Abstract
Background
The duration of protection conferred by prophylactic human papillomavirus (HPV) L1 virus-like particle vaccines is a critical determinant of their public health impact. A feature of vaccines that confer long-term immunity is their ability to induce immune memory.
Objectives
We evaluated antibody responses against HPV types 6, 11, 16 and 18 following administration of the quadrivalent HPV-6/11/16/18 vaccine to women who had previously received a monovalent HPV-16 vaccine.
Study design
As part of an extended follow-up study conducted between 2006 and 2009 in Seattle, Washington, we administered the quadrivalent HPV-6/11/16/18 vaccine to 52 women (19 vaccine and 33 placebo recipients) who had participated in a monovalent HPV-16 vaccine trial 8.5 years earlier. Serum samples were tested for anti-HPV antibodies using competitive Luminex immunoassay.
Results
Following administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine, the anti-HPV-16 geometric mean titer among monovalent HPV-16 vaccine recipients (GMT = 5024.0 milli-Merck units per milliliter [mMU/mL]; 95% confidence interval [CI]: 2710.1, 9313.6 mMU/mL) substantially exceeded that among the placebo recipients (GMT = 136.1; 95% CI: 78.5, 235.8 mMU/mL; p < 0.01) and their own highest anti-HPV-16 response observed during the original trial (GMT at month 7 of the original trial = 1552.7 mMU/mL; 95% CI: 1072.6, 2247.7 mMU/mL; p < 0.01).
Conclusions
The findings suggest that the administration of the three-dose regimen of the monovalent HPV-16 vaccine had produced memory lymphocytes, characterized by a heightened immune response following administration of the quadrivalent HPV-6/11/16/18 vaccine that effectively served as an antigen challenge.
Keywords: Human papillomavirus type 16, Vaccines, Immune memory
1. Background
In randomized controlled trials (RCTs), prophylactic human papillomavirus (HPV) L1 virus-like particle (VLP) vaccines have shown a high-level of efficacy against infection and cervical intraepithelial neoplasia associated with the vaccine-types.1–3 In these trials, all vaccine recipients seroconverted by one month after completion of the three-dose vaccine series and a large proportion of them remained seropositive at the end of the follow-up time. In addition, extended follow-up studies of participants in these trials have provided evidence of sustained immune response through at least 7.3 years.4,5 The basis for protection conferred by these vaccines is believed to be the production of type-specific neutralizing antibodies.6
The main target group of prophylactic HPV vaccines is children before sexual debut. Thus, it is important to assess how long antibodies generated following administration of these vaccines last. A feature of vaccines that confer long-term immunity is their ability to induce immune memory. Immune memory is defined as the generation of long-lived memory cells that, upon re-exposure to the same antigen, mount a rapid and robust immune response capable of preventing infection. Evidence exists that the quadrivalent HPV-6/11/16/18 vaccine is capable of inducing immune memory. In response to an antigen challenge given at 5 years following administration of that vaccine, participants mounted an anamnestic response characterized by rapid and robust antibody production against all four vaccine types.7
2. Objectives
As part of an extended follow-up study, we had a unique opportunity to assess the antibody response against HPV-16 following administration of the quadrivalent HPV-6/11/16/18 vaccine to women who had participated in a monovalent HPV-16 vaccine trial 8.5 years earlier. As a secondary objective, we assessed antibody responses against other vaccine types (i.e., HPV types 6, 11, and 18) among these women.
3. Study design
3.1. Study population
Between October 1998 and November 1999, 2391 women were enrolled in a multi-center, double-blind, phase IIb RCT of a monovalent HPV-16 vaccine in the United States. The monovalent vaccine was administered in 3 doses on day 1, month 2, and month 6. Administration of the vaccine resulted in the generation of a strong immune response as measured by anti-HPV-16 geometric mean titers (GMTs).2 The highest anti-HPV-16 GMT was observed at the month 7 (i.e., post-dose 3) visit.2 Of 2391 participants in that trial, 500 women were enrolled in Seattle, Washington. Beginning in February 2006, all these 500 women were offered participation in a new extended follow-up study with visits occurring every 6 months to assess the longer-term efficacy of the monovalent HPV-16 vaccine. According to the study protocol, after the quadrivalent HPV-6/11/16/18 vaccine was licensed in the U.S. in 2006, we offered it to all participants in the original trial. Therefore, we were able to assess antibody responses against HPV types 6, 11, 16, and 18 following administration of the quadrivalent HPV-6/11/16/18 vaccine to women who had already received the monovalent HPV-16 vaccine. The institutional review board of the University of Washington approved the study.
