Abstract
This 4-yr longitudinal study examined the different roles of perceived stress in the association between older adults’ physical activities and physical health. We hypothesized that physical activities would exert beneficial effects on physical health by preventing chronically high levels of perceived stress.
Design and Measures
We assessed baseline levels of physical activities and repeated measures of perceived stress and physical symptoms in three waves of data from a sample of 157 older adults.
Results
Among participants with high (but not low) baseline levels of perceived stress, physical activity predicted a 2-yr reduction of perceived stress and a 4-yr prevention of physical health symptoms. Moreover, the interaction effect on 4-yr changes in physical symptoms was mediated by 2-yr changes in perceived stress.
Conclusions
Physical health benefits of physical activity are particularly pronounced among older adults who perceive high levels of stress, and this effect is mediated by a prevention of chronically high perceptions of stress.
Keywords: physical health, perceived stress, physical activities, older adulthood
Research suggests that perceptions of stress play an important role in the link between physical activity and physical health. First, physical activity has been shown to reduce perceptions of stress (Salmon, 2001). Second, physical activity and low stress are generally associated with better physical health (Cohen, Janicki-Deverts, & Miller, 2007; Haskell et al., 2007). Third, the health benefits of activity are particularly evident among individuals who experience high, as compared to low, levels of stress (Brown & Siegel, 1988; Carmack et al., 1999). While these findings suggest that physical activity could benefit health by ameliorating chronically high levels of stress, this mechanism has not yet been demonstrated in longitudinal research. Here, we test this possibility in three waves of data from a 4-year longitudinal study of community-dwelling older adults. We expected that engaging in physical activity would ameliorate stress and thereby mediate good physical health in persons who perceive high levels of stress. By contrast, we did not expect these effects to be apparent among persons low in stress, as these individual are generally at a lower risk of developing health problems, and health benefits of physical activity may be less likely to operate through a reduction of low stress levels.
Physical Activities, Perceived Stress, and Physical Health
The maintenance of a physically active lifestyle is an important contributor to older adults’ physical health (Centers for Disease Control and Prevention, 2010; Haskell et al., 2007; Pate et al., 1995). While some of the benefits of physical activity are attributable to modulation of biological processes involved in disease (e.g., increased cardiorespiratory fitness, decreased blood pressure, more musculoskeletal strength, Dunn et al., 1997; Faulkner, Green, & White, 1994), there is also evidence that psychological mechanisms, such as the perception of stress, may explain beneficial health effects of physical activity. Perceived stress represents appraisals of person-environment interactions that can influence emotional and biological processes (Lazarus & Folkman, 1984). In addition, perceived stress is associated with a number of adverse physical health outcomes, likely in a causal fashion (Cohen, 1996; Cohen, Tyrrell, & Smith, 1991; Cohen, Kamarck, & Mermelstein, 1983).
In support of this process, research suggests that high levels of perceived stress contribute to negative affect (e.g., feelings of anxiety and depression) and biological and behavioral processes (e.g., dysregulated cortisol rhythms or non-adherence to medical regimes) that may heighten vulnerability to various physical health problems (Cohen et al., 2007; Cohen, Kessler, & Underwood, 1995, McEwen, 1998; Miller, Chen, & Cole, 2009; Miller, Chen, & Zhou, 2007). Moreover, physical activity is generally associated with decreased levels of perceived stress (King, Taylor, & Haskell, 1993; Norris, Carroll, & Cochrane, 1990; Salmon, 2001) and alters some of the behavioral and biological processes through which perceived stress can affect physical health outcomes (e.g., less smoking, better nutrition practice, less cortisol output or cardiovascular reactivity; Boutelle, Murray, Jeffery, et al., 2000; Crews & Landers, 1987; Fleshner, Kennedy, Johnson, et al., 2009; Rimmele et al., 2009; Wankel & Sefton, 1994).
These findings suggest that physical activity has physical health benefits, which may operate partially through amelioration of perceived stress. However, several longitudinal studies have shown that physical activity does not always explain large portions of variance in physical health outcomes (Guszkowska, 2005; Mead et al., 2007). Such a lack of stronger associations could be due to the possibility that physical activity is not equally adaptive among different groups of individuals. For example, physical activity may exert its adaptive effects on physical health particularly among individuals who are vulnerable to developing a disease, if it attenuates or counteracts some of the underlying risk factors or pathogenic mechanisms. By contrast, the health effects of physical activities may be less pronounced among individuals who are at a lower risk of developing physical disease. A corollary of this argument is that perceived stress itself could not only mediate, but also moderate, the link between physical activity and physical health (Brown, 1991; Brown & Siegel, 1988; Carmack et al., 1999). In this scenario, physical activity may be particularly beneficial for health among individuals who perceive high levels of stress, because it can ameliorate their maladaptive stress levels and some of its emotional, behavioral, and biological consequences.
