Abstract
Objective
Stress and stress-related concomitants, including hypothalamic-pituitary-adrenal (HPA) axis activation, are implicated in obesity and its attendant co-morbidities. Little is known about this relationship in adolescents. To begin to address this important knowledge gap, we studied HPA axis activity in 262 healthy adolescent girls aged 11, 13, 15, and 17. We hypothesized that obesity would be correlated with increased HPA axis activity and reactivity.
Methods
Measures of HPA axis activity included 3 blood samples obtained mid-day (between 1300 and 1400) over the course of 40 minutes and overnight urine free cortisol (UFC), and cortisol levels 0, 20, and 40 min after venipuncture (cortisol reactivity). Measures of adiposity included BMI, BMI-Z, percent body fat, and fat distribution (central adiposity) assessed by dual energy X-ray absorptiometry.
Results
Daytime levels of serum cortisol were inversely associated with BMI-Z and central adiposity (p < 0.05). The UFC excretion rate was positively correlated with BMI, BMI-Z, and central adiposity. There was blunting of cortisol response to venipuncture with increasing adiposity.
Conclusions
Our results suggest that there may be reduced cortisol levels during the day and increased levels at night with increasing degree of adiposity. This study provides preliminary findings indicating an alteration of the circadian rhythm of cortisol with obesity. We conclude that obesity is associated with altered HPA activity in adolescent girls. The clinical implications of our findings require further investigation.
Keywords: cortisol, adiposity, stress
Introduction
Obesity is widespread in the United States and other industrialized nations (1, 2), leading to significant health problems that impact nearly every organ system. Multiple endocrine disturbances, such as type 2 diabetes and polycystic ovary syndrome, often coexist with obesity. In addition, changes in the functioning of the hypothalamic-pituitary-adrenal (HPA) axis have been implicated as both a cause and a consequence of obesity (3, 4). Furthermore, obesity constitutes a chronic stressor with central and peripheral consequences. It has been proposed that a prolonged period of HPA axis stimulation secondary to the chronic stress of obesity is followed by a breakdown in the regulatory mechanisms of the HPA axis (5). For example, individuals with increased abdominal obesity have greater endogenous glucocorticoid responses to a meal (6) or adrenocorticotropic hormone (ACTH) challenge (7). It is also known that exogenous glucocorticoids increase food intake (8) and promote deposition of abdominal fat that is predictive of cardiovascular disease, particularly in women (5, 6). These observations support the notion that changes in the HPA axis in the setting of obesity may confer greater risk to develop obesity-related comorbidities including anxiety and depression, hypertension, hyperlipidemia, and diabetes.
Determining causality and directionality of the interactions between the HPA axis activity and obesity is hampered by difficulty, in characterizing a dynamic axis with a pronounced diurnal pattern. Not only is the cortisol rhythm diurnal, cortisol levels rise following meals or in response to illness, psychosocial challenge, and exercise. In the circulation, the greatest amount of cortisol is bound to plasma proteins (>90%), particularly corticosteroid binding globulin (CBG) and sex hormone binding globulin (SHBG), whereas tissues “see” only the free (unbound) fraction. Because the secretion of cortisol is diurnal and pulsatile, a single time point for cortisol is inherently unreliable in estimating the total cortisol secretion. Thus, it is not surprising that there are conflicting results concerning the association between cortisol and obesity using single cortisol measures (9, 10). More consistent findings were reported in studies using serial measures of the HPA axis (11-14). Strain and colleagues revealed an increased metabolic clearance rate of cortisol among obese compared with non-obese women (13) and a positive association between absolute cortisol production and relative body weight in men and women (14). Stimulated measures of the HPA axis reflect the reactivity (i.e., hypoactivity vs. hyperactivity) of the stress system. Only one study examined the association between cortisol reactivity and obesity in children (15), demonstrating a positive association between cortisol reactivity to a stressful situation and body mass index.
An individual’s reaction to acute and chronic stress has been hypothesized to be related to the development of obesity. Bjorntorp (16) postulated that a heightened vulnerability to psychosocial stress increases exposure to stress-induced cortisol, which in turn promotes central fat deposition. Other studies supported this theory, particularly among women with central body fat distribution (increased waist-to-hip ratio) (12, 17, 18). Additionally, research has evaluated whether increased stress contributed to the obesity epidemic in children (19). In Swedish infants (N=7443) followed through age 5 years, children in families reporting stress in at least two of the four domains assessed had significantly higher odds (OR, 2.6) of being obese (19).
