Abstract
Risperidone has been shown to improve serious behavioral problems in children with autism. Here we asked whether risperidone-associated improvement was related to changes in concentrations of inflammatory molecules in the serum of these subjects. Seven molecules were identified as worthy of further assessment by performing a pilot analysis of 31 inflammatory markers in 21 medication-free subjects with autism versus 15 healthy controls: epidermal growth factor (EGF), interferon-γ (IFN-γ), interleukin (IL)-13, IL-17, monocyte chemoattractant protein-1 (MCP-1), IL-1 and IL-1-receptor antagonist. Serum concentrations of these markers were then established in a different set of subjects that participated in a double-blind, clinical trial and an expanded group of healthy subjects. In the first analysis, samples obtained from subjects with autism at baseline visits were compared to visits after 8-week treatment with placebo (n=37) or risperidone (n=40). The cytokine concentrations remained stable over the 8-week period for both risperidone and placebo groups. In the second analysis, we explored further the differences between medication-free subjects with autism (n=77) and healthy controls (recruited independently; n=19). Serum levels of EGF were elevated in subjects with autism (median=103 pg/mL, n=75) in comparison to healthy controls (75 pg/mL, n=19; p<0.05), and levels of IL-13 were decreased in autism (median=0.8 pg/mL, n=77) in comparison to controls (9.8 pg/mL, n=19; p=0.0003). These changes did not correlate with standardized measures used for a diagnosis of autism. In summary, risperidone-induced clinical improvement in subjects with autism was not associated with changes in the serum inflammatory markers measured. Whether altered levels of EGF and IL-13 play a role in the pathogenesis or phenotype of autism requires further investigation.
Introduction
Autism is a childhood-onset neurodevelopmental disorder characterized by impairments in reciprocal social interactions and functional communication, as well as repetitive behaviors and restricted interests (Volkmar et al. 2005). Although the heritability of autism has been estimated to be as high as 90% (Levitt and Campbell 2009), interactions of environmental factors with genetic susceptibility factors are suspected to contribute to the etiology of autism (Geschwind 2008). At the interface between the genetically determined susceptibility and the external stimuli lies the immune system which has been implicated in autism (Goines and Van De Water 2010; Libbey et al. 2005; Pardo et al. 2005).
Elevated levels of inflammatory mediators in post-mortem brains of subjects with autism, as well as in cerebrospinal fluid suggest that enhanced activity of innate immunity and Thelper-1 (Th1) lymphocytes may exist (Chez et al. 2007; Li et al. 2009; Vargas et al. 2005; Zimmerman et al. 2005). It has not been established yet whether the inflammation represents an autoimmune process directed against healthy neuronal cells or results from clearance of degenerating neurons or other factors. In an attempt to understand the overall activity of the immune system in subjects with autism, immune parameters in peripheral blood of subjects with autism spectrum disorders have been analyzed. These studies also indicated increased activation of innate immunity and Th1 lymphocytes (Ashwood et al. 2011a; Ashwood et al. 2011b; Croonenberghs et al. 2002; Jyonouchi et al. 2005; Jyonouchi et al. 2002; Jyonouchi et al. 2001; Singh 1996; Singh et al. 1991; Sweeten et al. 2004). In addition, elevated levels of immunoglobulins (Singer et al. 2008; Singer et al. 2006; Zimmerman et al. 2007) and enhanced activity of natural killer cells (Enstrom et al. 2009) in peripheral blood have been reported, as well as decreased numbers of regulatory T cells (Ashwood et al. 2011b; Mostafa et al. 2009) and reduced serum levels of TGF-beta (a cytokine typically released by regulatory T cells) (Ashwood et al. 2008). All together, the findings imply an imbalance between effector and suppressor immune mechanisms, which could be due to an autoimmune process, but also due to chronic stress resulting from the ongoing psychiatric disorder (Corbett et al. 2009; Miyara and Sakaguchi 2007; Sapolsky et al. 2000).
