Abstract
The first human case with trichinellosis was reported in 1964 in Tibet, China. However, up to the present, the etiological agent of trichinellosis has been unclear. The aim of this study was to identify a Tibet Trichinella isolate at a species level by PCR-based methods. Multiplex PCR revealed amplicon of the expected size (173 bp) for Trichinella spiralis in assays containing larval DNA from Tibet Trichinella isolate from a naturally infected pig. The Tibet Trichinella isolate was also identified by PCR amplification of the 5S ribosomal DNA intergenic spacer region (5S ISR) and mitochondrial large-subunit ribosomal RNA (mt-lsrDNA) gene sequences. The results showed that 2 DNA fragments (749 bp and 445 bp) of the Tibet Trichinella isolate were identical to that of the reference isolates of T. spiralis. The Tibet Trichinella isolate might be classifiable to T. spiralis. This is the first report on T. spiralis in southwestern China.
Keywords: Trichinella spiralis, taxonomy, molecular identification, Tibet, China
INTRODUCTION
Trichinellosis is a cosmopolitan zoonotic disease caused by the ingestion of raw or undercooked meat containing Trichinella larvae. Currently, taxonomy of the genus Trichinella encompasses 8 species (T. spiralis, T. nativa, T. britovi, T. pseudospiralis, T. murrelli, T. nelsoni, T. papuae, and T. zimbabwensis) and 4 additional genotypes ("Trichinella T6", "T8", "T9" and "T12") [1,2]. Of all the species and genotypes, T. spiralis is the most widely distributed, often found in domestic pigs, and the most common cause of human trichinellosis [3].
In China, the first human case with trichinellosis was reported in 1964 in Tibet [4]. From this observation until 2009, a total of 15 outbreaks, consisting of 187 cases and 12 deaths, were recorded in Tibet [5,6]. Although trichinellosis is an important zoonotic diseases and a serious public health concern in Tibet, the etiological agent of trichinellosis has been unclear. All Trichinella isolates from the patients' biopsy muscle tissues or pork have not been identified at the species level although it was assumed to be T. spiralis. The species identification of Trichinella isolates is of great importance for etiological, epidemiological, and phylogenetic studies.
The aim of this study was to identify the species of Tibet Trichinella isolate by multiplex PCR, then to analyze its genetic variation within Trichinella species by PCR-based methods using 5S ribosomal DNA intergenic spacer region (5S ISR) and mitochondrial large-subunit ribosomal DNA (mt-lsrDNA) as genetic markers.
MATERIALS AND METHODS
Collection of Tibet Trichinella isolates and experimental infection
In February 2009, a small familial outbreak of trichinellosis due to consumption of raw pork occurred in a village of Linzhi prefecture of Tibet, southwestern China. The raw pork samples of the residue posterior leg during this outbreak were collected, and examined by the compression method and histological sections; the encapsulated Trichinella larvae were found. The pork was cut into pieces and digested with 0.33% pepsin (1:31,000) - 1% HCl for 4 hr at 43℃ [7,8]. The muscle larvae were recovered after artificially digesting the pork. After washing, individual muscle larvae were stored at -80℃ until used. This Tibet Trichinella isolate was also maintained by serial passage in 6-week-old, SPF male Kunming mice (Experimental Animal Center of Henan Province, China) at 6- to 8-month intervals in our department (Department of Parasitology, Medical College, Zhengzhou University). The reference Trichinella isolates used in this study was T. nativa (ISS10) and T. nelsoni (ISS29), which were obtained from the Trichinella Reference Center (IRC; Rome, Italy), and Henan isolate of T. spiralis (ISS534) from domestic pigs in Nanyang city of Henan Province, China.
Species identification of Tibet Trichinella isolate by multiplex PCR
For multiplex PCR, about 20 muscle larvae recovered from the naturally infected pork were each washed 3 times in Hank's Balanced Salt Solution, pelleted, and subjected to genomic DNA extraction utilizing a DNeasy Tissue Kit (Qiagen Inc., Valencia, California, USA). Trichinella larvae were identified at the species level by multiplex PCR, as previously described [9]. The PCR-amplified fragments were visualized by agarose gel electrophoresis (2% standard agarose).