The pediatric literature contains few studies involving the HPA axis and obesity. Chelew and colleagues evaluated serial serum cortisol concentrations over 20-24 hours in 16 obese males and females aged 5-16 years (20). Compared with lean children, the obese children had significantly lower mean cortisol concentrations. Another study reported no significant associations between the HPA axis and percent body fat in prepubertal obese children (21). However, when central adiposity was examined, a significant inverse association with salivary cortisol response to a meal was found among females but not males and a positive association between urinary glucocorticoid metabolites and central adiposity was also found among females (21). Although studies have begun to explore associations between the HPA axis and obesity in childhood, much remains unknown; thus, investigations in younger, non-adult cohorts may provide insight into the roots of metabolic disease that are expressed in adulthood.
In examining the association between measures of the HPA axis and degree of adiposity in adolescent girls, we hypothesized that measures of adiposity would be correlated with increased HPA axis activity and reactivity. As a secondary aim, we evaluated whether measures of the HPA axis are independently associated with central adiposity when controlling for percent body fat. This study is unique in capturing multiple measures of both the HPA axis and adiposity in a large cohort of adolescent girls.
Methods and Procedures
Two-hundred sixty-two healthy girls aged 11, 13, 15, and 17 years were enrolled in a cross-sequential longitudinal study evaluating the impact of psychological symptoms and smoking on reproductive and bone health (22). The cross-sequential design allows for a cost-effective and more efficient way to capture change across development. Participants are enrolled at different ages and followed for fewer years, compared to a traditional longitudinal design which enrolls all participants at the same age and follows the girls for many years. The current study is a secondary data analysis and includes only baseline data which are only available for girls (i.e., no boys were enrolled in the original study).
Participants were recruited from an academic medical center and the surrounding community of a Midwestern city. Exclusion criteria included pregnancy or breastfeeding within 6 months, primary (≥16 years) or secondary (<6 menstrual cycles/year and >2 years post-menarche) amenorrhea, body mass index (BMI) ≤1st percentile or weight >300 pounds (limitation of dual energy X-ray absorptiometry [DXA] table), medication or medical disorder influencing bone health, or severe psychological disabilities impairing comprehension or compliance with the protocol.
Institutional Review Board approval was obtained. Parents provided consent and adolescents assent for participation. Visits were conducted at the General Clinical Research Center of a children’s hospital. Eligible participants received a container in which to collect overnight urine on the night prior to their study visit. Instructions indicated to void prior to sleep and to save their urine from sleep onset through the first morning void. Time of sleep onset and awakening were recorded to determine the duration of the collection.
The study visit began between 1100 and 1230 hours; post-menarcheal girls were scheduled during days 5 through 9 of their menstrual cycle. Participants had a physical examination for pubertal maturation. A menstrual interview and medication history, including use of hormonal contraceptives, was conducted by the medical professional. Height and weight were measured. Following a two-hour fast, blood samples were obtained. Subsequently, a DXA scan was performed.
Measures
Anthropometric measures
BMI was determined by the mean of three measures of height and weight using a wall-mounted stadiometer (Holtan Ltd., United Kingdom) and digital scale (Scaletronix, Carol Stream, IL), respectively. BMI was computed as weight (kg)/height (m2).
BMI-Z score is a standard calculation to account for relative adiposity in growing children and adolescents (23). The Z score accounts for age and sex by generating a standard deviation score from reference data from Centers for Disease Control and Prevention growth curves (24, 25).
Percent body fat was determined from DXA Hologic QDR4500A scans (Hologic, Inc., Bedford, MA) analyzed using software version 12.4. Percent body fat was derived from total body fat (kg) divided by total body mass (kg).
Fat distribution, as a measure of central adiposity, was determined from DXA subregion analyses, with four regions described by anatomic landmarks: android subscapular, android waist, gynoid hip, and gynoid thigh. Fat distribution is defined as the ratio of fat mass in the upper (android) vs. lower (gynoid) body regions (26). This method has been used in prior pediatric studies to evaluate central adiposity (27, 28). Fat distribution is analogous to waist-to-hip ratio and provides an objective assessment of the pattern of fat deposition, with higher values corresponding to greater central adiposity.
