Abstract
Earlier work suggests that the area of space from which useful visual information can be extracted (useful field of view, UFoV) shrinks in old age. We investigated whether this shrinkage, documented previously with a visual search task, extends to a bimanual tracking task. Young and elderly subjects executed two concurrent tracking tasks with their right and left arms. The separation between tracking displays varied from 3 to 35 cm. Subjects were asked to fixate straight ahead (condition FIX) or were free to move their eyes (condition FREE). Eye position was registered. In FREE, young subjects tracked equally well at all display separations. Elderly subjects produced higher tracking errors, and the difference between age groups increased with display separation. Eye movements were comparable across age groups. In FIX, elderly and young subjects tracked less well at large display separations. Seniors again produced higher tracking errors in FIX, but the difference between age groups did not increase reliably with display separation. However, older subjects produced a substantial number of illicit saccades, and when the effect of those saccades was factored out, the difference between young and older subjects’ tracking did increase significantly with display separation in FIX. We conclude that the age-related shrinkage of UFoV, previously documented with a visual search task, is observable with a manual tracking task as well. Older subjects seem to partly compensate their deficit by illicit saccades. Since the deficit is similar in both conditions, it may be located downstream from the convergence of retinal and oculomotor signals.
Keywords: Aging, Attention, Oculomotor control, Arm motor control, Tracking, Dual-task
Introduction
Our everyday activities such as walking, driving a car, or using a tool depend critically on the ability to deploy visual attention (Broman et al. 2004; Baldauf and Deubel 2008; Owsley et al. 1998). As an example, manual actions are planned on the basis of attended visual information (Baldauf and Deubel 2010; Land 2005; Land and Hayhoe 2001). Subjects typically focus both their gaze (Hayhoe et al. 2009; Pelz et al. 2001) and their eyes at the goal of their activities (Hayhoe et al. 2003; Mennie et al. 2007), which indicates that attention, eye movements, and manual control are closely interlinked. This linkage can have implications for our everyday life; for example, persons with impaired visual attention are more likely than others to fall while walking (Owsley and McGwin 2004) and to cause car accidents (Ball et al. 1993; Myers et al. 2000; Owsley et al. 1998). It therefore is of substantial concern for our “graying” society that visual attention decays in old age (Madden 1990; McDowd and Shaw 1999), possibly due to a shrinkage of the “useful field of view” (UFoV). The UFoV is defined as the area from which a person can process complex visual stimuli rapidly and accurately (Ball et al. 1988; Sekuler and Ball 1986); it can be substantially smaller than the visual field as determined by perimetry.
In an influential study, Ball and colleagues quantified UFoV with a visual search task that presented targets and distracters at different eccentricities and directions in the visual field of young and older subjects, and asked them to identify each target direction as fast as possible. (Ball et al. 1988). Seniors performed less well than young participants and, most importantly, their deficit increased in the periphery, as expected if UFoV indeed shrinks in old age.1 This outcome has been confirmed by numerous other studies (Coeckelbergh et al. 2004; Sekuler and Ball 1986; Sekuler and Bennett 2000; Seiple et al. 1996).
One possible confounding factor in UFoV research is the role of eye movements. Some authors verbally instructed their subjects to look at the targets while others asked them to fixate straight ahead, but eye movements were not registered and the actual gaze behavior is therefore unknown. Importantly, oculomotor behavior changes in old age: the latency and duration of saccades increase while their accuracy decreases, thus necessitating corrective and re-fixation saccades (Bono et al. 1996; Irving et al. 2006; Moschner and Baloh 1994; Meza et al. 2009; Paquette and Fung 2011). In consequence, differences between age groups might reflect not only different processing of peripheral visual stimuli, but also a different contribution of eye movements. It has indeed been reported that the number of saccades correlates inversely with task performance (Becic et al. 2007; Scialfa et al. 1994) and that young and older subjects’ performance no longer diverges in the periphery when the number of saccades is factored out (Scialfa et al. 1994). We therefore believe that it is crucially important to register and analyze oculomotor behavior when studying UFoV.