During immune responses, multiple growth factors are released. In autism, several growth factors were implicated, including epidermal growth factor (EGF; Toyoda et al. 2007; Iseri et al. 2011; Suzuki et al. 2007); granulocyte-macrophage colony-stimulating factor (GM-CSF; Ashwood et al. 2011b; Li et al. 2009); hepatocyte growth factor (Sugihara et al. 2007), and vascular endothelial growth factor (Emanuele et al. 2010). Their role remains obscure, but their investigation may be clinically relevant since one of the most consistent findings in children with autism is overgrowth of their brain (Courchesne et al. 2011).
Thus far, the status of the immune system and serum levels of growth factors in autism has been addressed in cross-sectional studies not involving any treatment intervention. Previously, we had shown that eight weeks of treatment with risperidone significantly reduced irritability in children with autism (McCracken et al. 2002; McDougle et al. 2005). In the present study, we asked whether the concentrations of inflammatory markers in subjects with autism that we found to be altered compared to healthy control subjects are stable over time and whether the changes in behavior associated with treatment in these subjects are also associated with changes in inflammatory markers in their serum. Risperidone has been reported to reduce serum levels of inflammatory markers in patients with schizophrenia (Cazzullo et al. 2002; Crespo-Facorro et al. 2008; Erbagci et al. 2001; Song et al. 2009; Zhang et al. 2005; Zhang et al. 2009). Therefore, we hypothesized that the risperidone-induced clinical improvement in subjects with autism may also be associated with reduced levels of inflammatory serum markers. To address the hypothesis, we analyzed archived, longitudinally collected serum samples of medication-free children with autism and severe behavioral problems who had participated in a National Institute of Mental Health-sponsored multisite, randomized, double-blind, placebo-controlled trial of risperidone.
Methods
Design
As a pilot analysis, we compared levels of 31 cytokines in sera of 21 subjects with autism and 15 healthy controls using R&D Luminex based kits. We then selected seven cytokines and examined their serum levels in different set of subjects with autism and an expanded set of healthy controls using Millipore Luminex based kit. In the first analysis, we compared levels of cytokines in subjects with autism at baseline and after 8 weeks of treatment with risperidone (n=40) or placebo (n=37). In the second analysis, we explored further the differences in serum levels of the inflammatory markers in subjects with autism at the baseline visit (n=77) versus 19 healthy control subjects (15 of these controls were also used in the pilot analysis).
Subjects with autism
We analyzed archived serum samples of 98 medication-free subjects with a DSM-IV diagnosis of autistic disorder (79 boys, 19 girls, mean age 8.8±2.63 years, range 5.2–16.9 years) who participated in a randomized clinical trial of risperidone versus placebo conducted by the Research Units on Pediatric Psychopharmacology Autism Network (McCracken et al. 2002) (Table 1). The original sample included 101 subjects, but archived serum samples were available for 98 subjects at baseline and for 77 subjects at endpoint (Week 8). Serum samples from 21 subjects (18 boys, 3 girls, mean age 9.5±2.6 years, range 5.2–15.2 years) were available only at screening visit. These samples were employed in the pilot comparison of cytokine levels in subjects with autism versus healthy subjects. The 77 subjects, who had archived samples for both baseline and the Week 8 visit, met all criteria for trial entry and were randomized for the treatment study. Forty subjects were treated with risperidone (32 boys, 8 girls, mean age 8.3±2.7 years, range 5.2– 16.9 years) and 37 subjects received placebo (29 boys, 8 girls, mean age 9±2.5 years, range 5.5–14.8 years) (McCracken et al. 2002). Blood samples were collected at each site from 1998 to 2001. Blood was drawn into 5 mL polypropylene tubes and then kept on ice or at 4° C. Samples were centrifuged at the sites for 5 min at 400 g and then shipped on ice to the Semel Institute, where they arrived within 24-48 hours from the collection. Blood samples were then centrifuged again (400 g, 5 min), serum was collected, aliquoted into microcentrifuge tubes and stored at −80° C. One aliquote of each sample was shipped to Yale University on dry ice in 2007. The original protocol was approved by the institutional review board at each site.
Table 1.