3.2. Laboratory methods
Ten milliliters of blood were drawn at each visit and shipped to Merck Research Laboratories (MRL), West Point, Pennsylvania. At the laboratory, specimens were tested using a competitive Luminex immunoassay (cLIA).8,9 This is the primary assay used by MRL to evaluate the serological response to the vaccine. In this assay, yeast-derived VLPs are coupled to a set of distinct fluorescent Luminex microspheres. Antibody titers are determined in a competitive format, where known type-specific phycoerythrin (PE)-labeled neutralizing monoclonal antibodies (mAbs) compete with the subject’s serum antibodies for binding to conformationally sensitive neutralizing epitopes on the VLPs. The concentration of HPV antibody measured by this assay is reported in milli-Merck units per milliliter (mMU/mL); the fixed cutoff points to determine HPV-6, HPV-11, HPV-16, and HPV-18 cLIA seropositivity are 20, 16, 20, and 24 mMU/mL, respectively. All specimens from the original trial and the extended follow-up study included in the present analysis were tested by this assay in the same fashion. As part of the extended follow-up study, women were also tested for the presence of HPV DNA in labial, vulvar, lateral vaginal, ecto- and endocervical, perineal, and perianal specimens to determine their infection status.5
3.3. Statistical analysis
Anti-HPV GMTs and their corresponding 95% confidence intervals were calculated at 2 distinct time-points of interest relative to the administration of the quadrivalent vaccine. These time-points are referred to as pre-dose 1 and post-dose 1 visit throughout this manuscript. The pre-dose 1 visit refers to the last visit before administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine or the visit during which the first dose of this vaccine was administered following blood draw, whichever came last. The post-dose 1 visit refers to the visit following administration of the first dose and before administration of the second dose of the quadrivalent HPV-6/11/16/18 vaccine. Paired-coordinate plots were created to show the trajectory of change in antibody levels from the pre-dose 1 to post-dose 1 visit for each individual. Women who were found to be infected with HPV types 6, 11, 16, or 18 during either the pre-dose 1 or post-dose 1 visit were excluded from the analysis to remove the effect of current infection on antibody levels.
4. Results
A total of 52 women (19 monovalent HPV-16 vaccine and 33 placebo recipients in the original trial) were included in the final analysis (Figure 1). At the pre-dose 1 visit, the mean age of participants was 29 years (range: 26–32 years) and the mean follow-up time since enrollment in the original trial was 8.5 years (range: 7.7–9.6 years) in both groups. The post-dose 1 visit occurred, on average, 6 months following administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine in both groups reflecting the design of the extended follow-up study in which visits occurred every 6 months. However, there was a large variability in the number of days between administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine and post-dose 1 visit (range: 0.7–18 months) because vaccine visits did not necessarily coincide with the routine scheduled visits during the extended follow-up study.
Figure 1.
Flow chart of the study population
At the pre-dose 1 visit, 89.5% of monovalent HPV-16 vaccine recipients and 9.1% of placebo recipients were HPV-16 seropositive (Table 1). Following administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine, HPV-16 antibody levels rose sharply among both monovalent HPV-16 vaccine and placebo recipients (Figure 2). Monovalent HPV-16 vaccine recipients mounted potent anti-HPV-16 responses (GMT = 5024.0 mMU/mL; 95% confidence interval [CI]: 2710.1, 9313.6 mMU/mL) that substantially exceeded anti-HPV-16 responses observed among the placebo recipients (GMT = 136.1; 95% CI: 78.5, 235.8 mMU/mL; p < 0.01). Anti-HPV-16 responses among monovalent HPV-16 vaccine recipients at post-dose 1 even exceeded their own highest anti-HPV-16 responses observed during the original trial with a 3-fold increase (Month 7 GMT = 1552.7 mMU/mL; 95% CI: 1072.6, 2247.7 mMU/mL; p < 0.01).
