Abstract
Children with Down syndrome (DS) bear an increased risk of acute lymphoblastic leukemia (ALL) and treatment complications. We compared blood counts and toxicities in 22 DS and 44 non-DS ALL patients. Patients with DS had deeper, longer neutrophil and monocyte count nadirs; more toxicities (HR 2.0, p = 0.0005); longer hospitalizations (HR 1.4, p < 0.0001); and more frequent microbiologically documented infections (HR 5.7, p = 0.0019), mucositis (HR 29.0, p = 0.0006), and cellulitis (HR 3.0, p= 0.033). Severe neutropenia, monocytopenia and increased cellulitis in DS-ALL suggest the importance of skin hygiene, vigilance and aggressive treatment of cutaneous infections.
Keywords: Down syndrome, ALL, infections
Introduction
Children with Down syndrome (DS) have a 20-fold increased risk of acute lymphoblastic leukemia (ALL) [1]. Their inferior outcomes may be partially attributable to infrequent favorable cytogenetic features [2]. However, treatment-related toxicities, particularly infections, are more frequent and severe, and remain a formidable challenge [2]. The Children’s Oncology Group (COG) and British Medical Research Council recently modified protocols due to excessive deaths in patients with DS-ALL, primarily overwhelming sepsis during myelosuppression [3, 4]. The degree and pattern of myelosuppression in DS-ALL has not been characterized, and may contribute to increased infections. Here we compare peripheral blood counts and toxicities in patients with DS- versus NDS-ALL during the first six months of therapy.
Methods
All DS-ALL and control NDS-ALL cases at Texas Children’s Cancer Center from 1998-2007 were retrospectively reviewed, with local institutional review board approval. Controls were matched 2:1 (NDS:DS) by treatment protocol. Data abstracted from medical records included: clinical/demographic features; white blood count (WBC); absolute neutrophil, lymphocyte, and monocyte counts (ANC, ALC, AMC); peripheral blast counts weekly ×8 and then monthly ×4; and grade 3-4 toxicities [5]. Data were censored at relapse, death, admission for stem cell transplantation, or date of last contact.
ANC, ALC, and AMC were compared over time between the two groups with fixed effects including group, time, and group*time using a generalized linear mixed model with natural log-transformed variables. To compare hospitalizations and toxicities, natural log-transformed counts per 1,000 patient follow-up days were used, assuming a Poisson distribution. Time to first toxicity event was analyzed using the Cox proportional hazard model. To evaluate the correlation between blood counts and toxicities, a Cox regression model was employed to analyze how time to the first toxicity event related to each CBC variable, using the last CBC value recorded prior to occurrence of the toxicity event. Analyses were conducted using SAS software (version 9.2; SAS Institute Inc., Cary, North Carolina).
Results
Characteristics of the 22 DS-ALL cases and 44 NDS-ALL controls are summarized in Table I. Patients received treatment on 14 protocols, with 77% (17/22) treated on either Pediatric Oncology Group (POG) protocol 9905 or COG protocol AALL0331. POG protocols 9905 and 9906 included six courses of intermediate-dose methotrexate, modified for DS patients to initially receive a 50% dose reduction, with subsequent courses at full dose if tolerated without grade 3-4 mucositis or delayed excretion. DS-specific modifications on AALL0331 included leucovorin rescue following intrathecal methotrexate prior to maintenance, omission of a second interim maintenance and delayed intensification for “standard risk-high” patients, and conservative management recommendations for myelosuppression, illness, and fever. None of the patients with DS included in this study received antifungal or antibacterial prophylaxis, and only a single patient received routine gammaglobulin replacement.
TABLE I.