If the age-related shrinkage of UFoV is a fundamental phenomenon, it should be observable not only in the visual search task used in previous literature, but also in other tasks that require peripheral visual processing. To find out, the present study employs a bimanual tracking task, which, in our view, is related to real-life scenarios such as car driving and tool use. We compare a condition where subjects are asked to fixate straight ahead with one where they are free to look around, since humans rarely fixate a given object for more than a few hundred milliseconds in real life. To control for the effects of eye movements, eye position is registered along with manual performance and is factored out in a similar way as in previous work (Scialfa et al. 1994).
Methods
Subjects
Fourteen young (♀ = 8, ♂ = 6; mean age: 22.0 ± 2.1 years) and 14 older (♀ = 9, ♂ = 4; mean age: 69.4 ± 3.3 years) subjects participated. Since the eye data of one elderly subject were incomplete due to equipment malfunction, they were discarded. Among the remaining subjects, two young and two old ones reported to prefer their left hand for writing; the others indicated to prefer their right hand. All subjects lived independently in the community and had not participated in research on motor control or cognition within the preceding 6 months. All subjects had normal or corrected-to-normal vision, and all reported to be free of orthopedic and muscular impairment in a questionnaire completed before participating in the actual study. Since all subjects arrived without help at the agreed-upon time in the agreed-upon place, properly followed our instructions, and correctly completed questionnaire items requiring memory and orientation (e.g., address, date of birth, medication used), we deemed them to be free of gross cognitive impairment. Before participating, all signed an informed consent statement for this study, which was pre-approved by the authors’ institutional Ethics Committee.
Bimanual tracking task
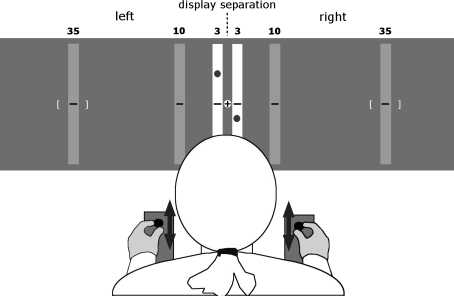
As illustrated in Fig. 1, subjects sat at about 70 cm distance from two vertical display areas, located symmetrically to the left and right of their body midline. The center of the display areas was 104 cm above ground. The display areas were 3, 10, or 35 cm apart, which corresponds to a viewing angle of about 2.5°, 8.2°, or 28.0°, respectively. Two joysticks, mechanically constrained to fore-aft movements, were placed at shoulder distance on a table, that is, the joystick boxes were just inside the edges of each subject’s shoulders. The right joystick controlled the vertical position of a cursor in the right display area and the left one that of a cursor in the left display area. The control law for either joystick was an unstable divergent function with added noise (Jex et al. 1966), and the cursors were presented as white dots of 0.85 cm diameter, shown against a black background on two 17′′ TFT monitors. Subjects grasped the joysticks with the thumb and index finger of their respective hand and were instructed to move them such as to keep both cursors near the display center, that is, to compensate the noise. For each display separation, subjects completed one trial of 60 s. It should be noted that this is a compensatory tracking task and not a pursuit tracking task; if subjects perform it well, the cursors will hardly move up or down and rather remain quite still in the display center.
Fig. 1.
Schematic drawing of our experimental setup. Black dots represent the cursors that the subjects had to keep centered, and black arrows indicate the possible movement of the two joysticks. The vertical target display areas are plotted in white (for 3 cm display separation) and gray (for 10 and 35 cm display separations). The center cross represents the fixation point; it was displayed only in condition FIX and the white brackets represent the calculated target zone (for exemplary individual and 35 cm display separation)
The subjects’ head and body movements were not mechanically constrained. The seat height was not adjustable, and the subjects’ eye height therefore varied between 126 and 130 cm depending on body size; to look at the display center, subjects thus had to lower their gaze by 17–20°, which is well within the comfort range of 10–25° stated in German ergonomic guidelines. In condition FIX, subjects were instructed to fixate continuously a dot mid-between the two display areas, but this instruction was lifted in condition FREE. The two conditions, and the three display separations within each condition, were administered to the subjects in a mixed order.