Demographic Characteristics of Subjects with Autism and Healthy Controls
| Subjects with autism-pilot study (n=21) | Subjects with autism (n=77) | Healthy subjects (n=19) | |
|---|---|---|---|
| Male sex [no./total no. (%)] | 17/20 (85) | 61/77 (79) | 12/19 (63) |
| Age [mean±SD (range)] | 9.6±2.6 (5.2–15.2) | 8.6±2.6 (5.1–15.2) | 13.7±2.3 (9.6–17.4) |
| Annual household income [no./total no. (%)] | |||
| <$20,000 | 3/20 (15) | 7/75 (9) | |
| $20,001–$40,000 | 5/20 (25) | 23/75 (31) | |
| $40,001–$60,000 | 5/20 (25) | 13/75 (17) | |
| >$60,001 | 7/20 (35) | 32/75 (43) | |
| Education of parent or primary caregiver [no./total no. (%)] | |||
| High school or less | 3/20 (15) | 15/77 (19) | |
| Trade school or college | 14/20 (70) | 51/77 (66) | |
| Advanced degree | 3/20 (15) | 11/77 (14) | |
| Educational placement of child [no./total no. (%)] | |||
| Regular class | 3/20 (15) | 5/77 (6) | |
| Special-education program | 13/20 (65) | 70/77 (90) | |
| Residental school | 1/20 (5) | 2/77 (3) | |
| Mental development [no./total no. (%)] | |||
| IQ HIGH (>70) | 7/9 (78) | 65/77 (84) | |
| IQ LOW (<70) | 2/9 (22) | 12/77 (16) | |
| Score on Autism Diagnostic Interview – Revised (mean±SD) | |||
| Social domain | 24.7±3.3 | 26.2±3.5 | |
| Score on Aberrant Behavior Checklist (mean±SD) | |||
| Irritatibility | 21.3±9.1* | 27.4±6.6 | |
| Social withdrawal | 14.4±8.1* | 18.1±8.1 | |
| Stereotype | 7.1±5.2* | 10.4±4.9 | |
| Hyperactivity | 27.1±9.9* | 32.8±8.7 | |
| Inappropriate speech | 5.4±4.0 | 5.8±4.0 | |
| Score on Vineland Adaptive Behavior Scales (mean±SD) | |||
| Communication | 40.4±15.5 | 44.5±16.0 | |
| Socialization | 44.4±12.6 | 49.5±13.7 | |
| Daily living | 34.0±16.7 | 39.5±19.4 | |
Demographic information was not available for one subject of the pilot group.
Healthy subjects
Nineteen healthy control subjects (12 boys and 7 girls, mean age 13.7±2.3 years, range 9.6–17.4 years) were recruited by sending invitation letters to families in the New Haven, Connecticut area that had children between 7–17 years of age. The list of these families was purchased from a telemarketing company “USA Data”. At the time of blood draw, parents and children were interviewed. Children with any signs of inflammatory conditions, elevated temperature within 7 days of the blood draw, malignancy, or history of developmental disability or psychiatric illness were excluded. Informed consents were obtained, and the blood collection was performed according to protocol approved by the Human Investigation Committee of Yale University. Blood samples from these subjects were collected between the years 2002 and 2006. Blood was drawn into tubes with red-grey tops containing clotting agent (BD Biosciences, catalogue number 367988), allowed to clot for at least two hours and then centrifuged (5 min, 400 g). Collected serum was aliquoted into microcentrifuge tubes and stored in −80° C freezer.
Clinical assessments
The assessment of subjects in the trial has been described in detail elsewhere (Network R.A., 2002; McDougle et al., 2005). Measures of autism employed in the current study included the Aberrant Behavior Checklist (ABC) (Brown et al. 2002), the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), the Vineland Adaptive Behavior Scales (VABS) (Sparrow and Cicchetti 1984), and standardized tests of intelligence. Further clinical characterization involved a medical history of previous and current illness, which was recorded at baseline. Children with serious acute or chronic medical problems were excluded from the trial. After initiation of treatment with risperidone (mean dose 1.8±0.7 mg per day) or placebo (Network R.A., 2002), adverse effects were recorded throughout the 8-week trial.