HPA axis measures
Serum cortisol was determined from three samples obtained via an indwelling catheter every 20 minutes beginning at 1300-1400 hours. Girls fasted for 2 hours prior to the venipuncture. Radioimmunoassay (Coat-A-Count; Diagnostic Products Corporation, Los Angeles, CA) was used for cortisol measurement, with a detection limit of 0.3 μg/dL and standard curve range of 0.5-60 μg/dL. Inter- and intra-assay coefficients of variation were 6.6% and 6.0%, respectively. Cortisol area under the curve with respect to ground (AUCg) and increase (AUCi) were computed (29) to represent integrated measures. Computation of the AUC is a method used to comprise repeated measurements over time, and in this instance to estimate overall hormone secretion over a specific time (i.e. 40 minutes). AUCg is a method which incorporates baseline hormone levels; AUCi is an alternative method which does not incorporate baseline hormone levels and therefore represents the change (i.e., increase or decrease) in overall hormone secretion.
UFC excretion rate was obtained from an overnight urine collection, which is highly correlated with a 24-hour collection (30). Subjects were instructed to void before going to bed, collect any urine eliminated overnight, and collect the first morning void. UFC was measured using a modified radioimmunoassay (Coat-A-Count). UFC concentration, sleep duration, and volume of urine collected were the components used to determine the UFC excretion rate (nmol/h).
Cortisol reactivity cluster groupings were created using the K-means method (29, 31) based on participants’ serial serum cortisol measures in response to venipuncture (32, 33). Cluster centers are chosen in a first pass of the data. Subsequent iterations grouped an observation based on the distance to the mean, minimizing within-cluster variance and maximizing variability between clusters (29, 31). K-means does not depend on the order of the observations as do hierarchical cluster analyses methods. We anticipated three reactivity groupings based on previous literature (32). Participants were grouped into the following three clusters based on their serial serum cortisol concentrations: (1) low concentration, stable; (2) moderate concentration, slightly increasing; and (3) high concentration, increasing.
Covariates
Tanner stage breast (34) was assessed by a trained medical professional using inspection and palpation; two clinician researchers demonstrated 100% agreement on the Tanner stage in a nonrandom sample (n=23). Socioeconomic status (SES) was determined using the Hollingshead Four Factor Index of Social Status, with a higher score corresponding to higher SES (35). Physical activity was assessed with the Physical Activity Questionnaire for Older Children (PAQ-C). Participants recalled amount of moderate to vigorous activity over the preceding 7 days. An average was used to create a score ranging from 1 (low) to 5 (high) activity (36). Use of hormone contraceptives in the previous two weeks was ascertained by interview.
Statistical analyses
Distributions were assessed for all primary variables and covariates. Adiposity and HPA axis measures were all normally distributed with the exception of UFC excretion rate. Thus, the Log10 of the UFC excretion rate was used in subsequent analyses. Multiple linear regression was used to evaluate associations between adiposity and (a) integrated serum cortisol (AUCg, AUCi) and (b) Log10 UFC excretion rate. Along with age, potential covariates were considered based on the literature. Only significant covariates (p<.10) were retained in the models. Analysis of variance (ANOVA) was used to evaluate group differences in adiposity (BMI-Z) among the three cortisol reactivity clusters.
Results
Participants
Two-hundred sixty-two girls were enrolled. Demographic characteristics are summarized in Table 1. Table 2 reports descriptive statistics for adiposity and HPA axis measures.
Table 1.
Sample characteristics of the participants (N=262)
| Characteristic | Value |
|---|---|
| Race/ethnicity, No. (%) | |
| White | 162 (61.8) |
| Black | 86 (32.8) |
| Other | 14 (5.3) |
| Age, mean (SD), y | 14.9 (2.2) |
| Socioeconomic status, mean (SD) a | 37.3 (13.7) |
| Tanner breast stage, No. (%) | |
| 1 | 4 (1.5) |
| 2 | 5 (1.9) |
| 3 | 27 (10.3) |
| 4 | 39 (14.9) |
| 5 | 187 (71.4) |
| Postmenarcheal, No. (%) | |
| Yes | 209 (79.8) |
| No | 53 (20.2) |
| Weight category b | |
| Normal weight | 155 (59.4) |
| Overweight | 49 (18.7) |
| Obese | 58 (22.1) |
| Currently using hormonal contraceptive (any type) | |
| Yes | 67 (25.6) |
| No | 195 (74.4) |
Abbreviation: SD = standard deviation
Hollingshead AB (35);
normal weight = BMI <85th percentile; overweight = BMI ≥85th to <95th percentile; obese = BMI ≥ 95th percentile.
Table 2.