We note that this process would be consistent with research indicating that people can be stressed over different periods of time, and that the chronic rather than the temporary perception of stress is detrimental to a person’s physical health. Such consequences of chronic stress may be due to its harmful long-term effects on emotional, physiological, and behavioral responses that influence susceptibility to, and course of, disease (Kiecolt-Glaser et al., 1996, 2003; Miller et al., 2002, 2008, 2009; Schulz, Kirschbaum, Pruessner, & Hellhammer, 1998). Transient perceptions of stress, by contrast, should be less problematic for a person’s physical health as they may not give rise to sustained patterns of biological dysregulation or maladaptive health behaviors.
Further support for the proposed process stems from research documenting that physical activity is associated with better physical health particularly among individuals who experienced many stressful life events, and that this association can be explained by psychological distress (Brown, 1991; Brown & Siegel, 1988; Carmack et al., 1999). In addition, longitudinal research showed that the deleterious effects of widowhood on functional health declines were more pronounced among community-dwelling older adults who engaged in low, as compared to high, levels of physical activities (Unger, Johnson, & Marks, 1997). Finally, experimental intervention research suggests that engaging obese women or cancer survivors in exercise programs can decrease their experience of stress (Cramer, Nieman, & Lee, 1991; Hughes, Leung, & Naus, 2008; for a review, see Salmon, 2001).
These studies demonstrate that health benefits of physical activity can occur particularly among individuals who perceive high levels of stress, and that physical activity can reduce stress levels in vulnerable populations that confront problematic life circumstances. Nonetheless, the present literature did not document the complete process that links physical activity, perceived stress, and physical health outcomes over time. In particular, it has not been shown that the beneficial effects of physical activity on the prevention of chronically high levels of stress can mediate subsequent levels of physical health. From our theoretical perspective, however, such associations are likely to occur, and demonstrating this process in longitudinal research may contribute to our understanding of the adaptive health effects of physical activity.
The Present Research
This research examined whether physical activity would have differential health benefits in higher vs. lower stress individuals. We expected the health benefits to be most apparent in the former group, in whom physical activity would reduce high stress levels over time. By contrast, we did not expect these benefits of physical activity to be apparent in the low-stress individuals. To start examining this possibility, we analyzed three waves of data from a 4-year longitudinal study of older adults. Our analysis focused on predicting physical health symptoms (e.g., chest pain, joint pain, or difficulty breathing) because such symptoms can be signs of a variety of underlying or developing physical diseases and have been associated with distress and biological markers of stress in previous research (Wrosch, et al., 2002, 2007, 2008). More specifically, we examined whether the interaction between baseline levels of physical activity and perceived stress would predict changes in physical symptoms over time. In addition, we expected that changes in perceived stress would mediate this interaction effect.
Method
Participants
This study is based on a sample of community-dwelling older adults who took part in the longitudinal Montreal Aging and Health Study (MAHS; Wrosch, Schulz, Miller, & Dunne, 2007). Participants were recruited through newspaper advertisement. The only inclusion criterion was that participants had to be older than 60 years because we were interested in examining a normative sample of older adults. In 2004, we conducted the first wave of the MAHS by assessing a heterogeneous sample of 215 older adults from the Montreal area. The 2-yr follow-up included 184 participants and 164 subjects participated in the 4-yr follow-up. Reasons for non-participation were being deceased (n = 13), having problems that prevented participation (n =17), refusing further participation (n = 8), and being unable to locate participants (n = 13). Seven additional participants were excluded from the analyses because they did not participate in the 2-year follow-up. The final sample included 157 participants who were on average 72 years old (SD = 5.55) and 48% of the sample was male. Study attrition over four years was not significantly associated with baseline measures of the study variables, except for age. Older participants were more likely to discontinue their study participation, t(213) = −2.30, p < .05.
Materials
The main study variables included repeated measures of physical health symptoms and perceived stress, as well as baseline measures of physical activities. In addition, the study included a number of covariates associated with health-relevant sociodemographic characteristics (i.e., age, sex, education) and baseline levels of chronic health problems (see Tables 1 and 2 for zero-order correlations, means, standard deviations, and frequencies of main study variables).
Table 1.
Zero-Order Correlations Between Main Constructs.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Physical health symptoms (baseline) | ||||||||||
| 2. Physical health symptoms (2-yr follow up) | .58** | |||||||||
| 3. Physical health symptoms (4-yr follow up) | .48** | .57** | ||||||||
| 4. Perceived stress (baseline) | .34** | .28** | .26** | |||||||
| 5. Perceived stress (2-yr follow up) | .25** | .29** | .31** | .63** | ||||||
| 6. Perceived stress (4-yr follow up) | .33** | .31** | .30** | .68** | .76** | |||||
| 7. Physical activities (baseline) | −.08 | −.09 | −.17* | −.11 | −.07 | −.17* | ||||
| 8. Age | −.08 | −.07 | −.10 | −.11 | .03 | −.01 | −.12 | |||
| 9. Sex a | .09 | .03 | .10 | .11 | .12 | .16* | −.09 | .06 | ||
| 10. Education | −.20* | −.16* | −.07 | −.21** | −.14 | −.21** | .09 | −.20* | −.14 | |
| 11. Chronic health problems | .24** | .31** | .21** | .08 | .08 | .12 | −.08 | −.06 | −.03 | −.05 |
Note.