Data recording
The vertical distance of each cursor from the display center was registered at the frame rate of the display, 60 Hz. From these data, we calculated the root mean square tracking error (RMSE) as the root mean square distance between cursor and display center during the last 50 s of each trial averaged across the left and right cursor. We calculated this parameter separately for each subject and display separation.
Vertical and horizontal positions of the head and the left eye were registered with the video-based EyeLink 1000 device (SR Research Ltd.) at a sampling rate of 500 Hz and a spatial resolution of <0.1°. Preliminary analyses revealed that saccades from the left to the right display area and vice versa sometimes under- or overshot by a small amount, that is, horizontal eye position was outside the display area. We felt that these saccades should still count as “looking at the display areas” and not as “looking somewhere else.” We therefore decided to classify subjects’ gaze direction with respect to two target zones extending horizontally about the left and right display center, respectively. The width of these target zones was determined individually for each subject as ±2 standard deviations of horizontal eye position when repeatedly looking at the same object (see Fig. 1 for an illustration of target zones). Having established the target zones, we calculated the following parameters for the last 50 s of each trial:
Number of fixations within each of the two target zones
Fixation time within each of the two target zones
Fixation range: width of each target zone
Number of crossings, that is, of saccades that crossed the screen midline from left to right or right to left.
With the 3 cm display separation, the two target zones overlapped such that some fixations could not unequivocally be classified as within or between the target zones. We therefore decided to limit the analysis of eye parameters to the display separations of 10 and 35 cm.
Data analysis
RMSE and eye movement parameters were submitted to analyses of variance (ANOVAs) using the within-factors display Separation and Side (dominant, non-dominant Hand), and the between-factor Age.
Similar to the approach of other groups (Becic et al. 2007; Scialfa et al. 1994), we partialled out the effects of eye movements on seniors’ RMSE by linear regression analysis. As a first step, we calculated the multiple linear regression of individual seniors’ RMSE on their eye movement parameters and stored the residuals r i of each senior i. As a second step, we entered the mean eye movement parameters of young subjects into the regression equation to yield Y, the predicted mean RMSE of seniors if their eye movement parameters equaled those of young subjects. As a third step, we calculated the normalized RMSE of each individual senior, predicted whether that person’s eye movement parameters equaled those of young subjects:
| 1 |
This analysis was done separately for each condition and for each display separations at which eye movements could be analyzed (see above). The normalized RMSE′ scores were submitted to the same ANOVAs as were the original RMSE scores.
Results
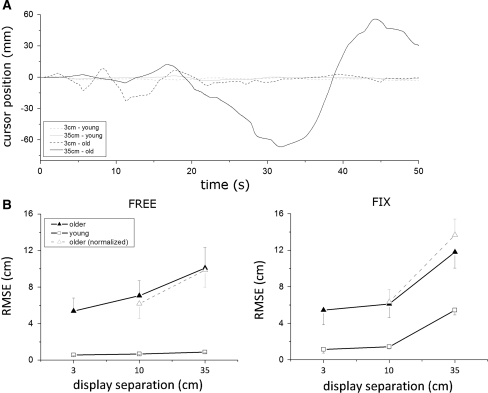
The solid lines in Fig. 2b depict the tracking error of both age groups for the three display separations and both viewing conditions; Table 1 summarizes the corresponding ANOVA outcome. In condition FREE, young subjects had relatively low RMSE scores for all display separations. The scores of elderly subjects were generally higher and increased distinctly at larger display separations (effects of Age, Separation and Age * Separation in Table 1). In contrast to condition FREE, young subjects in condition FIX again produced relatively low RMSE scores, but this time the difference between age groups did not increase significantly with display separation (effect of Age and Separation, but not Age * Separation, in Table 1). Side and its interactions showed no significant effects in condition FREE, and only Side * Separation (F(2,50) = 4.05; P < 0.05) and Side * Separation * Age (F(2,50) = 5.23; P < 0.01) were significant in condition FIX.