Anthropometric and hormonal characteristics of participants (N=262)
| Mean (SD)
|
|
|---|---|
| Obesity measure | |
| BMIa | 24.0 (6.2) |
| BMI-Zb | 0.7 (1.0) |
| Percent body fat (DXA) | 29.2 (7.7) |
| Fat distributionc | 0.6 (0.2) |
| Measures of the HPA axis | |
| Daytime serum cortisol (n=255) | |
| Cortisol μg/dL (mean 3 samples) | 9.7 (4.9) |
| Cortisol AUC (AUCg)d | 19.5 (10.0) |
| Cortisol net response (AUCi)d | 1.2 (7.5) |
| Overnight urine free cortisol (n=218) | |
| Urine cortisol excretion, nmol/h | 3.0 (2.5) |
| Cortisol reactivity | |
| Cortisol reactivity cluster, mean cortisol (μg/dL) | |
| Cluster 1: low, stable (n=168) | 6.9 (2.0) |
| Cluster 2: moderate, slightly increasing (n=69) | 14.4 (2.8) |
| Cluster 3: high, increasing (n=18) | 27.4 (5.5) |
Abbreviations: SD=standard deviation; DXA=dual energy X-ray absorptiometry; AUC= area under the curve; BMI=body mass index;
Calculated as weight (kg)/height (meters2);
Calculated as fat mass in upper/lower body regions (DXA);
Calculated as described by Pruessner et al (29)
Measures of the HPA axis and adiposity
Results of multiple regression for associations between cortisol AUCg and AUCi with all measures of adiposity are shown in Table 3. Serum cortisol AUCg was inversely associated with BMI-Z (B = −.014, P = .004) and fat distribution (B = −.003, P = .02). Specifically, a 10-unit decrease in cortisol AUCg was associated with a .14-unit increase in BMI-Z and a .03 increase in the ratio of fat mass in upper vs. lower body regions. Cortisol AUCi was inversely associated with BMI-Z (B = −.019, P = .02), and there was a trend toward an inverse association with fat distribution (B = −.004, P = .07).
Table 3.
Associations between hypothalamic-pituitary-adrenal (HPA) axis and obesity measures using multiple regression
| Serum cortisol | Serum cortisol | Urine free cortisol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area under the curve (AUCg) | Net response (AUCi) | Log10 excretion rate (nmol/h) | ||||||||||
| β | B | SE (B) | p | β | B | SE (B) | p | β | B | SE (B) | p | |
| Dependent variable | ||||||||||||
| BMIa | −.103 | −.049 | .028 | .09 | −.076 | −.062 | .048 | .20 | .181 | 3.544 | 1.237 | .005 |
| BMI-Zb | −.174 | −.014 | .005 | .004 | −.144 | −.019 | .008 | .02 | .178 | .560 | .205 | .007 |
| Percent body fatc | −.046 | −.027 | .038 | .48 | −.045 | −.045 | .065 | .49 | .109 | 2.597 | 1.658 | .12 |
| Fat distributiond,e | −.146 | −.003 | .001 | .02 | −.114 | −.004 | .002 | .07 | .146 | .110 | .050 | .03 |
Abbreviations: BMI=body mass index; SES=socioeconomic status; DXA=dual energy X-ray absorptiometry;
Model adjusted for age, race, Tanner stage, and SES.;
Model adjusted for race, Tanner stage, and SES.;
Model adjusted for age, race, and SES.;
Model adjusted for Tanner stage and SES.;
Fat distribution=fat mass in upper/lower body regions (DXA).
Associations between the UFC excretion rate and measures of adiposity are displayed in Table 3. After controlling for the stated covariates, overnight UFC (Log10) excretion rate was positively associated with BMI, BMI-Z, and fat distribution. Higher UFC excretion rates were associated with higher BMI, BMI-Z, and ratio of fat mass in upper vs. lower body regions.
Cortisol reactivity and adiposity
Figure 1 displays the mean cortisol concentrations by computed cortisol reactivity clusters. Results of ANOVA, controlling for covariates, revealed significant differences (P = .04) in mean BMI-Z scores between Cluster 1 (“low, stable”) and Cluster 3 (“high, increasing”). Specifically, the “high, increasing” reactivity cluster had a lower BMI-Z compared with the “low, stable” cluster. There was a trend toward a significant difference (P = .09) in the mean BMI-Z between Cluster 2 (“moderate, slightly increasing”) and Cluster 3.
Figure 1.