Higher values represent female participants.
p < 0.05.
p < 0.01.
Table 2.
Means, Standard Deviations, and Frequencies of Main Study Variables (N = 157).
| Constructs | Mean (SD) or Percentage a |
|---|---|
| Physical health symptoms | |
| Baseline | 2.59 (3.20) |
| 2 Years | 2.87 (3.50) |
| 4 Years | 3.42 (4.04) |
| Perceived stress | |
| Baseline | 2.42 (0.65) |
| 2 Years | 2.42 (0.65) |
| 4 Years | 2.47 (0.73) |
| Hours of weekly physical activity (baseline) | 2.10 (3.25) |
| Number of chronic health problems (baseline) | .80 (.81) |
| Arthritis (%) | 29.90 |
| Diabetes (%) | 14.10 |
| Cancer (%) | 3.20 |
| Lung or other respiratory disease (%) | 10.80 |
| Heart condition (%) | 17.20 |
| Difficulty bathing (%) | 2.50 |
| Difficulty managing finances (%) | 2.50 |
| Age | 71.72 (5.55) |
| Male (%) | 48.40% |
| Education (baseline) | 2.09 (1.07) |
| None (%) | 4.00 |
| High school (%) | 31.30 |
| Trade (%) | 28.70 |
| Bachelor (%) | 24.00 |
| Masters or Doctorate (%) | 12.00 |
Note.
Mean (SD) are presented for continuous variables.
Physical health symptoms were measured at baseline, 2-yr, and 4-yr follow-up by administering a symptom checklist that has been validated in previous research with older adults (Wrosch, Schulz, & Heckhausen, 2002). Participants reported at bedtime on three typical days whether they had experienced each of 12 physical health symptoms during the day (e.g., chest pain, joint pain, or shortness of breath; PRIME MD: Spitzer et al., 1994). Daily measures of physical symptoms were significantly correlated with each other (rsT1 = .65 to .75, psT1 < .01; rsT2 = .61 to .77, psT2 < .01; rsT3 = .44 to .67, psT3 < .01), and we obtained indicators of physical health symptoms for baseline, 2-yr, and 4-yr follow up by counting the total number of symptoms experienced across all three days (see Table 2). Levels of physical symptoms showed a linear increase across waves, F(1, 156) = 7.71, p < .01.
Perceived stress was measured at baseline, 2-yr, and 4-yr follow-up by administering the 10-item version of the perceived stress scale (Cohen et al., 1983). Respondents were asked to rate how frequently they experienced 10 different situations over the past month (e.g., “How often have you been upset because of something that happened unexpectedly?” or “How often have you felt difficulties were piling up so high that you could not overcome them?”), by using a five-point Likert-type scale ranging from 1 (never) to 5 (very often). Indicators of perceived stress were obtained by averaging the ratings of the 10 items separately for baseline, 2-yr, and 4-yr follow up, αs > .87. Levels of perceived stress did not significantly change across waves, F(1, 156) = 1.05, p > .05.
Participants’ engagement in physical activity was assessed at baseline by administering an open-response format questionnaire used in previous research (Miller, Cohen, & Herbert, 1999; Paffenbarger, Blair, Lee, & Hyde, 1993). Participants were asked if they engage in any regular activity (e.g., walking, jogging, bicycling, etc), long enough to work up a sweat. In addition, they reported how many days per week and for how long each time they engage in physical activities. This measure has been validated by previous research, showing that it predicts objective markers of fitness, such as oxygen uptake during pedal ergometry (Siconolfi, Lasater, Snow, & Carleton, 1985). In order to obtain a measure of participants’ weekly physical activity, we multiplied the number of days participants were physically active by the hours participants usually engaged each time in physical activities. On average, participants engaged approximately two hours per week in physical activities (see Table 2). Note that based on the physical activity guidelines from the Centers for Disease Control and Prevention (2010), 69.4% of our sample can be classified as insufficiently active (less than 150 min per week of total activity) and 30.6% as active (at least 150 minutes per week of total activity).
Covariates
To reduce the possibility of spurious associations, this study incorporated a number of covariates that were associated in previous research with activity engagement, perceived stress, or physical health problems. These variables included participants’ age, sex, education, and chronic health problems (Denton, Prus, & Walters, 2004; Lee, Lindquist, Segal, & Covinsky, 2006). Education was measured by asking participants to report their highest educational degree completed (0 = none, 1 = high school, 2 = trade, 3 = undergraduate degree, 4 = graduate degree). A measure of chronic health problems was derived based on research identifying conditions associated with premature mortality in older adults (Lee et al., 2006). It included six conditions - diabetes, cancer, lung or other respiratory disease, heart condition, difficulty bathing, and difficulty managing finances (Lee et al., 2006). In addition, we incorporated the presence of arthritis into our measure, given that this disease is associated with both perceived stress and daily symptoms (Wrosch & Schulz, 2008; Thomason, 1992).