Analysis of serum samples
Using the Luminex-based multiplex kits A and B (Kit A: Cat. number LUH000; Kit B: Cat. number LUB000; R&D Systems, Inc., Minneapolis, MN), we examined serum levels of 31 inflammatory molecules in a pilot analysis of sera samples of 21 subjects with autism and 15 healthy controls (listed in Table 2). For further evaluation, we selected seven cytokines based on three criteria. First, cytokines characteristic for CD4 T-helper subtypes that play critical role in the regulation of the immune system [interferon (IFN)-γ, interleukin (IL)-17, IL-13) but were not detectable by Luminex kits of R&D Systems. Second, epidermal growth factor (EGF) was selected since results of the pilot analysis suggested its increase in subjects with autism. Third, MCP-1 is a marker of chronic inflammation that was previously found significantly elevated in cerebrospinal fluid of subjects with autism (Vargas et al. 2005), and balance of IL-1 and IL-1RA is also often disturbed in ongoing inflammatory processe, and increased levels of IL-RA were reported in children with autism (Croonenberghs et al. 2002). In the subsequent more focused analysis, we employed the Lincoheptaplex kit (Cat. number HCYTO-60K, Millipore Corp., St. Charles, MO) and measured the levels of cytokines in 77 subjects at baseline and after 8 weeks of treatment with placebo and risperidone, as well as 19 healthy control subjects (Table 3A).
Table 2.
Pilot Analysis of Cytokine Levels in Serum Samples of Subjects with Autism (n=21) Versus Healthy Age-Matched Control Subjects (n=15).
| |
Subjects |
Control subjects |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mediator | Mean (pg/mL) | Median (pg/mL) | SD (pg/mL) | N | Mean (pg/mL) | Median (pg/mL) | SD (pg/mL) | n | p* | Kit detection limit (pg/mL) |
| IL-5 | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 0.33 |
| IL-6 | 0.7 | 0.7 | 0.3 | 21 | 0.7 | 0.7 | 0.2 | 15 | 0.9 | 0.39 |
| IL-10 | 1.0 | 0.9 | 0.2 | 21 | 0.9 | 0.9 | 0.1 | 15 | 0.5 | 0.13 |
| IL-13 | 245 | 303 | 191 | 21 | 186 | 258 | 150 | 15 | 0.4 | 6.0 |
| IL-17 | ND | ND | ND | 21 | 4.8 | 0 | 12.1 | 15 | NA | 0.39 |
| IFN-γ | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 0.31 |
| IL-2 | ND | ND | ND | 21 | 5.0 | 0 | 9.0 | 15 | NA | 0.89 |
| IL-4 | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 1.8 |
| CD40Ligand | 1335 | 0 | 2848 | 21 | 1067 | 0 | 1711 | 15 | 1 | 7.6 |
| TNF-α | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 0.47 |
| IL-12p70 | 36 | 27 | 16 | 21 | 40 | 27 | 20 | 15 | 0.6 | 5.1 |
| IL-1α | 80 | 82 | 28 | 21 | 103 | 100 | 25 | 15 | 0.03 | 1.2 |
| IL-1RA | 253 | 168 | 250 | 21 | 165 | 157 | 74 | 15 | 0.6 | 2.1 |
| IL-1β | ND | ND | ND | 21 | ND | ND | ND | 15 | 0.9 | 0.27 |
| Tpo | 88 | 87 | 34 | 21 | 81 | 79 | 25 | 15 | 0.7 | 2.8 |
| MCP-1/CCL2 | 43 | 43 | 19 | 21 | 38 | 37 | 14 | 15 | 0.4 | 0.95 |
| MIP-1α /CCL3 | 105 | 62 | 201 | 21 | 62 | 67 | 28 | 15 | 0.7 | 3.1 |
| MIP-1β/CCL4 | 23 | 19 | 9 | 21 | 22.0 | 21.0 | 7.0 | 15 | 0.9 | 2.1 |
| RANTES/CCL5 | 2351 | 1749 | 1851 | 21 | 2759 | 2002 | 1767 | 15 | 0.4 | 1.1 |
| Eotaxin/CCL11 | 49 | 0 | 147 | 21 | 33 | 0 | 65 | 15 | 0.4 | 2.6 |
| ENA-78/CXCL5 | 389 | 284 | 354 | 21 | 370 | 319 | 231 | 15 | 0.7 | 2.7 |
| IP-10/CXCL10 | 7.6 | 2.7 | 9.9 | 21 | 7.7 | 2.8 | 7.3 | 15 | 0.7 | 0.4 |
| I-TAC/CXCL11 | 18 | 0 | 74 | 21 | 11 | 0 | 25 | 15 | 0.4 | 2.1 |
| IL-8 | 2.7 | 1.8 | 3.0 | 21 | 2.6 | 2.9 | 1.9 | 15 | 0.6 | 0.39 |
| EGF | 141 | 88 | 215 | 21 | 24 | 16 | 20 | 15 | 0.001 | 0.57 |
| G-CSF | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 0.57 |
| GM-CSF | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 1.1 |
| HGF | 430 | 311 | 314 | 21 | 288 | 220 | 185 | 15 | 0.09 | 2.1 |
| FGF basic | ND | ND | ND | 21 | ND | ND | ND | 15 | NA | 1.8 |
| VEGF | 20 | 18 | 13 | 21 | 30 | 29 | 21 | 15 | 0.2 | 0.8 |
| Leptin | 2655 | 0 | 7439 | 21 | 4373 | 0 | 9658 | 15 | 0.6 | 7.7 |
Mann-Whitney U-test, alpha=0.0016.