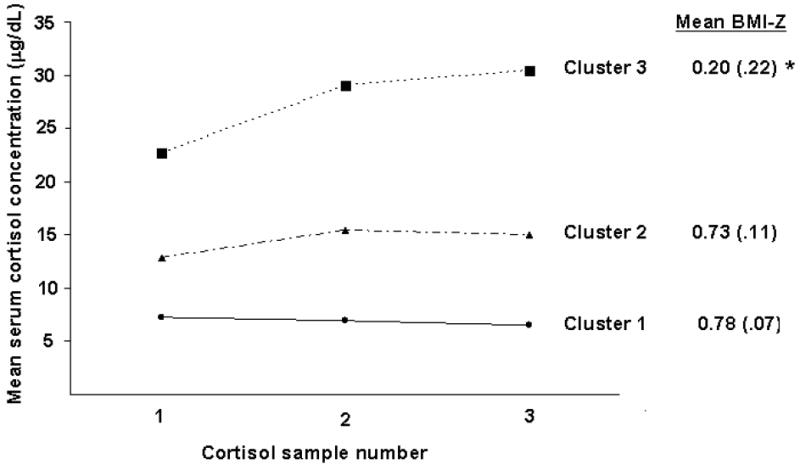
Mean daytime serum cortisol concentrations for cortisol reactivity cluster groupings in response to venipuncture.
* Denotes significant difference in mean BMI-Z between Clusters 1 and 3 (p = .04);
Cortisol clusters: 1=“low, stable” (n=168); 2=“moderate, slightly increasing” (n=69); 3=“high, increasing” (n=18); Clusters were created using the K-means method (31) based on three serial serum cortisol samples (every 20 minutes) from participants in response to venipuncture.
HPA axis and central adiposity
To determine whether measures of the HPA axis were independently associated with central adiposity, percent body fat was included in the models with fat distribution. Serum cortisol AUCg was independently and inversely associated with fat distribution (β = −.111, B = −.002, P =.007). That is, higher AUCg was associated with decreased fat distribution or less fat mass in the upper (central) body. There also was a trend toward an inverse association of serum cortisol AUCi with fat distribution (P = .06) and a positive association with overnight UFC excretion rate (P = .09).
Discussion
To our knowledge, this is the first study to evaluate associations between various measures of the HPA axis and adiposity in a community sample of adolescent girls. We found that serum cortisol measures (AUCg, AUCi) were inversely associated with adiposity (BMI-Z, fat distribution) and that the UFC excretion rate was positively associated with adiposity. Surprisingly, greater cortisol reactivity (i.e., hyperactivity of the HPA axis) was associated with decreased adiposity, highlighting that individuals respond differently to stress and suggesting that this response may be weight-related and/or associated with weight-related consequences. Our study contributes to the literature by demonstrating that adiposity is associated with altered HPA activity in early and mid-adolescence. The causality and directionality of our findings require further investigation to put into clinical perspective.
Measures of the HPA axis and adiposity
In the literature, an inverse association between daytime serum cortisol and adiposity has been shown in adult women (12, 13) and in a small sample of obese pre-adolescent males and females (20). In the current study, serum cortisol measures (AUCg, AUCi) were also inversely associated with BMI-Z and fat distribution, extending this finding to adolescent females.
Several existing theories support an inverse association between serum cortisol measures and adiposity. First, it is well documented that obesity is associated with insulin resistance, even during childhood (37). Cortisol and other hormones (e.g., growth hormone) antagonize insulin action to raise blood glucose levels. Thus, lower serum cortisol concentrations in the context of adolescent obesity may be an early adaptive mechanism to assist in the maintenance of glucose and metabolic homeostasis when one is insulin resistant. Second, inverse associations of HPA axis measures and adiposity may be related to the properties of cortisol and its binding mechanisms. Serum cortisol reflects the total concentration of cortisol in circulation, including that bound to proteins. Most cortisol circulates while bound to CBG or other serum proteins (SHBG, albumin); only about 10% is unbound or “free.” CBG and SHBG levels have been shown to be inversely correlated with body mass index and waist-to-hip ratio among adult men and women (38, 39). Because serum cortisol includes that bound to CBG and other serum proteins, it is not surprising that total cortisol levels would be lower in obese women. Finally, there is evidence that androgens (i.e., testosterone) attenuate HPA axis activity (40). Obesity contributes to increased circulating androgen levels either through decreased SHBG, stimulation of ovarian stroma production of testosterone via insulin, or increased peripheral conversion of estradiol to testosterone by aromatase in fat tissues. Thus, interaction between obesity and androgen levels may occur during the HPA axis reactivity to stress. Whether these mechanisms explain the inverse association between serial serum cortisol measures and obesity remains an empirical question. Due to the secondary nature of these analyses we were not able to explore these mechanisms in this study.