Results
Data Analyses
The hypotheses were tested by conducting two sets of analyses. In the first set, we examined whether baseline levels of perceived stress would moderate the effect of baseline levels of physical activities on changes in older adults’ physical health symptoms over time. To this end, we conducted two separate regression analyses predicting 2-yr and 4-yr levels in physical health symptoms by baseline levels of physical symptoms, physical activities, and perceived stress (step 1), and the interaction between physical activities and perceived stress (step 2). All analyses controlled for a number of covariates (i.e., age, sex, education, and chronic health problems), and predictor variables were standardized prior to conducting the analyses.
In the second set, we investigated whether the interaction between baseline levels of physical activities and perceived stress in predicting changes in physical health symptoms would be mediated by a reduction of perceived stress. To test this hypothesis, we conducted bootstrap analyses (Preacher & Hayes, 2008), which examined whether 2-yr changes in perceived stress exert an indirect effect on the interaction effect between physical activity and perceived stress in predicting changes in physical health symptoms. The analysis was based on 5000 bootstraps and the indirect effect was evaluated as significant if the bias-corrected 95% confidence interval did not cross zero (see Preacher & Hayes, 2008).
Perceived Stress as a Moderator
The results of the first set of analyses showed that baseline levels of physical symptoms were significantly associated with 2-yr levels, F(1, 149) = 48.32, B = .50, SE = .07, R2 = .20, p < .01, and 4-yr levels of physical symptoms, F(1, 149) = 29.16, B = .42, SE = .08, R2 = .14, p < .01. Moreover, the first step of the analyses indicated that of the covariates, only chronic health symptoms significantly predicted 2-yr increases in physical symptoms, F(1, 149) = 7.19, B = .18, SE = .07, R2 = .03, p < .01. The other covariates and the main effects of physical activity and perceived stress were unrelated to 2-yr or 4-yr changes in physical symptoms, Fs(1, 149) < 3.00, ps > .05. However, the second step of the analyses confirmed a significant interaction between physical activities and perceived stress in predicting 4-yr changes in physical symptoms, F(1, 148) = 4.20, B = −.16, SE = .08, R2 = .02, p < .05. We note that the interaction between physical activity and perceived stress remained significant if we controlled in additional analyses for 2-yr or 4-yr changes in physical activity, and that this interaction was not found in the analysis of 2-yr changes in physical symptoms, F(1, 148) = .94, p > .05. Further, neither the quadratic main effect for physical activity nor the quadratic interaction effect between physical activity and perceived stress predicted 4-yr changes in physical symptoms.
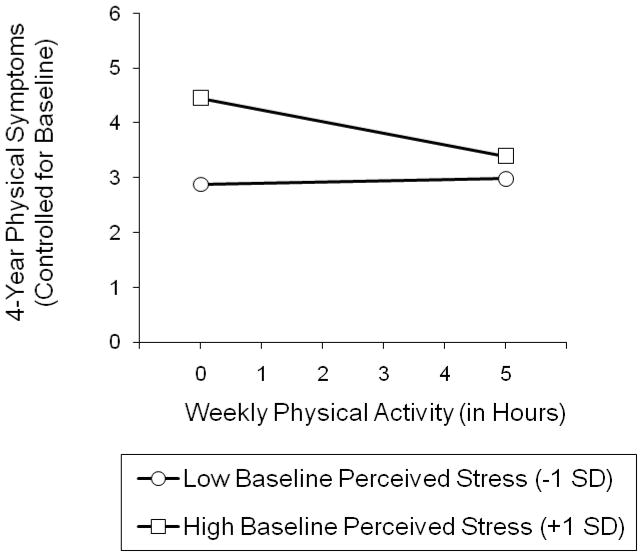
Figure 1 illustrates the significant interaction effect. We plotted the association between hours of weekly physical activity (0 to 5 hours, where 5.35 hours represented one standard deviation above the sample mean) and 4-yr changes in physical health symptoms for participants who perceived high (+1 SD) and low (−1 SD) baseline levels of stress (Aiken & West, 1991). The pattern of results indicated that, above and beyond baseline levels of physical health symptoms, the highest symptom levels after 4 years were found among participants who reported high baseline levels of perceived stress and did not engage in physical activities. A calculation of the simple slopes confirmed this interpretation by demonstrating that physical activity was significantly associated with fewer increases in physical symptoms over time among participants who perceived high baseline levels of stress, B = −.34, SE = .16, p < .01, but not among their counterparts who perceived low levels of stress, B = −.04, SE = .10, p > .05.
Figure 1.
Associations between baseline levels of physical activity and 4-yr changes in physical health symptoms, separately for participants who experienced high and low baseline levels of perceived stress.
