NIH grants to RGO, R01 DK070950 and R21 DE018522.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 3.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007;1772:907–914. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Jin M, Pochynyuk O, O’Neil GR. TRPV4 channel activation is required for flow-dependent K+ secretion/BK channel activation in mouse cortical collecting duct (CCD) FASEB J. 2011;25:1041.1029. [Google Scholar]
- 6.Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 7.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegierski T, Lewandrowski U, Muller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Wu L, O’Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 10.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Elias A, I, Lorenzo M, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283:31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- 12.Strotmann R, Semtner M, Kepura F, Plant TD, Schoneberg T. Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4. PLoS ONE. 2010;5:e10580. doi: 10.1371/journal.pone.0010580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin M, Wu Z, Chen L, Jaimes J, Collins D, Walters ET, et al. Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLoS ONE. 2011;6:e16713. doi: 10.1371/journal.pone.0016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuajungco MP, Grimm C, Oshima K, D’Hoedt D, Nilius B, Mensenkamp AR, et al. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem. 2006;281:18753–18762. doi: 10.1074/jbc.M602452200. [DOI] [PubMed] [Google Scholar]
- 15.Shukla AK, Kim J, Ahn S, Xiao K, Shenoy SK, Liedtke W, et al. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem. 2010;285:30115–30125. doi: 10.1074/jbc.M110.141549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol. 2009;88:141–152. doi: 10.1016/j.ejcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol. 2007;292:F667–673. doi: 10.1152/ajprenal.00458.2005. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol. 2007;293:F227–235. doi: 10.1152/ajprenal.00057.2007. [DOI] [PubMed] [Google Scholar]
- 19.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol. 2010;21:634–645. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 21.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 23.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, et al. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 24.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 26.Marrelli SP, O’Neil GR, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1390–1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 27.Reiter B, Kraft R, Gunzel D, Zeissig S, Schulzke JD, Fromm M, et al. TRPV4-mediated regulation of epithelial permeability. FASEB J. 2006;20:1802–1812. doi: 10.1096/fj.06-5772com. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Fernandez JM, Andrade YN, Arniges M, Fernandes J, Plata C, Rubio-Moscardo F, et al. Functional coupling of TRPV4 cationic channel and large conductance, calcium-dependent potassium channel in human bronchial epithelial cell lines. Pflugers Arch. 2008;457:149–159. doi: 10.1007/s00424-008-0516-3. [DOI] [PubMed] [Google Scholar]
- 29.Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2010 doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 31.Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 33.Hu Z, Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci U S A. 2007;104:16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–729. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 35.Liedtke W. TRPV channels’ role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007:473–487. doi: 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- 36.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropa noyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamid e (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 38.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Gordon E, Lin Z, Lozinskaya IM, Chen Y, Thorneloe KS. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin) 2009;3:156–160. doi: 10.4161/chan.3.3.8555. [DOI] [PubMed] [Google Scholar]
- 40.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient Receptor Potential Vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse esophageal keratinocytes. J Physiol. 2011 doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modregger J, Ritter B, Witter B, Paulsson M, Plomann M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci. 2000;113(Pt 24):4511–4521. doi: 10.1242/jcs.113.24.4511. [DOI] [PubMed] [Google Scholar]
- 42.D’Hoedt D, Owsianik G, Prenen J, Cuajungco MP, Grimm C, Heller S, et al. Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem. 2008;283:6272–6280. doi: 10.1074/jbc.M706386200. [DOI] [PubMed] [Google Scholar]
- 43.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 44.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens. 2011 doi: 10.1097/MNH.0b013e3283488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weatherall KL, Goodchild SJ, Jane DE, Marrion NV. Small conductance calcium-activated potassium channels: from structure to function. Prog Neurobiol. 2010;91:242–255. doi: 10.1016/j.pneurobio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Faber ES. Functions and modulation of neuronal SK channels. Cell Biochem Biophys. 2009;55:127–139. doi: 10.1007/s12013-009-9062-7. [DOI] [PubMed] [Google Scholar]
- 51.Millership JE, Heard C, Fearon IM, Bruce JI. Differential regulation of calcium-activated potassium channels by dynamic intracellular calcium signals. J Membr Biol. 2010;235:191–210. doi: 10.1007/s00232-010-9266-1. [DOI] [PubMed] [Google Scholar]
- 52.Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. Am J Physiol. 1997;272:C657–666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]