Fig. 2.
a Exemplary tracking performance of young (gray lines) and older (black lines) subject in condition FREE in its original state. Dashed lines represent cursor movements with 3 cm display separation, solid lines represent cursor movements with 35 cm display separation. b Tracking error of young and elderly subjects. Symbols represent means and bars represent the appropriate standard errors. Display separations of 3, 10, and 35 cm are plotted
Table 1.
ANOVA outcome for RMSE and RMSE′
| FREE | FIX | |
|---|---|---|
| RMSE | ||
| Age | F(1,25) = 19.77*** | F(1,25) = 10.90** |
| Separation | F(2,50) = 4.54* | F(2,50) = 60.03*** |
| Age * separation | F(2,50) = 3.45* | F(2,50) = 2.07 n.s. |
| RMSE′ | ||
| Age | F(1,25) = 23.78*** | F(1,25) = 20.24*** |
| Separation | F(2,50) = 6.53* | F(2,50) = 96.22*** |
| Age * separation | F(2,50) = 5.15* | F(2,50) = 8.19** |
n.s., *, **, and *** indicate P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively
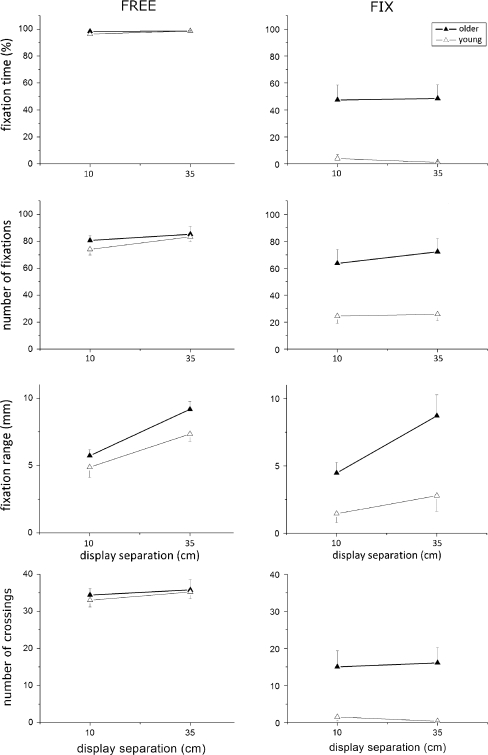
As shown in Fig. 3 and Table 2, eye parameters in condition FREE were similar in both age groups, with fixation range increasing at the larger display separation (effect of Separation in Table 2). Again, the performance of subjects did not differ between their dominant and non-dominant hand for either of the measured eye parameters (no effects of Side and its interaction with Age and Separation). In contrast, eye parameters in condition FIX were distinctly age-dependent (effect of Age for all parameters in Table 2): elderly subjects produced more saccades than younger ones into the target areas and spent more time there, thus disobeying our instructions for that condition. The fixation range and the number of crossings in young subjects, but not that of elderly subjects was much lower than in condition FREE which, however, could well be an artifact due to the small number of saccades in young participants. Comparable to condition FIX, Side again influenced subjects number of fixations as a function of Separation * Side (F(1,25) = 5.81; P < 0.05) and Age * Separation * Side (F(1,25) = 6.54; P < 0.05). Results show that subjects did not prefer looking at the side controlled by their dominant hand nor did they improve their performance of the non-dominant hand by permanently focusing the appropriate display side.
Fig. 3.
Eye movement parameters, with symbols representing mean values and error bars representing the appropriate standard error. Only display separations of 10 and 35 cm are plotted, since data from the 3 cm separation were not unequivocal
Table 2.