Perceived Stress as a Mediator
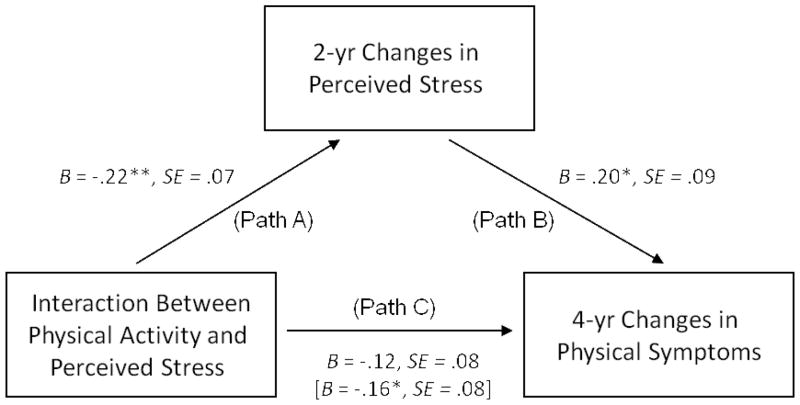
To examine whether 2-yr changes in perceived stress would mediate the observed interaction effect on 4-yr changes in physical symptoms, we conducted bootstrap analyses. To this end, we used the “indirect SPSS macro” (Preacher & Hayes, 2008), and repeated the above-reported analysis for predicting 4-yr changes in physical health symptoms, and additionally incorporated 2-yr levels of perceived stress as a potential mediator. Note that this analysis controlled for baseline perceived stress, which implies that the mediating variable was independent of its baseline levels and represents 2-yr changes in perceived stress.
Figure 2 illustrates the mediation model tested. The results of the analysis demonstrated that the interaction between baseline levels of physical activity and perceived stress significantly predicted changes in perceived stress over 2 years, F(1, 148) = 10.10, B = −.22, SE = .07, R2 = .04, p < .01 (see path A in Figure 2). The obtained pattern for this interaction effect closely resembled the results found for predicting 4-yr changes in physical symptoms. Physical activity was significantly associated with a reduction of perceived stress over 2 years. This association was apparent among participants with high, but not low, baseline perceived stress (+1 SD: B = −.29, SE = .02, p< .01 vs. −1 SD: B = .13, SE = .02, p > .05).
Figure 2.
Mediation model examining the effect of 2-yr changes in perceived stress on the association between baseline levels of physical activity and perceived stress on 4 yr-changes in physical health symptoms.
The findings further showed that increased levels of perceived stress over 2 years were independently associated with increases in physical symptoms over 4 years, F(1, 147) = 4.67, B = .20, SE = .09, R2 = .02, p < .05 (see path B in Figure 2). Moreover, the analyses demonstrated that the interaction effect between baseline levels of physical activities and perceived stress in predicting 4-yr changes in physical symptoms was rendered non-significant, F(1, 147) = 2.13, B = −.12, SE = .08, R2 = .01, p = .15, if 2-yr changes in perceived stress were included as a potential mediator into the model (see path C in Figure 2). In fact, the bootstrap analyses confirmed that 2-yr changes in perceived stress exerted a significant indirect effect on the interaction between physical activities and perceived stress in predicting 4-yr changes in participants’ health symptoms (95% BCI [−.196, −.002]). This pattern of results suggests that the reduction of perceived stress mediated the beneficial effect of physical activity on fewer physical symptoms among participants who perceived high baseline levels of stress.
Discussion
This longitudinal study examined the roles of perceived stress in the association between physical activities and older adults’ physical health. We hypothesized that engaging in physical activities would have health benefits among individuals who perceived high (but not low) levels of stress. The study’s results supported our hypotheses by demonstrating significant interaction effects between baseline levels of physical activity and perceived stress on 2-yr changes in perceived stress and 4-yr changes in physical symptoms. These results showed that among participants with high baseline levels of perceived stress, those who frequently engaged in physical activities experienced a reduction of perceived stress over 2 years, and fewer increases in physical health symptoms over 4 years. No effects of physical activity on changes in perceived stress or physical symptoms were obtained among participants who perceived only low stress levels at baseline.
In addition, 2-yr reductions in perceived stress exerted a significant indirect effect on the interaction between baseline levels of physical activities and perceived stress in predicting 4-yr changes in physical health problems. These findings demonstrate that physical activity has the potential to ameliorate chronically high perceptions of stress and thereby produce long-term benefits on physical health.