Table 1.
HPV antibody responses following administration of the quadrivalent HPV-6/11/16/18 vaccine to women who had participated in the monovalent HPV-16 vaccine trial
| Monovalent HPV-16 Vaccine
|
Placebo
|
|||||
|---|---|---|---|---|---|---|
| n | GMT (95% CI) | % Seropositive | n | GMT (95% CI) | % Seropositive | |
| HPV-6 | ||||||
| Pre-dose 1 | 19 | 11.7 (7.7, 17.7) | 21.0 | 33 | 9.4 (7.2, 12.2) | 12.1 |
| Post-dose 1 | 19 | 139.1 (48.1, 402.6) | 89.5 | 33 | 98.8 (49.3, 198.1) | 75.7 |
| HPV-11 | ||||||
| Pre-dose 1 | 19 | 8.0 (8.0, 8.0) | 0.0 | 33 | 8.0 (8.0, 8.0) | 0.0 |
| Post-dose 1 | 19 | 106.7 (50.5, 225.3) | 94.7 | 33 | 82.5 (53.8, 126.4) | 91.0 |
| HPV-16 | ||||||
| Pre-dose 1 | 19 | 73.6 (46.5, 116.5) | 89.5 | 33 | 12.7 (10.4, 15.5) | 9.1 |
| Post-dose 1 | 19 | 5024.0 (2710.1, 9313.6) | 100.0 | 33 | 136.1 (78.5, 235.8) | 91.0 |
| HPV-18 | ||||||
| Pre-dose 1 | 19 | 11.0 (9.0, 13.4) | 5.3 | 33 | 11.3 (9.5, 13.4) | 3.0 |
| Post-dose 1 | 19 | 13.6 (9.1, 20.3) | 10.5 | 33 | 17.7 (11.5, 27.1) | 15.1 |
GMT: Geometric mean titer; CI: Confidence interval
Note: The pre-dose 1 visit refers to the last visit before administration of the first dose of the quadrivalent vaccine or the visit during which the first dose of this vaccine administered, whichever came last. The post-dose 1 visit refers to the visit following administration of the first and before administration of the second dose of the quadrivalent vaccine.
Figure 2.
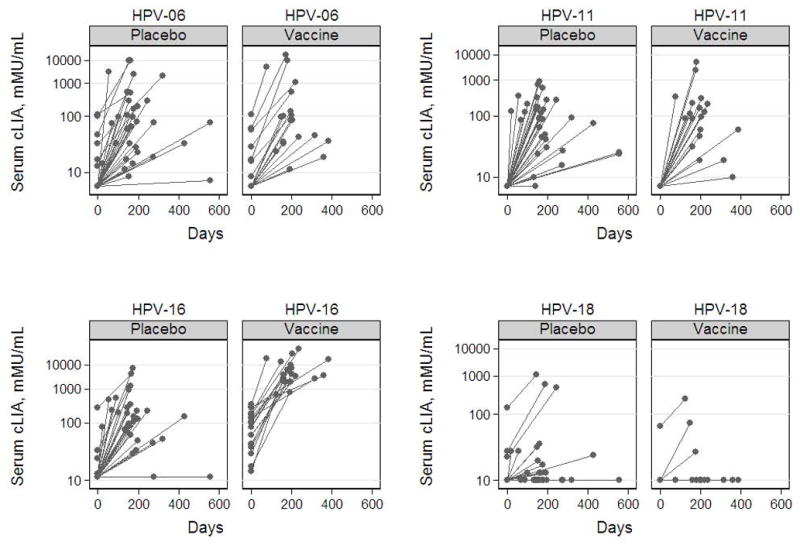
Antibody responses against HPV type 6, 11, 16, and 18 following administration of the quadrivalent HPV-6/11/16/18 vaccine to women who had participated in the monovalent HPV-16 vaccine trial. The pre-dose 1 visit refers to the last visit before administration of the first dose of the quadrivalent vaccine or the visit during which the first dose of this vaccine was administered before blood draw, whichever came last. The post-dose 1 visit refers to the visit following administration of the first and before administration of the second dose of the quadrivalent vaccine.