The UFC excretion rate was positively associated with measures of adiposity in our study, corroborating earlier findings in adults (11, 41). Furthermore, our finding of increased UFC excretion with greater fat mass in upper vs. lower body regions is in agreement with previous adult studies that demonstrated increased nighttime UFC excretion in the setting of central compared with peripheral fat distribution using waist-to-hip ratio to discriminate body fat distribution (11). The physiologic impact of greater overnight excretion of cortisol with increased adiposity remains unclear. The current study also corroborates Barat and colleagues’ findings of increased 24-hour total glucocorticoid metabolites in the urine of pre- and post-adolescent girls with a greater distribution of body fat in upper body regions (21). Our findings should be interpreted with caution in that increased UFC excretion or clearance rate does not necessarily translate to overall increased cortisol production. This study utilized an overnight urine collection vs. a 24-hour collection. Although the two are highly correlated they are not identical.(30) Additionally, other factors may impact cortisol excretion in the urine including consumption of caffeine or alcohol, medications, and hydration status.
Cortisol reactivity and adiposity
The HPA axis is involved in the stress response, and cortisol reactivity has not been widely studied in the setting of obesity. To our knowledge, the only study that evaluated the association between cortisol reactivity and obesity using a laboratory stressor was conducted by Dockray and colleagues who reported heightened salivary cortisol response (hyperactivity of the HPA axis) to the Trier Social Stress Test with greater adiposity (BMI) (15). Our findings stand in opposition to the Dockray study although such differences may be evident because the Dockray study measured salivary cortisol (free). In the current study, serum cortisol (total; free + bound) was measured and girls in the “high, increasing” cortisol cluster had a lower mean BMI-Z score compared with those in the “low, stable” cortisol cluster. Mean BMI-Z for the “high, increasing” cluster was 0.20, representing a normal and healthy weight. This outcome suggests that the HPA axis in these girls may be responding in a more “normative” manner to a potentially stressful situation while other patterns of cortisol reactivity may be abnormally attenuated; girls with obesity may demonstrate hypoactivity of the HPA axis in response to a stressor.
There may be other differences about the girls in the “high, increasing” cortisol cluster that resulted in activation of their stress axis. For example, Loucks and colleagues described differential responses to a submaximal exercise challenge among women with functional hypothalamic amenorrhea (FHA) compared with eumenorrheic ovulatory women (EW) (42). Women with FHA had a significantly greater increase in cortisol in response to the exercise challenge compared with EW women. Therefore, it is possible that the girls in our study who showed “high, increasing” cortisol in response to venipuncture may exhibit some other factor resembling FHA that is influencing their HPA axis. Another possible explanation of our findings may be related to the differential perception of venipuncture as a stressor. For example, obese girls may have more experience with venipuncture in a clinical setting compared with normal-weight girls, thereby minimizing activation of the stress response differentially among obese girls in this study. However, using venipuncture as a stressor has been successful among adolescent girls, and group differences have been shown (32, 33). There are likely behavioral and genetic influences that further influence the stress response that were not evaluated in this study.
In spite of the strengths of the study, additional limitations should be noted. First, these analyses were cross-sectional and thus causality cannot be determined. Second, use of waist circumference is the standard for measuring central adiposity. However, we did not have measures of waist circumference and therefore used fat distribution from DXA regions which has been used in other pediatric samples. Third, there was a wide range in urine volume and duration involving the overnight urine collection. Adolescent sleep patterns are more varied than adult patterns in terms of time to bed and awakening, and this study purposely did not impose a set bedtime and wake-up time. To account for some of this variability in duration of collection, we calculated cortisol excretion as a rate in nmol/h. Finally, this is a secondary data analysis and we did not have many of the other hormonal variables of interest that could differentially impact cortisol levels and the cortisol response to stress.