ANOVA outcome for eye parameter
| FREE | FIX | |
|---|---|---|
| Number of fixations | ||
| Age | F(1,25) = 0.67 n.s | F(1,25) = 20.59*** |
| Separation | F(1,25) = 3.48 n.s. | F(1,25) = 0.10 n.s. |
| Age * separation | F(1,25) = 0.47 n.s. | F(1,25) = 0.74 n.s. |
| Fixation time | ||
| Age | F(1,25) = 2.44 n.s. | F(1,25) = 8.10** |
| Separation | F(1,25) = 9.31** | F(1,25) = 1.27 n.s. |
| Age * separation | F(1,25) = 0.19 n.s. | F(1,25) = 3.83 n.s. |
| Fixation range | ||
| Age | F(1,25) = 3.77 n.s. | F(1,25) = 19.41*** |
| Separation | F(1,25) = 47.88*** | F(1,25) = 11.30** |
| Age * separation | F(1,25) = 0.86 n.s. | F(1,25) = 5.14* |
| Number of crossings | ||
| Age | F(1,25) = 0.15 n.s. | F(1,25) = 15.78*** |
| Separation | F(1,25) = 1.13 n.s. | F(1,25) = 0.00 n.s. |
| Age * separation | F(1,25) = 0.06 n.s. | F(1,25) = 0.35 n.s. |
n.s., *, **, and *** indicate P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively
The dashed lines in Fig. 2b depict the normalized tracking scores RMSE′, which partial out the effects of eye movements. Clearly, the normalized scores increased with display separation even more than did the original scores. The bottom part of Table 1 shows that in condition FREE, the effect of Age * Separation remained significant for RMSE′ as it was for RMSE; in condition FIX, it became significant for RMSE′ which it was not for RMSE. In other words, when age-related differences of eye movements were taken into account, the tracking performance of young and elderly subjects diverged with increasing display separation not only in FREE but also in FIX.
In additional analyses, we evaluated whether looking at one target zone had a differential effect on RMSE at the same and at the opposite side. This was done with a generalized linear model (GLM) approach with the factors Age and Side, and the regressor “time spend with gaze in dominant target zone.” We yielded significance for age (F(1,24) = 18.64; P < 0.001) and the regressor (F(1,24) = 12.01; P < 0.01), but not for other effects, notably not for the Side*regressor interaction. In a second GLM approach, we changed the regressor to “time spend with gaze in non-dominant target zone”; this is not trivial, since subjects could also spend time outside both target zones. The analysis yielded only one significant effect, that of age (F(1,24) = 12.83; P < 0.01). GLM outcomes were corrected for multiple comparisons using Bonferroni corrections. From this we conclude that looking at the dominant zone improved tracking with the dominant and with the non-dominant arm, while looking at the non-dominant zone had no reliable effect on tracking with each hand. Since this holds for both age groups, it has no explanatory value for the age-related UFoV shrinkage.
Discussion
Our study compares the performance of young and elderly subjects in a bimanual tracking task, when tracking displays are presented at different eccentricities with respect to the egocentric straight ahead, while the hands remain at the same eccentricity. In condition FREE, subjects were allowed to look around. We found that the manual tracking error of young subjects was low and did not depend on display eccentricity, while that of elderly subjects was higher and increased with display eccentricity. Thus, the manual tracking data of the two age groups diverged with increasing eccentricity, as did the visual search data in previous studies where subjects were asked to fixate straight ahead (Ball et al. 1988; Coeckelbergh et al. 2004; Seiple et al. 1996; Sekuler and Ball 1986; Sekuler and Bennett 2000). Factoring out the effects of eye movements did not reduce this divergence as it did in an earlier study on visual search (Scialfa et al. 1994), possibly because visual search requires fairly accurate eye movements to distinguish targets from distracters, while manual tracking of easily discernible targets does not require high oculomotor precision. In other words, we propose that the control of eye movements is degraded in old age to an extent that is critical for visual search, but not yet for manual control.
In condition FIX, subjects were asked to keep their gaze straight ahead. Young persons obeyed this instruction well, while older ones spent about half of the time glancing at the targets. This lack of compliance could reflect deficits in the ability to inhibit automated but undesired behavior, and/or in the ability to concurrently control fixation and manual tracking: response inhibition as well as multitasking is an important component of executive functions, which are known to decay in old age (Andres et al. 2008; Brennan et al. 1997; Gunning-Dixon and Raz 2003; Somberg and Salthouse 1982). Manual tracking was again poorer in elderly subjects, but unlike in condition FREE, the errors of both groups increased at the largest display eccentricity.