Patient Characteristics
| DS-ALL, n (%) |
NDS-ALL, n (%) |
|
|---|---|---|
| Number | 22 | 44 |
| Gender | ||
| Male | 5 (23%) | 25 (57%) |
| Female | 17 (77%) | 19 (43%) |
| Age | ||
| 1-10 years | 20 (91%) | 37 (84%) |
| 10-18 years | 2 (9%) | 7 (16%) |
| NCI risk group | ||
| Standard | 19 (86%) | 38 (86%) |
| High | 3 (14%) | 6 (14%) |
| Ethnicity | ||
| Caucasian | 14 (64%) | 21 (48%) |
| Hispanic | 8 (36%) | 17 (39%) |
| African American | 0 (0%) | 5 (11%) |
| Asian | 0 (0%) | 1 (2%) |
| Mean BMI (kg/m2), (s.d.) | 17.1 (5.0) | 17.6 (2.9) |
| Treatment protocol | ||
| POG 9005 | 1 (5%) | 2 (5%) |
| POG 9405 | 1 (5%) | 2 (5%) |
| POG 9406 | 2 (9%) | 4 (9%) |
| POG 9905 | 11 (50%) | 22 (50%) |
| POG 9906 | 1 (4%) | 2 (4%) |
| COG AALL0331 | 6 (27%) | 12 (27%) |
DS, Down syndrome; ALL, acute lymphoblastic leukemia; NDS, non-Down syndrome; NCI, National Cancer Institute; BMI, body mass index; s.d., standard deviation; POG, Pediatric Oncology Group; COG, Children’s Oncology Group.
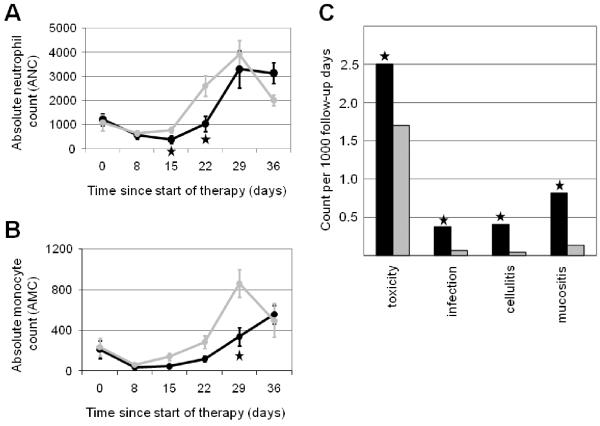
As expected, all patients demonstrated an induction nadir in blood counts, with lower mean ANC and AMC in DS-ALL at induction day 15, 22, and 29 (Figures 1A and 1B), reaching statistical significance for ANC at day 15 and 22 (p = 0.02 and 0.0001, respectively) and for AMC at day 29 (p = 0.005). There were no significant differences in ALC, blast clearance, or post-induction ANC or AMC.
Figure 1. Mean absolute neutrophil and monocyte counts and treatment-related toxicities in patients with DS-ALL and NDS-ALL during early therapy.
A. Mean absolute neutrophil counts (ANC) in patients with DS-ALL (black) and NDS-ALL (gray). B. Mean absolute monocyte counts (AMC) in patients with DS-ALL (black) and NDS-ALL (gray). Asterisks indicate time-points at which ANC and AMC significantly differed between groups (p < 0.05). C. The number of toxicities per 1000 follow-up days is shown for patients with DS-ALL (black) versus NDS-ALL (gray). Overall toxicities are shown, as well as the specific categories of microbiologically documented infections, cellulitis, and mucositis. Asterisks indicate significant differences between groups (p < 0.01). DS, Down syndrome; ALL, acute lymphoblastic leukemia; NDS, non-Down syndrome.
Patients with DS-ALL were hospitalized significantly longer (mean 34.1 versus 24.9 days; hazard ratio (HR) 1.4; 95% confidence interval (CI) 1.3, 1.6; p < 0.0001). They also experienced significantly more grade 3-4 toxicities (mean 2.5 versus 1.4 per patient; HR 2.0; 95% CI 1.4, 3.0, p = 0.0005) [5]. Notably, only one of 22 patients with DS-ALL (4.5%) experienced no grade 3-4 toxicities, compared to 15 of 44 NDS-ALL (34.1%) (Fisher’s exact p = 0.013).