The study’s findings are important for different reasons. First, they substantiate previous research suggesting that associations between physical activity and physical health can be more pronounced among individuals with high, as compared to low, levels of stress (Brown, 1991; Brown & Siegel, 1988; Carmack et al., 1999; Unger, Johnson, & Marks, 1997). The present study extends this line of work by demonstrating that such effects also appear among community-dwelling older adults. This implies that physical activity may be especially beneficial for physical health among vulnerable older adults who perceive stress. By contrast, older adults who are better adjusted psychologically may have more favorable health trajectories, and as such the benefits of physical activity in them are less pronounced. However, it is important to note that the majority of our sample consisted of “young-old” individuals and many of the more severe health stressors occur in later phases of older adulthood (Smith & Baltes, 1997). Accordingly, it is possible that physical activity could become more adaptive among participants with low baseline levels of stress, when new age-related stressors emerge in their future. A corollary of this argument is that the observed interaction effect between physical activity and perceived stress may become smaller in later stages of the life course when individuals increase their likelihood of encountering more physical stressors based on age-normative biological declines (Baltes, 1997). In such circumstances, we would expect to observe strong health effects of physical activity -independent of participants’ baseline stress levels - given that physical activity can ameliorate the psychological impact of emerging stressors and provide direct biological benefits (e.g., musculoskeletal strength, Faulkner et al., 1994) to counteract age-normative health declines (for research showing general effects of physical activity on physical health, see also Simonsick et al., 1993).
Second, the findings document an important mechanism that can explain health-related variability in old age. This mechanism is associated with the prevention of chronically high levels of perceived stress. In particular, it shows that physical activity can not only reduce elevated perceptions of stress over time, but such reductions in stress levels can further protect older adults’ physical health. This pattern of findings provides an explanation for the above-discussed stronger health effects of physical activity among individuals who perceive high (as compared to low) levels of stress. Physical activity may prevent stressed individuals from entering into a downward spiral, characterized by chronically high perceptions of stress and subsequent increases in physical health problems.
Finally, we think that our findings have implications for models of psychosocial determinants of illness, as they point to complex and dynamic interactions between health-promoting behaviors (e.g., physical activity) and psychological risk factors (e.g., perceived stress). Our study suggests that the presence of a psychological risk factor can generally enhance a person’s likelihood of developing physical symptoms and thus increases the importance and adaptive value of engaging in health-promoting behaviors. In turn, the engagement in health-promoting behaviors can contribute to long-term physical health outcomes if the behavior has the potential to attenuate the psychological risk factor. We therefore suggest that theory and research should extend the present analysis in long-term longitudinal studies and examine additional psychological risk factors (e.g., depression or anxiety) as well as other health-promoting behaviors (e.g., adaptive sleeping or eating patterns) to identify the health behaviors that are particularly well suited to prevent chronic levels of different psychological problems (for effects of physical activity and stressors on different psychological problems, see Carmack et al., 1999). Research along these lines may not only contribute to improved psychological theories of physical health, but may also provide important information that can be used to promote the physical health of individuals who experience psychologically problematic situations.
Limitations and Future Research
There are limitations to this research that need to be addressed in future studies. First, the predictor and outcome variables were assessed with self-report measures. In this regard, we note that our study did not assess participants’ memory capacity, which could influence the validity of self-report measures. However, given that the reliability of our perceived stress measure remained high across time (alphas > .87), and that our sample of older adults was on average relatively healthy at baseline, we think that it is unlikely that our findings are due to memory impairments. In addition, self-reports could be influenced by other dispositional constructs, such as negative affectivity or neuroticism (Portella, Harmer, Flint, Cowen, & Goodwin, 2005; Watson & Pennebaker, 1989). While such general biases are less likely to occur in longitudinal analyses because change scores should be less affected by disposition-based individual biases, we note that our study included measures of neuroticism and negative affectivity (Costa & McCrae, 1992; Watson, Clark, & Tellegon, 1988), and follow-up analyses revealed that these variables did not affect the reported interaction effects. Nonetheless, we suggest that future studies should use objective measures of memory capacity, physical health, and activities (e.g., Mora, DiBonaventura, Idler, et al. 2008; Nasreddine et al., 2005; Parker, Strath, & Swartz, 2008) to substantiate the conclusions drawn from our study.
Second, our findings suggest that perceived stress and physical activity can be relatively independent from each other, which allowed us to demonstrate significant interaction effects. However, future research is needed to identify the variables that determine individual differences in the association between perceived stress and activity engagement. In this regard, it would be interesting to identify psychological factors that help maintain high levels of physical activity among older adults who encounter stressful life circumstances. Such factors could be related to social support or perceptions of control and should be included in future studies.
Third, our analyses did not identify the mechanisms through which physical activities attenuated perceived stress, and through which the latter predicted subsequent health problems. In this regard, the stress-reducing effects derived from physical activity may be associated with changes in attentional focus or improved coping patterns. Physical activity typically turns people’s attention away from stressful circumstances (Bahrke & Morgan, 1978) and therefore could provide a temporary respite from life stress, or physical activity may serve a beneficial restorative function that allows people to deal with stressful circumstances more effectively. In addition, exercise could counteract some of the endocrine and immune dysregulation that is often associated with high stress levels (see Cohen, Janicki-Deverts, & Miller, 2007).