This study offers initial insight into the complex and inter-related associations of the HPA axis and obesity during adolescence. Although we did not perform dynamic testing of the HPA axis, the cortisol reactivity analysis suggests that among obese adolescent girls, a relative hypoactivity of the HPA axis may exist in response to a stressor (venipuncture). We demonstrated reduced cortisol levels during the day and increased levels at night with increasing degree of adiposity, possibly indicating alteration of the circadian rhythm of cortisol with adiposity as described by Nader (43). Furthermore, our finding of an inverse association of cortisol AUCg with fat distribution, after controlling for percent body fat, has not previously been shown among adolescent females. This is an important association because it suggests that as early as adolescence, changes in the HPA axis in the setting of obesity may have occurred and that the pattern of fat distribution is relevant to this association. In support of this, a recent study demonstrated that cortisol excess (UFC excretion) and central adiposity were associated with greater concentrations of markers of cardiovascular disease among adolescent girls (44). Future research is critical to confirm and replicate our findings and to extend this observation toward establishing a link between the HPA axis and obesity-related comorbidities in this age group.
Acknowledgments
This research was supported by grant R01 DA 016402 from the National Institute of Drug Abuse (PI: Dr. Dorn); in part by USPHS grant UL1RR026314 from the National Center for Research Resources, NIH; and by grant 1K12 HD051953 from the National Institute of Health/Office of Research on Women’s Health (Dr. Hillman).
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- UFC
urinary free cortisol
- BMI
body mass index
- BMI-Z
body mass index z-score
- ACTH
adrenocorticotropic hormone
- DXA
Dual Energy X-ray Absorptiometry
- AUCg
area under the curve with respect to the ground
- AUCi
area under the curve with respect to the increase
- SES
socioeconomic status
- CBG
cortisol binding globulin
- SHBG
sex hormone binding globulin
- FHA
functional hypothalamic amenorrhea
- EW
eumenorrheic ovulatory women
- GR
glucocorticoid receptors
Footnotes
Author contributions Dr. Hillman was responsible for the conceptualization of the secondary study aims, the data analyses and interpretation, and drafting the manuscript. Dr. Dorn conceptualized the original parent study, received grant funding, and collected data from the participants. Dr. Dorn also assisted in developing the conceptual organization of the aims and the current manuscript, interpretation of analyses, and revising the manuscript. Tammy Loucks and Dr. Berga completed the urine free cortisol (UFC) assays and provided guidance on how to analyze the UFC data, interpret the findings, and revise the manuscript.
Disclosures The authors have no conflicts of interest or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S1–8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. Journal of the American Medical Association. 2008 May 28;299(20):2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 3.Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009 Oct;16(5):340–6. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008 May 23;94(2):169–77. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes. 2000;24(2):S80–S5. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 6.Korbonits M, Trainer PJ, Nelson ML, Howse I, Kopelman PG, Besser GM, et al. Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: relation to body fat distribution. Clin Endocrinol (Oxf) 1996 Dec;45(6):699–706. doi: 10.1046/j.1365-2265.1996.8550865.x. [DOI] [PubMed] [Google Scholar]
- 7.Duclos M, Gatta B, Corcuff JB, Rashedi M, Pehourcq F, Roger P. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clin Endocrinol (Oxf) 2001 Oct;55(4):447–54. doi: 10.1046/j.1365-2265.2001.01384.x. [DOI] [PubMed] [Google Scholar]
- 8.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996 Aug;271(2 Pt 1):E317–25. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 9.Cakir M, Sari R, Tosun O, Karayalcin U. Cortisol levels during an oral glucose tolerance test in lean and obese women. Endocr Res. 2005;31(3):213–8. doi: 10.1080/07435800500373199. [DOI] [PubMed] [Google Scholar]
- 10.Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. Int J Obes Relat Metab Disord. 2004 Sep;28(9):1168–73. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- 11.Duclos M, Corcuff JB, Etcheverry N, Rashedi M, Tabarin A, Roger P. Abdominal obesity increases overnight cortisol excretion. J Endocrinol Invest. 1999 Jun;22(6):465–71. doi: 10.1007/BF03343591. [DOI] [PubMed] [Google Scholar]
- 12.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992 Aug;41(8):882–6. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 13.Strain GW, Zumoff B, Kream J, Strain JJ, Levin J, Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism. 1982 Mar;31(3):209–12. doi: 10.1016/0026-0495(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 14.Strain GW, Zumoff B, Strain JJ, Levin J, Fukushima DK. Cortisol production in obesity. Metabolism. 1980 Oct;29(10):980–5. doi: 10.1016/0026-0495(80)90043-8. [DOI] [PubMed] [Google Scholar]