A detailed analysis of grade 3-4 infectious complications and mucositis was performed since they cause significant morbidity and mortality in children with DS-ALL. Infectious complications were categorized as microbiologically documented infections, clinically documented infections, or fever of unknown origin. Upon initial qualitative review of the data, a notable prevalence of cellulitis was observed, with 10 episodes among the 22 cases of DS-ALL. Therefore, cellulitis, including events documented either microbiologically or clinically, was also analyzed as a separate category. Patients with DS-ALL were significantly more likely to experience at least one episode of mucositis (HR 29.0, p = 0.0006), microbiologically documented infections (HR 5.7, p = 0.0019), and cellulitis (HR 3.0, p = 0.033) (Figure 1C). There were no statistically significant differences in clinically documented infections (HR 2.28, p = 0.16) or fever of unknown origin (HR 1.16, p = 0.65). Clinical descriptions of toxicities are provided in Supplementary Table I.
Last, we investigated whether toxicities tended to be correlated with periods of low leukocyte counts. Overall toxicity events were significantly correlated with low AMC (HR 0.65 for each 2.7-fold increase in AMC, p = 0.014). Mucositis showed a trend toward correlation with low ANC (HR 0.41, p = 0.088).
Discussion
This study confirms the frequency and severity of treatment-related toxicities in DS-ALL, and provides new information on patterns of myelosuppression during chemotherapy, expanding beyond the existing literature on hematologic abnormalities in healthy children with DS [6-10]. The lower and more prolonged induction nadirs of both ANC and AMC likely contribute to the heightened risk of bacterial and fungal infections for patients with DS. Interestingly, ALC does not appear disproportionately affected. Recent literature suggests that early ALC recovery is associated with favorable prognosis in ALL [11]. The comparable ALC recovery during induction in DS-ALL is consistent with their comparable overall survival despite increased toxicities. Increased sensitivity to methotrexate and cytarabine in patients with DS has long been recognized [12, 13] and the mechanisms characterized [14]. However, these agents are absent in phases when patients with DS-ALL are at highest risk of toxicities. Our previous work demonstrated no significant increase in cytotoxicity of ALL chemotherapeutic agents in DS cell lines [15]. The current findings suggest that enhanced vulnerability to toxicities may be due to a general susceptibility to myelosuppression rather than differences in pharmacokinetics and pharmacodynamics of specific agents.
Our findings agree with others’ in demonstrating increased infections, mucositis, and hospitalization days in patients with DS-ALL [2, 16, 17]. While increased hospitalization days could be due partly to more conservative discharge criteria, the concomitant increase in toxicities suggests that increased treatment morbidity is a key factor. An increased risk of cellulitis has been reported previously in patients with DS and AML, but not in ALL [18]. Interestingly, grade 3-4 skin infections in patients with DS and ALL in this study are even more prevalent than reported in AML (40.9% versus 3.7%). Folliculitis has been reported in 21% of healthy subjects with DS [19], and may predispose to more significant cutaneous infections when chemotherapy suppresses the immune system and disrupts the skin barrier. Routine chlorhexidine cleansing, which has gained attention as a means of reducing inpatient bacterial skin colonization and infections [20], might be a beneficial preventive measure in patients with DS-ALL. The power of the current study is limited by the small sample size. Further studies are needed for validation in a larger population and for understanding effects of ALL therapy on other measures of immune function in DS. However, in light of the finding of an increased risk of cellulitis in DS-ALL, particular care for oral and skin hygiene, and vigilance for cutaneous infections, may be warranted in patients with DS.
Supplementary Material
Acknowledgments
We thank Xiaoying Yu for statistical support. Dr. Rabin was supported in part by the National Institutes of Health Pediatric Oncology Clinical Research Training Grant CA90433-06; the Kurt Groten Family Research Scholars’ Program; and a St. Baldrick’s Career Development Award.