ND=not detectable; NA=not applicable; SD=standard deviation; EGF=epidermal growth factor; HGF=hepatocyte growth factor; FGF=fibroblast growth factor; G-CSF=granulocyte colony-stimulating factor; GM-CSF=granulocyte-macrophage colony-stimulating factor; VEGF=vascular endothelial growth factor; MCP-1=monocyte chemoattractant protein-1; MIP=macrophage inflammatory protein; RANTES=regulated upon activation normal T-cell expressed and secreted protein; ENA-78=epithelial-cell-derived neutrophil attractant-78 protein; IP-10=interferon-inducible protein 10; I-TAC=interferon-inducible T-cell alpha chemo-attractant; IFN=interferon; TNF=tumor necrosis factor; IL-1RA=IL-1 receptor antagonist; Tpo=thrombopoietin).
Table 3.
Serum Levels of Inflammatory Mediators of Medication-Free Subjects with Autism
| A. Versus Healthy Age-Matched Control Subjects | ||||||||
|---|---|---|---|---|---|---|---|---|
| |
Subjects |
Control subjects |
|
|
||||
| Mediator | Mean (pg/mL) | SD (pg/mL) | N | Mean (pg/mL) | SD (pg/mL) | n | p* | Kit detection limit (pg/mL) |
| EGF | 162 | 148 | 75 | 104 | 131 | 19 | 0.048 | 4.8 |
| IL-13 | 17 | 81 | 77 | 58 | 107 | 19 | 0.0003 | 3.3 |
| IL-1α | 777 | 1129 | 77 | 437 | 480 | 19 | 0.2 | 1.3 |
| IL-1RA | 280 | 757 | 77 | 725 | 2261 | 19 | 0.7 | 7.7 |
| IL-17 | 9.6 | 36 | 77 | 32 | 103 | 19 | 0.2 | 1.0 |
| IFN-γ | 17 | 104 | 77 | 17 | 70 | 19 | 0.1 | 1.3 |
| MCP-1 | 286 | 140 | 77 | 240 | 104 | 19 | 0.1 | 1.3 |
| B. At Baseline Visit Versus After Eight-Week Treatment with Placebo | |||||||
|---|---|---|---|---|---|---|---|
| |
Week 1 |
Week 8 |
|
|
|
||
| Mediator | Mean (pg/mL) | SD (pg/mL) | Mean (pg/mL) | SD (pg/mL) | n | p* | r, p+ |
| EGF | 156 | 147 | 142 | 149 | 33 | 0.94 | 0.54, 0.001 |
| IL-13 | 5 | 11 | 3 | 5 | 35 | 0.35 | 0.77, 0.0001 |
| IL-1α | 805 | 1121 | 781 | 1114 | 34 | 0.26 | 0.98, 0.0001 |
| IL-1RA | 220 | 349 | 105 | 99 | 34 | 0.08 | 0.60, 0.0001 |
| IL-17 | 12 | 38 | 5 | 10 | 36 | 0.28 | 0.67, 0.0001 |
| IFN-γ | 4 | 5 | 3 | 5 | 35 | 0.34 | 0.67, 0.0001 |
| MCP-1 | 287 | 140 | 277 | 158 | 35 | 0.87 | 0.62, 0.0001 |
| C. At Baseline Visit Versus After Eight-Week Treatment with Risperidone | |||||||
|---|---|---|---|---|---|---|---|
| |
Week 1 |
Week 8 |
|
|
|
||
| Mediator | Mean (pg/mL) | SD (pg/mL) | Mean (pg/mL) | SD (pg/mL) | n | p* | r, p+ |
| EGF | 168 | 150 | 171 | 173 | 38 | 0.94 | 0.54, 0.0006 |
| IL-13 | 29 | 110 | 26 | 99 | 38 | 0.27 | 0.80, 0.0001 |
| IL-1α | 751 | 1149 | 786 | 1146 | 38 | 0.33 | 0.95, 0.0001 |
| IL-1RA | 344 | 1010 | 301 | 712 | 37 | 0.47 | 0.54, 0.0005 |
| IL-17 | 13 | 48 | 13 | 34 | 38 | 0.86 | 0.60. 0.0001 |
| IFN-γ | 29 | 144 | 22 | 96 | 38 | 0.34 | 0.76, 0.0001 |
| MCP-1 | 276 | 128 | 277 | 144 | 38 | 0.94 | 0.65, 0.0001 |
The difference in numbers of subjects for individual cytokines was determined by the availability of the reliable duplicate data for both time points; the investigator who performed the selection of the data was blinded to the identity of the samples.
SD=standard deviation; EGF=epidermal growth factor; MCP-1=monocyte chemoattractant protein-1; IFN=interferon; IL=interleukin; RA=receptor antagonist.