A substantial proportion of women seroconverted against HPV types 6 and 11, but not 18, following administration of the first dose of the quadrivalent HPV-6/11/16/18 vaccine (Table 1). At the post-dose 1 visit, the antibody responses against HPV types 6 (p = 0.56), 11 (p = 0.51), and 18 (p = 0.41) were not significantly different between women who had received the monovalent HPV-16 vaccine and placebo in the original trial. At that visit, women who had received the monovalent HPV-16 vaccine in the original trial mounted significantly stronger antibody responses against HPV type 16 than against HPV types 6, 11, and 18 (p < 0.01).
5. Discussion
The heightened immune response observed in this study suggests that the administration of the 3-dose regimen of the monovalent HPV-16 vaccine had produced memory lymphocytes, characterized by high antibody levels following an antigen challenge. The antigen challenge in this study was re-exposure to HPV-16 L1 VLPs through administration of the quadrivalent HPV-06/11/16/18 vaccine to women who had received the monovalent HPV-16 vaccine 8.5 years earlier. Previous studies had shown heightened antibody responses to quadrivalent HPV vaccine among women who were seropositive for vaccine HPV types at enrollment.10,11 The results of this study add to the existing body of knowledge that prophylactic HPV vaccines may generate long-term immune memory.7
No monovalent vaccine recipients, including those who had become seronegative during the course of follow-up, developed breakthrough HPV-16 infection in the extended follow-up study. More information on the efficacy of the monovalent vaccine through 8.5 years of follow-up can be found elsewhere.5 It is conceivable that upon natural exposure to HPV-16 in genital tract or other mucosal surfaces, monovalent vaccine recipients mounted an anamnestic response which augmented protection against infection. Such speculation is plausible since the host cell-bound papillomaviruses appear to be susceptible to inactivation by neutralizing antibodies.12 It is likely that anamnestic response plays a central role in preventing infection and that any potential breakthrough infections are not a direct result of waning immunity; however, conclusive evidence in support of this hypothesis has yet to be demonstrated.13
It is not known whether a certain threshold antibody level is required to prevent HPV infection. In fact, no correlation may exist between the frequency of L1 VLP-specific memory lymphocytes and the corresponding serum antibody titers. Memory lymphocyte-mediated protection against infection may persist despite low serum antibody titers, as has been shown for the hepatitis B vaccine.14 In the current study, monovalent HPV-16 vaccine recipients who were cLIA seronegative at the pre-dose 1 visit also mounted a robust immune response following administration of the quadrivalent HPV-6/11/16/18 vaccine. This suggests that, although these women maintained a low immune response over time, they likely did have immune memory lymphocytes capable of providing a robust immune response. Alternatively, the laboratory assays used in this study may not have been sensitive enough to detect protective antibody levels in some women.
This study is subject to some limitations. First, the time-frame used for the serum collection post-vaccination was reflective of routine scheduled visits separated by 6 months; it was not designed to capture peak immune response which is typically measured 1 month following administration of each dose. Therefore, anti-HPV GMTs reported in this study almost certainly underestimated peak post-dose titers. An example is the observed low antibody responses against HPV-18 in this study. It was previously shown that antibody responses against HPV-18 diminished more rapidly than other types; however, the efficacy against infection and cervical lesions associated with this type remained remarkably high following administration of all three doses.13 In our study, the anamnestic response, characterized by rapid and robust antibody production, was inferred through comparing antibody levels; it was not directly measured in the conventional time-frame following immunization. Second, the mode of antigen challenge in this study (i.e., intramuscular administration of the quadrivalent HPV-6/11/16/18 vaccine) was different from that of a natural antigen challenge (i.e., HPV exposure via sexual contact). It is not clear whether the anamnestic response following natural exposure would exhibit the same features of the one observed in this study. Third, while the increase in humoral immune response in an anamnestic fashion suggests the presence of circulating memory lymphocytes, the actual lymphocytes were not directly studied. Fourth, standardized units have not yet been formally established for reporting antibody responses against all four HPV types investigated in this study. Therefore, we were not able to report antibody responses using international units (IUs). While this limitation may make comparisons across studies where the same unit is not used challenging, it does not jeopardize the internal validity of the study. The World Health Organization has established a global HPV laboratory network with a main goal of the harmonization of HPV laboratory procedures in support of consistent monitoring and reporting of HPV studies in the future.15–17
If prophylactic HPV vaccines confer long-lasting immunity, they can potentially prevent a large number of cases of cervical cancer globally. Long-term immunity conferred by these vaccines is particularly important because the cost and programmatic implications of administration of a booster dose to young women could be overwhelming in the developing world where the HPV vaccines are most needed. Population-based follow-up studies should provide valuable information on the long-term effect of prophylactic HPV vaccines.18,19
Acknowledgments
Funding
National Institute of Allergy and Infectious Diseases (Grant R01 AI38383)
The authors wish to thank the staff of the Harborview Medical Center Women’s Research Clinic for their dedication to this project.