Finally, while our measure of physical symptoms may represent a reliable non-specific proxy for developing disease and mortality among the elderly (Sha et al., 2005), the analyses did not examine the development of more severe health problems (e.g., cancer, functional limitations, or mortality) or the health benefits deriving directly from physical activity (e.g., cardiorespiratory fitness or musculoskeletal strength, Dunn et al., 1997; Faulkner et al., 1994). We argue that our approach has some important advantages because it has the potential to detect physical health changes across different diseases. However, we note that supplemental analyses showed that our predictor variables were unrelated to changes in chronic health problems, functional limitations, or mortality. Nonetheless, future research should conduct long-term follow-ups of individuals’ chronic health problems, given that our sample was just approaching a life phase during which the likelihood of experiencing chronic health problems rapidly increases (Smith & Baltes, 1997). In addition, such an approach should examine a wider range of psychosocial, cognitive, and health variables (e.g., cardiorespiratory fitness) to provide a more comprehensive picture of the pathways to healthy aging.
Acknowledgments
Preparation of this manuscript was supported by a doctoral fellowship from Concordia University to Rebecca Rueggeberg, grants and awards from the Canadian Institutes of Health Research to Carsten Wrosch and grants from the Canadian Institutes of Health Research (89736), the National Institute of Child Health and Human Development (058502), and the Heart and Stroke Foundation of Canada awarded to Gregory Miller.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
Contributor Information
Rebecca Rueggeberg, Department of Psychology and Centre for Research in Human Development, Concordia University, Montreal, Canada.
Carsten Wrosch, Department of Psychology and Centre for Research in Human Development, Concordia University, Montreal, Canada.
Gregory E. Miller, Department of Psychology University of British Columbia, Vancouver, Canada
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bahrke MS, Morgan WP. Anxiety reduction following exercise and meditation. Cognitive Therapy and Research. 1978;2:323–333. doi: 10.1007/BF01172650. [DOI] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogenesis: Selection, optimization, and compensation as foundations of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037/0003-066X.52.4.366. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Murray D, Jeffery RW, Hennrikus D, Lando H. Associations between exercise and health behaviors in a community sample of working adults. Preventive Medicine. 2000;30:217–224. doi: 10.1006/pmed.1999.0618. [DOI] [PubMed] [Google Scholar]
- Brown JD. Staying fit and staying well: Physical fitness as a moderator of life stress. Journal of Personality and Social Psychology. 1991;60:555–561. doi: 10.1037/0022-3514.60.4.555. [DOI] [PubMed] [Google Scholar]
- Brown JD, Siegel JM. Exercise as a buffer of life stress: A prospective study of adolescent health. Health Psychology. 1988;7:341–353. doi: 10.1037/0278-6133.7.4.341. [DOI] [PubMed] [Google Scholar]
- Carmack CL, Boudreaux E, Amaral-Melendez M, Brantley PJ, Moor C. Aerobic fitness and leisure physical activity as moderators of the stress-illness relation. Annals of Behavioral Medicine. 1999;21:251–257. doi: 10.1007/BF02884842. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Physical activity guidelines for older adults. 2010 Available at: http://www.cdc.gov/nccdphp/dnpa/physical/pdf/PA_Fact_Sheet_OlderAdults.pdf.
- Cohen S. Psychological stress, immunity, and upper respiratory infections. Current Direction in Psychological Science. 1996;5:86–89. doi: 10.1111/1467-8721.ep10772808. [DOI] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA: Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:358–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Underwood L, editors. Measuring stress: A guide for health and social scientists. London: Oxford University Press; 1997. [Google Scholar]
- Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. New England Journal of Medicine. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO-PI-R (Personality Inventory Revised) manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cramer SR, Nieman DC, Lee JW. The effects of moderate exercise on psychological well-being and mood state in women. Journal of Psychosomatic Research. 1991;35:437–449. doi: 10.1016/0022-3999(91)90039-Q. [DOI] [PubMed] [Google Scholar]
- Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Medicine and Science in Sports and Exercise. 1987;19:114–120. doi: 10.1249/00005768-198710001-00004. [DOI] [PubMed] [Google Scholar]
- Denton M, Prus S, Walters V. Gender differences in health: A Canadian study of the psychosocial, structural and behavioural determinants of health. Social Science & Medicine. 2004;58:2585–2600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Reduction in cardiovascular disease risk factors: 6-month results from project active. Preventive Medicine. 1997;26:883–892. doi: 10.1006/pmed.1997.0218. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Green HJ, White T. Response and adaptation of skeletal muscel to changes in physical activity. In: Bouchard C, Shephard RJ, Stephens T, editors. Physical activity, fitness, and health: International proceedings and consensus statement. Champaign, IL, US: Human Kinetics; 1994. pp. 343–357. [Google Scholar]
- Fleshner M, Kennedy SL, Johnson JD, Day HEW, Greenwood BN. Exercise and stress resistance: Neural-Immune mechanisms. In: Siegel A, Zalcman SS, editors. The neuroimmunological basis of behavior and mental disorders. New York, NY, US: Springer Science + Business Media; 2009. pp. 87–107. [Google Scholar]