PCR and sequencing of 5S ISR and mt-lsrDNA of Tibet Trichinella isolate
For further confirmation of molecular identification of the Tibet Trichinella isolate, the 5S ISR and mt-lsrDNA fragments of the Tibet isolate and reference T. spiralis Henan isolate were amplified using the following primers: 5' GCGAATTCTTGGATCGGAGACGGCCTG and 5' GCTCTAGACGAGATGTCGTGCTTTCAACG for 5SISR, 5' WACAATGGTCCTTTCGTACT and 5' TGAGGACATTAAGGTAGC for mt-lsrDNA [10,11]. PCR products of the Tibet Trichinella isolate and reference T. spiralis Henan isolate were delivered to Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China), which were purified and directly sequenced using 15 pmol of the PCR primers with an ABI PRISM 3730 Genetic Analyzer. The sequencing reagent was BigDye terminator v3.1. The primers used for sequencing were the same as those used for PCR amplification. The products of 2 independent amplification tubes for the 2 genes were completely sequenced on both strands to confirm the nucleotide sequences.
RESULTS
Morphological features
Examination of pork samples obtained from the posterior legs of the pig showed infection with the coiled intracellular Trichinella larvae. These pork samples were subsequently examined by the artificial digestion method, and the coiled and motile muscle larvae were collected with an intensity of infection of 12 larvae per gram of muscle tissues.
Multiplex PCR
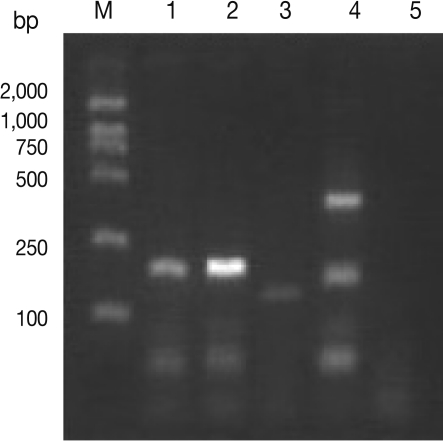
Multiplex PCR revealed amplicon of the expected size (173 bp) for T. spiralis in assays containing larval DNA from the Tibet Trichinella isolate from a naturally infected pig (Fig. 1).
Fig. 1.
Agarose gel separation of multiplex PCR products using DNA from the Tibet Trichinella isolate from a naturally infected pig. M, molecular weight markers. Lane 1, Tibet Trichinella isolate from a naturally infected pig. Lane 2, T. spiralis isolate (ISS534) control. Lane 3, T. nativa isolate (ISS10) control. Lane 4, T. nelsoni isolate (ISS29) control. Lane 5, Double-distilled water control.
PCR analysis of 5S-ISR DNA and mt-lsrDNA
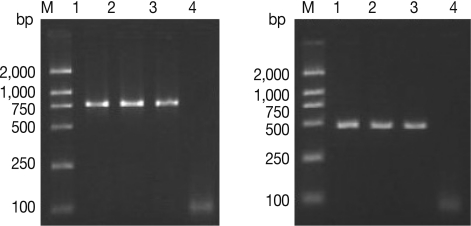
Results of agarose gel electrophoresis with primers derived from 5S ISR and mt-lsrDNA using genomic DNA from different Trichinella isolates are shown in Fig. 2. Two DNA fragments (749 bp and 445 bp) of the Tibet Trichinella isolate were identical to that of the reference isolate of T. spiralis. The BLAST analysis with DNA sequences of the Tibet Trichinella isolate showed that 5S ISR DNA and mt-lsrDNA regions of the Tibet isolate most closely resembled those of T. spiralis sequences available in GenBank. Homologous analysis showed that the consistence of 5S ISR sequences of the Tibet Trichinella isolate and T. spiralis (accession number AY009946) was 100%; the consistency of mt-lsrDNA sequences of the Tibet Trichinella isolate and T. spiralis (accession number AY851277) was 99.75%, with just 1 nucleotide difference (53 A-G).
Fig. 2.
Agarose gel separation of PCR products of 5S ISR (left) and mt-lsrDNA (right) of the Tibet Trichinella isolate. M, molecular weight markers. Lanes 1-2, Tibet Trichinella isolate. Lane 3, T. spiralis isolate (ISS534) control. Lane 4, Double-distilled water control.
DISCUSSION
Two species of Trichinella (T. spiralis from domestic pigs in central and northeastern areas and T. nativa from dogs in northeastern areas) have been described in China [12,13]. However, the Trichinella isolates in southwestern China were not identified at the species level. Our results showed that the Trichinella isolate from a naturally infected pig in Tibet was identified as T. spiralis by multiplex PCR and PCR using 5S ISR as well as mt-lsrDNA as genetic markers. This is the first species identification of Trichinella isolates in southwestern China.