Footnotes
Conflicts of Interest Statement: There are no conflicts to disclose.
References
- 1.Rabin KR, Whitlock JA. Malignancy in children with trisomy 21. Oncologist. 2009;14:164–173. doi: 10.1634/theoncologist.2008-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock JA. Down syndrome and acute lymphoblastic leukaemia. Br J Haematol. 2006;135:595–602. doi: 10.1111/j.1365-2141.2006.06337.x. [DOI] [PubMed] [Google Scholar]
- 3.de la Fuente J, Richards S, Webb DK, et al. Acute lymphoblastic leukaemia has a poor outcome in children with Down Syndrome due to infective death in remission (results of UK MRC ALL 97 trial) Blood. 2005;106:256a. [Google Scholar]
- 4.Maloney KW, Larsen E, Mattano LA, et al. Increased infection-related mortality for children with Down syndrome in contemporary Children’s Oncology Group acute lymphoblastic leukemia clinical trials. Blood. 2006;108:1865a. [Google Scholar]
- 5.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2003.
- 6.de Hingh YC, van d V, Gemen EF, et al. Intrinsic abnormalities of lymphocyte counts in children with down syndrome. J Pediatr. 2005;147:744–747. doi: 10.1016/j.jpeds.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi G, Mastrocola M, Capelli M, et al. Immunological patterns in young children with Down syndrome: is there a temporal trend? Acta Paediatr. 2007;96:1479–1482. doi: 10.1111/j.1651-2227.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 8.Kusters MA, Verstegen RH, Gemen EF, de VE. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156:189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloemers BL, van Bleek GM, Kimpen JL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr. 2010;156:804–9. 809. doi: 10.1016/j.jpeds.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Kivivuori SM, Rajantie J, Siimes MA. Peripheral blood cell counts in infants with Down’s syndrome. Clin Genet. 1996;49:15–19. doi: 10.1111/j.1399-0004.1996.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 11.De AG, Yuen C, Palla SL, et al. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer. 2008;112:407–415. doi: 10.1002/cncr.23168. [DOI] [PubMed] [Google Scholar]
- 12.Garre ML, Relling MV, Kalwinsky D, et al. Pharmacokinetics and toxicity of methotrexate in children with Down syndrome and acute lymphocytic leukemia. J Pediatr. 1987;111:606–612. doi: 10.1016/s0022-3476(87)80131-2. [DOI] [PubMed] [Google Scholar]
- 13.Peeters M, Poon A. Down syndrome and leukemia: unusual clinical aspects and unexpected methotrexate sensitivity. Eur J Pediatr. 1987;146:416–422. doi: 10.1007/BF00444952. [DOI] [PubMed] [Google Scholar]
- 14.Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer. 2005;44:33–39. doi: 10.1002/pbc.20092. [DOI] [PubMed] [Google Scholar]
- 15.Valle M, Plon SE, Rabin KR. Differential in vitro cytotoxicity does not explain increased host toxicities from chemotherapy in Down syndrome acute lymphoblastic leukemia. Leuk Res. 2009;33:336–339. doi: 10.1016/j.leukres.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N, Al-Ahmari A, Al-Yamani A, et al. Outcome and toxicity of chemotherapy for acute lymphoblastic leukemia in children with Down syndrome. Pediatr Blood Cancer. 2009;52:14–19. doi: 10.1002/pbc.21737. [DOI] [PubMed] [Google Scholar]
- 17.Afzal S, Ethier MC, Dupuis LL, et al. Risk factors for infection-related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J. 2009;28:1064–1068. doi: 10.1097/INF.0b013e3181aa6eae. [DOI] [PubMed] [Google Scholar]
- 18.Gamis AS, Woods WG, Alonzo TA, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891. J Clin Oncol. 2003;21:3415–3422. doi: 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 19.Schepis C, Barone C, Siragusa M, et al. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205:234–238. doi: 10.1159/000065859. [DOI] [PubMed] [Google Scholar]
- 20.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46:274–281. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.