In contrast to condition FREE, the tracking data of young and elderly subjects in condition FIX did not reliably diverge with increasing display eccentricity. However, the divergence became statistically significant when the effects of seniors’ illicit eye movements were factored out. From this we conclude that our elderly subjects partly compensated their peripheral deficits by disobeying our instructions and directing their gaze at the display areas.
It should be noted that our condition FIX, with eye movements factored out, is analogous to previous UFoV paradigms where the effects of eye movements were restricted. The main difference is that we used manual tracking, while previous studies were based on visual search. Since the performance of young and older subjects diverged with increasing eccentricity not only in the earlier studies but in the present condition FIX as well, our findings suggest that age-related UFoV shrinkage is not an isolated phenomenon limited to visual search, but rather extends to manual skill tasks.
Our condition FREE differs from previous UFoV paradigms in that subjects could direct their gaze at the stimuli. Our eye registrations show that both age groups took advantage of this in a similar fashion. In spite of this fact, tracking performance of young and older subjects again diverged with increasing eccentricity, and Fig. 2 illustrates that the divergence was comparable to that in condition FIX when the effects of eye movements were factored out. In fact, defining divergence as the difference between young and old subjects at 35 cm display separation minus that at 10 cm separation, the amount of 3.5 cm calculated for FREE is very similar to 3.3 cm calculated for FIX. It therefore is conceivable that the divergence in both conditions, as well as that in previous visual search tasks, is due to age-related decrements of a central stage, accessible for visual search, manual tracking, and possibly many other visuomotor tasks. The divergence seems not to be explainable by oculomotor deficits in old age, since it persisted in condition FREE—and only emerged in condition FIX—after the effects of eye movements were factored out.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The divergence is not visible in the published data of Ball et al. (1988), but can be uncovered by back-transforming the arc-sine transformed plots. We are thankful to an anonymous reviewer for pointing this out.
References
- Andres P, Guerrini C, Phillips L, Perfect T. Differential effects of aging on executive and automatic inhibition. Dev Neuropsychol. 2008;33(2):101–123. doi: 10.1080/87565640701884212. [DOI] [PubMed] [Google Scholar]
- Baldauf D, Deubel H. Visual attention during the preparation of bimanual movements. Vis Res. 2008;48(4):549–563. doi: 10.1016/j.visres.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Baldauf D, Deubel H. Attentional landscapes in reaching and grasping. Vis Res. 2010;50(11):999–1013. doi: 10.1016/j.visres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ball K, Beard B, Roenker D, Miller R, Griggs D. Age and visual search: expanding the useful field of view. J Opt Soc Am A. 1988;5(12):2210–2219. doi: 10.1364/JOSAA.5.002210. [DOI] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane M, Roenker D, Bruni J. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- Becic E, Kramer A, Boot W. Age-related differences in visual search in dynamic displays. Psychol Aging. 2007;22(1):67–74. doi: 10.1037/0882-7974.22.1.67. [DOI] [PubMed] [Google Scholar]
- Bono F, Oliveri R, Zappia M, Aguglia U, Puccio G, Quattrone A. Computerized analysis of eye movements as a function of age. Arch Gerontol Geriatr. 1996;22(3):261–269. doi: 10.1016/0167-4943(96)00698-X. [DOI] [PubMed] [Google Scholar]
- Brennan M, Welsh M, Fisher C (1997) Aging and executive function skills: an examination of a community-dwelling older adult population. Percept Mot Skills 84(3 Pt 2):1187–1197 [DOI] [PubMed]
- Broman A, West S, Munoz B, Bandeen-Roche K, Rubin G, Turano K. Divided visual attention as a predictor of bumping while walking: the salisbury eye evaluation. Invest Ophthalmol Vis Sci. 2004;45(9):2955–2960. doi: 10.1167/iovs.04-0219. [DOI] [PubMed] [Google Scholar]