At present, Trichinella is a serious food safety risk to consumers of porks in southwestern China [6]. In Tibet, pigs are often raised in backyards under poor hygienic condition or in open areas where pigs are pastured freely and feed on raw waste products or animal carcasses. The local minor ethnic groups slaughter their self-raised pigs at home without veterinary inspection [14]. In addition, because of the high altitude with 3,000 m above the sea level and low air pressure, the meat is often insufficiently cooked; the ethnic groups enjoy eating raw meat. So, outbreaks of trichinellosis occurred often in Tibet. From 2004 to 2009, all of 4 outbreaks occurred in Tibet were caused by eating raw or undercooked porks [6]. Our results showed that the etiological agent of human trichinellosis in Tibet is T. spiralis, and suggested that domestic pigs play an important role in the maintenance of the life cycle of T. spiralis. Hence, implementation of a Trichinella control programme, together with general health education, will be necessary for the local ethnic villagers [15].
In conclusion, the Trichinella isolate from a naturally infected pig in Tibet was identified as T. spiralis. This is the first report of species identification of Trichinella isolates in southwestern China.
ACKNOWLEDGMENTS
We thank Dr. Ci Ren for collaborating in collection of pork samples. This work was supported by the National Basic Research Program of China (No.2010CB530000) and the National Natural Science Foundation of China (No. 30972492, 30972579).
References
- 1.Pozio E, Zarlenga DS. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int J Parasitol. 2005;35:1191–1204. doi: 10.1016/j.ijpara.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Krivokapich SJ, Prous CL, Gatti GM, Confalonieri V, Molina V, Matarasso H, Guarnera E. Molecular evidence for a novel encapsulated genotype of Trichinella from Patagonia, Argentina. Vet Parasitol. 2008;156:234–240. doi: 10.1016/j.vetpar.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dupouy-Camet J. Presidential address of ICT12 Conference: "Trichinella and trichinellosis-A never ending story". Vet Parasitol. 2009;159:194–196. doi: 10.1016/j.vetpar.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Wang ZQ, Cui J. The epidemiology of human trichinellosis in China during 1964-1999. Parasite. 2001;8:S63–S66. doi: 10.1051/parasite/200108s2063. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZQ, Cui J, Xu BL. The epidemiology of human trichinellosis in China during 2000-2003. Acta Trop. 2006;97:247–251. doi: 10.1016/j.actatropica.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Cui J, Wang ZQ, Xu BL. The epidemiology of human trichinellosis in China during 2004-2009. Acta Trop. 2011;118:1–5. doi: 10.1016/j.actatropica.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, Schenone H, Zhu X. International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol. 2000;93:393–408. doi: 10.1016/s0304-4017(00)00354-x. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Cui J, Wang ZQ, Jiang P. Sensitivity and optimization of artificial digestion in the inspection of meat for Trichinella spiralis. Foodborne Pathog Dis. 2010;7:879–885. doi: 10.1089/fpd.2009.0445. [DOI] [PubMed] [Google Scholar]
- 9.Zarlenga DS, Chute MB, Martin A, Kapel CM. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int J Parasitol. 1999;29:1859–1867. doi: 10.1016/s0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 10.Rombout YB, Bosch S, van Der Giessen JWB. Detection and identification of eight Trichinella genotypes by reverse line blot hybridization. J Clin Microbiol. 2001;39:642–646. doi: 10.1128/JCM.39.2.642-646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozio E, Foggin CM, Marucci G, La Rosa G, Sacchi L, Corona S, Rossi P, Mukaratirwa S. Trichinella zimbabwensis n.sp.(Nematoda), a new non-encapsulated species from crocodiles (Crocodylus niloticus) in Zimbabwe also infecting mammals. Int J Parasitol. 2002;32:1787–1799. doi: 10.1016/s0020-7519(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZQ, Cui J, Shen LJ. The epidemiology of animal trichinellosis in China. Vet J. 2007;173:391–398. doi: 10.1016/j.tvjl.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Fu BQ, Liu MY, Yao CY, Li WH, Li YG, Wang YH, Wu XP, Zhang DL, Cai XP, Blaga R, Boireau P. Species identification of Trichinella isolates from China. Vet Parasitol. 2009;159:214–217. doi: 10.1016/j.vetpar.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Wang HJ, Li CC, Jiang J, Bai J, Li XJ, Ci R. Investigation on outbreak of trichinellosis in Milin county of Linzhi prefecture, Tibet. Strait J Prev Med. 2008;14:47. [Google Scholar]
- 15.Gajadhar AA, Pozio E, Gambie HR, Nökler K, Maddox-Hyttel C, Forbes LB, Vallée I, Rossi P, Marinculić A, Boireau P. Trichinella diagnostics and control: Mandatory and best practices for ensuring food safety. Vet Parasitol. 2009;159:197–205. doi: 10.1016/j.vetpar.2008.10.063. [DOI] [PubMed] [Google Scholar]