- Guszkowska M. Physical fitness as a resource in coping with stress among high school students. Journal of Sports Medicine and Physical Fitness. 2005;45:105–111. [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Hughes DC, Leung P, Naus MJ. Using single-system analyses to assess the effectiveness of an exercise intervention on quality of life for Hispanic breast cancer survivors: A pilot study. Social Work in Health Care. 2008;47:73–91. doi: 10.1080/00981380801970871. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan JF. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Taylor CB, Haskell WL. Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychology. 1993;12:292–300. doi: 10.1037/0278-6133.12.4.292. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: A Randomized Trial of Exercise or Relaxation. Journal of the American Geriatrics Society. 2007;55:892–899. doi: 10.1111/j.1532-5415.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin TJ, Doll R, Ma R, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF- κB signaling. Biological Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou E. If it goes up must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosomatic Medicine. 1999;61:850–860. doi: 10.1097/00006842-199911000-00021. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mora PA, DiBonaventura MD, Idler E, Leventhal EA, Leventhal H. Psychological factors influencing self-assessments of health: Toward an understanding of the mechanisms underlying how people rate their own health. Annals of Behavioral Medicine. 2008;36:292–303. doi: 10.1007/s12160-008-9065-4. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of aerobic and anaerobic training on fitness, blood pressure and psychological stress and well-being. Journal of Psychosomatic Research. 1990;34:367–375. doi: 10.1016/0022-3999(90)90060-H. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Blair SN, Lee I, Hyde RT. Measurement of physical activity to assess health effects in a free-living population. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Strath SJ, Swartz AM. Physical activity measurement in older adults: Relationships with mental health. Journal of Aging and Physical Activity. 2008;16:369–380. doi: 10.1123/japa.16.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Portella MJ, Harmer CJ, Flint J, Cowen P, Goodwin GM. Enhanced early morning salivary cortisol in neuroticism. American Journal of Psychiatry. 2005;162:807–809. doi: 10.1176/appi.ajp.162.4.807. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes A. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediation models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34:190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review. 2001;21:33–61. doi: 10.1016/S0272-7358(99)00032-X. [DOI] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer DH. Increased free cortisol secretion after awakening in chronically-stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. doi: 10.1002/(SICI)1099-1700(199804)14:2<91::AID-SMI765>3.0.CO;2-S. [DOI] [Google Scholar]
- Sha MC, Callahan CM, Counsell SR, Westmoreland GR, Stump TE, Kroenke K. Physical symptoms as a predictor of health care use and mortality among older adults. The American Journal of Medicine. 2005;118:301–306. doi: 10.1016/j.amjmed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Siconolfi SF, Lasater TM, Snow CK, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. American Journal of Epidemiology. 1985;122:101–105. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Lafferty ME, Phillips CL, Mendes de Leon CF, Kasl SV, Seeman TE, et al. Risk due to inactivity in physically capable older adults. American Journal of Public Health. 1993;83:1443–1450. doi: 10.2105/AJPH.83.10.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Baltes PB. Profiles of psychological functioning in the old and oldest old. Psychology and Aging. 1997;12:458–472. doi: 10.1037/0882-7974.12.3.458. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. Journal of the American Medical Association. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Thomason BT, Brantley PJ, Jones GN, Dyer HR, Morris JL. The relations between stress and disease activity in rheumatoid arthritis. Journal of Behavioral Medicine. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- Unger JB, Johnson CA, Marks G. Functional decline in the elderly: Evidence for direct and stress-buffering protective effects of social interactions and physical activity. Annals of Behavioral Medicine. 1997;19:152–160. doi: 10.1007/BF02883332. [DOI] [PubMed] [Google Scholar]
- Wankel LM, Sefton JM. Physical activity and other lifestyle behaviors. In: Bouchard C, Shephard RJ, Stephens T, editors. Physical activity, fitness, and health: International proceedings and consensus statement. Champaign, IL, US: Human Kinetics; 1994. pp. 530–550. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. Journal of Personality and Social Psychology. 1988;45:1063–70. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295X.96.2.234. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Lupien S, Pruessner JC. Diurnal cortisol secretion and 2-year changes in older adults’ physical symptoms: The moderating roles of negative affect and sleep. Health Psychology. 2008;27:685–693. doi: 10.1037/0278-6133.27.6.685. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Schulz R. Health engagement control strategies and 2-year changes in older adults’ physical health. Psychological Science. 2008;19:537–541. doi: 10.1111/j.1467-9280.2008.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: The importance of health engagement control strategies. Health Psychology. 2002;21:340–348. doi: 10.1037/0278-6133.21.4.340. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Schulz R, Miller GE, Lupien S, Dunne E. Physical health problems, depressive mood, and cortisol secretion in old age: Buffer effects of health engagement control strategies. Health Psychology. 2007;26:341–349. doi: 10.1037/0278-6133.26.3.341. [DOI] [PubMed] [Google Scholar]