- 15.Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. J Adolesc Health. 2009 Oct;45(4):344–50. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. 1991 Sep;230(3):195–201. doi: 10.1111/j.1365-2796.1991.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 17.Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005 Jul;13(7):1157–66. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- 18.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000 Sep-Oct;62(5):623–32. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Koch FS, Sepa A, Ludvigsson J. Psychological stress and obesity. J Pediatr. 2008 Dec;153(6):839–44. doi: 10.1016/j.jpeds.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Chalew SA, Lozano RA, Armour KM, Zadik Z, Kowarski AA. Reduction of plasma cortisol levels in childhood obesity. J Pediatr. 1991 Nov;119(5):778–80. doi: 10.1016/s0022-3476(05)80302-6. [DOI] [PubMed] [Google Scholar]
- 21.Barat P, Gayard-Cros M, Andrew R, Corcuff JB, Jouret B, Barthe N, et al. Truncal distribution of fat mass, metabolic profile and hypothalamic-pituitary adrenal axis activity in prepubertal obese children. J Pediatr. 2007 May;150(5):535–9. 9 e1. doi: 10.1016/j.jpeds.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Dorn LD, Susman EJ, Pabst S, Huang B, Kalkwarf H, Grimes S. Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Arch Pediatr Adolesc Med. 2008 Dec;162(12):1181–8. doi: 10.1001/archpedi.162.12.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002 Jan;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun;8(314):1–27. [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002 May;(246):1–190. [PubMed] [Google Scholar]
- 26.Walton C, Lees B, Crook D, Worthington M, Godsland IF, Stevenson JC. Body fat distribution, rather than overall adiposity, influences serum lipids and lipoproteins in healthy men independently of age. Am J Med. 1995 Nov;99(5):459–64. doi: 10.1016/s0002-9343(99)80220-4. [DOI] [PubMed] [Google Scholar]
- 27.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999 Feb 2;99(4):541–5. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 28.Hillman JB, Dorn LD, Bin H. Association of anxiety and depressive symptoms and adiposity among adolescent females, using dual energy X-ray absorptiometry. Clin Pediatr (Phila) 2010 Jul;49(7):671–7. doi: 10.1177/0009922810363155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003 Oct;28(7):916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 30.Corcuff JB, Tabarin A, Rashedi M, Duclos M, Roger P, Ducassou D. Overnight urinary free cortisol determination: a screening test for the diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf) 1998 Apr;48(4):503–8. doi: 10.1046/j.1365-2265.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 31.Romesburg C. Cluster Analysis for Researchers: Lulu.com. 2004 [Google Scholar]
- 32.Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior, and behavioral and psychological problems in young adolescents: A longitudinal perspective. Journal of Research on Adolescence. 1997;7(1):81–105. [Google Scholar]
- 33.Sontag LM, Dorn LD, Tissot A, Susman EJ, Beers S, Rose S. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Development and Psychopathology. doi: 10.1017/S0954579411000782. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollingshead AB. Four-Factor Index of Social Status. New Haven: Yale University CT Press; 1975. [Google Scholar]
- 36.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997 Oct;29(10):1344–9. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002 Mar 14;346(11):802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Real JM, Pugeat M, Grasa M, Broch M, Vendrell J, Brun J, et al. Serum corticosteroid-binding globulin concentration and insulin resistance syndrome: a population study. J Clin Endocrinol Metab. 2002 Oct;87(10):4686–90. doi: 10.1210/jc.2001-011843. [DOI] [PubMed] [Google Scholar]
- 39.Pasquali R, Casimirri F, Plate L, Capelli M. Characterization of obese women with reduced sex hormone-binding globulin concentrations. Hormone and Metabolic Research. 1990 May;22(5):303–6. doi: 10.1055/s-2007-1004907. [DOI] [PubMed] [Google Scholar]
- 40.Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007 Sep-Nov;32(8-10):1052–61. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993 Aug;77(2):341–6. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 42.Loucks T, Dube J, Laychak K, Robertson R, Berga S. Metabolic and endocrine responses to submaximal exercise challenge in women with functional hypothalamic amenorrhea (FHA) Presented at the 51st Annual Meeting of the Society for Gynecologic Investigation. 2004 [Google Scholar]
- 43.Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010 May;21(5):277–86. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell M, Bredella M, Tsai P, Mendes N, Miller KK, Klibanski A, et al. Relative growth hormone deficiency and cortisol excess are associated with increased cardiovascular risk markers in obese adolescent girls. J Clin Endocrinol Metab. 2009 Aug;94(8):2864–71. doi: 10.1210/jc.2009-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]