Abbreviations
- RCT
randomized controlled trial
- HPV
human papillomavirus
- VLP
virus-like particle
- GMT
geometric mean titer
- MRL
Merck Research Laboratories
- cLIA
competitive Luminex immunoassay
- CI
confidence interval
Footnotes
Ethical approval
The institutional review board of the University of Washington approved the study.
Conflict of interest
The University of Washington has received funding from Merck & Co., Inc. to support HPV vaccine studies conducted by L.A.K. F.B.A. and J.T.B. were employees of Merck & Co., Inc. at the time of the conduct of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 2.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 4.De Carvalho NS, Roteli-Martins CM, Teieira J, Nahud P. Immunogenicity and safety of HPV-16/18 ASO4-adjuvanted vaccine up to 7.3 years. Presented at the 25th International Papillomavirus Conference; Malmö, Sweden. 8 – 14 May 2009. [Google Scholar]
- 5.Rowhani-Rahbar A, Mao C, Hughes JP, Alvarez FB, Bryan JT, Hawes SE, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27:5612–9. doi: 10.1016/j.vaccine.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109:S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–9. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–69. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poland GA, Jacobson RM, Koutsky LA, Tamms GM, Railkar R, Smith JF, et al. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2-year randomized controlled clinical trial. Mayo Clin Proc. 2005;80:601–10. doi: 10.4065/80.5.601. [DOI] [PubMed] [Google Scholar]
- 11.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–83. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 12.Christensen ND, Cladel NM, Reed CA. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology. 1995;207:136–42. doi: 10.1006/viro.1995.1059. [DOI] [PubMed] [Google Scholar]
- 13.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–51. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Ward SM, Phalora P, Bradshaw D, Leyendeckers H, Klenerman P. Direct ex vivo evaluation of long-lived protective antiviral memory B cell responses against hepatitis B virus. J Infect Dis. 2008;198:813–7. doi: 10.1086/591094. [DOI] [PubMed] [Google Scholar]
- 15.Eklund C, Unger ER, Nardelli-Haefliger D, Zhou T, Dillner J. International collaborative proficiency study of Human Papillomavirus type 16 serology. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.10.096. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Eklund C, Zhou T, Dillner J. Global proficiency study of human papillomavirus genotyping. J Clin Microbiol. 2010;48:4147–55. doi: 10.1128/JCM.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson M, Wilkinson DE, Zhou T. WHO meeting on the standardization of HPV assays and the role of the WHO HPV Laboratory Network in supporting vaccine introduction held on 24–25 January 2008, Geneva, Switzerland. Vaccine. 2009;27:337–47. doi: 10.1016/j.vaccine.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Lehtinen M, Apter D, Dubin G, Kosunen E, Isaksson R, Korpivaara EL, et al. Enrolment of 22,000 adolescent women to cancer registry follow-up for long-term human papillomavirus vaccine efficacy: guarding against guessing. Int J STD AIDS. 2006;17:517–21. doi: 10.1258/095646206778145550. [DOI] [PubMed] [Google Scholar]
- 19.Lehtinen M, Idanpaan-Heikkila I, Lunnas T, Palmroth J, Barr E, Cacciatore R, et al. Population-based enrolment of adolescents in a long-term follow-up trial of human papillomavirus vaccine efficacy. Int J STD AIDS. 2006;17:237–46. doi: 10.1258/095646206776253453. [DOI] [PubMed] [Google Scholar]