- Coeckelbergh T, Cornelissen F, Brouwer W, Kooijman A. Age-related changes in the functional visual field: further evidence for an inverse age × eccentricity effect. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P11–P18. doi: 10.1093/geronb/59.1.P11. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/S0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hayhoe MM, Shrivastava A, Mruczek R, Pelz JB. Visual memory and motor planning in a natural task. J Vis. 2003;3(1):49–63. doi: 10.1167/3.1.6. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Gillam B, Chajka K, Vecellio E. The role of binocular vision in walking. Vis Neurosci. 2009;26(1):73–80. doi: 10.1017/S0952523808080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006;47(6):2478–2484. doi: 10.1167/iovs.05-1311. [DOI] [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV (1966) A “critical” tracking task for man-machine research related to the operator’s effective delay time. I. Theory and experiments with a first-order divergent controlled element. NASA CR-616. NASA Contract Rep NASA CR:1–105 [PubMed]
- Land MF. Eye-hand coordination: learning a new trick. Curr Biol. 2005;15(23):R955–R956. doi: 10.1016/j.cub.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vis Res. 2001;41(2001):3559–3565. doi: 10.1016/S0042-6989(01)00102-X. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Adult age differences in the time course of visual attention. J Gerontol. 1990;45(1):P9–P16. doi: 10.1093/geronj/45.1.p9. [DOI] [PubMed] [Google Scholar]
- McDowd J, Shaw R. Attention and aging: a functional perspective. In: Craik C, Salthouse T, editors. The handbook of aging and cognition. Mahwah: Lawrence Erlbaum Assoc; 1999. pp. 221–292. [Google Scholar]
- Mennie N, Hayhoe M, Sullivan B. Look-ahead fixations: anticipatory eye movements in natural tasks. Exp Brain Res. 2007;179(3):427–442. doi: 10.1007/s00221-006-0804-0. [DOI] [PubMed] [Google Scholar]
- Meza M, Patterson P, Nakayasu H. Comparing visual performance and useful field of view of older and younger drivers. Biomed Sci Instrum. 2009;45:83–88. [PubMed] [Google Scholar]
- Moschner C, Baloh RW. Age-related changes in visual tracking. J Gerontol. 1994;49(5):M235–M238. doi: 10.1093/geronj/49.5.m235. [DOI] [PubMed] [Google Scholar]
- Myers RS, Ball KK, Kalina TD, Roth DL, Goode KT. Relation of useful field of view and other screening tests to on-road driving performance. Percept Mot Skills. 2000;91(1):279–290. doi: 10.2466/pms.2000.91.1.279. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G. Association between visual attention and mobility in older adults. J Am Geriatr Soc. 2004;52:1901–1906. doi: 10.1111/j.1532-5415.2004.52516.x. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Sloane ME, Roenker DL, White MF, Overley ET. Visual processing impairment and risk of motor vehicle crash among older adults. Jama. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Paquette C, Fung J. Old age affects gaze and postural coordination. Gait Posture. 2011;33(2):227–232. doi: 10.1016/j.gaitpost.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Pelz J, Hayhoe M, Loeber R. The coordination of eye, head, and hand movements in a natural task. Exp Brain Res. 2001;139(3):266–277. doi: 10.1007/s002210100745. [DOI] [PubMed] [Google Scholar]
- Scialfa CT, Thomas DM, Joffe KM. Age differences in the useful field of view: an eye movement analysis. Optom Vis Sci. 1994;71(12):736–742. doi: 10.1097/00006324-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Seiple W, Szlyk JP, Yang S, Holopigian K (1996) Age-related functional field losses are not eccentricity dependent. Vision Res 36 (12):1859–1866 [DOI] [PubMed]
- Sekuler R, Ball K. Visual localization: age and practice. J Opt Soc Am A. 1986;3(6):864–867. doi: 10.1364/JOSAA.3.000864. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Bennett PJ. Effects of aging on the useful field of view. Exp Aging Res. 2000;26(2):103–120. doi: 10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- Somberg BL, Salthouse TA. Divided attention abilities in young and old adults. J Exp Psychol Hum Percept Perform. 1982;8(5):651–663. doi: 10.1037/0096-1523.8.5.651. [DOI] [PubMed] [Google Scholar]