Sensitivity for the individual cytokines is listed in Table 2 for the Luminex kits, and in Table 3A for the Lincoheptaplex kit. Measurements were performed in 50 μL undiluted serum in kit A and diluted serum (1:4) in kit B (R&D Systems, Inc., Minneapolis, MN) or 25 μL of undiluted serum (Linco- Millipore Corp., St. Charles, MO) in duplicates according to the manufacturer's instructions. The samples were then analyzed using Biorad system (Luminex 100 system; Luminex Corporation, Austin, TX, USA), with Bio-Plex Manager 4.0.0.325 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Detection limits of this instrument for cytokines ranged from 0.2 pg/mL to 32 ng/mL.
Samples were measured in duplicate. Intra-assay and inter-assay variability was monitored by using the same quality controls on each plate. The inter-assay variability was 14.6% and the intra-assay variability less than 5.5%. If the duplicates significantly differed (mostly due to the low number of antibody-coated beads in the well), the samples were remeasured. If it was not possible to repeat the measurement due to a limited amount of serum, we excluded that sample value from the analysis, resulting in slight differences in sample size for individual cytokines. Thus, the number of subjects may be slightly reduced for some comparisons.
Statistical analysis
Cross-sectional analyses comparing healthy control subjects to subjects with autistic disorder were performed using the nonparametric Mann-Whitney U test (medians are presented). In the pilot analysis, an alpha value of 0.05 was divided by 31 to correct for multiple comparisons, which yielded a critical alpha (p) value for statistical significance of 0.0016. A group of seven markers was selected for subsequent analysis comparison of medication-free subjects with autism versus healthy controls, and alpha <0.05 was considered statistically significant. Correlations among cytokines were assessed by the Spearman's rank correlation coefficient and after correction for 22 comparions, alpha <0.0024 was considered significant. Analyses exploring the relationship between cytokine levels and clinical characteristics were carried out using a median split of cytokine levels and t-tests on the resulting high and low groups; correction for multiple comparisons yielded alpha <0.001 indicating significance. To evaluate the effects of risperidone and the stability of selected inflammatory factors from baseline to Week 8 in the RUPP trial subjects, we computed Spearman's rank correlation coefficients and performed paired t-tests. Alpha values were corrected for 14 comparions and an alpha <0.0035 was considered significant. The effects of three groups' anti-inflammatory drugs on serum levels of seven cytokines were evaluated by comparison of subjects taking the medication with those who did not. Correction for 21 comparisons yielded alpha <0.0024.
Results
Pilot analysis of cytokine levels in subjects with autism and healthy control subjects
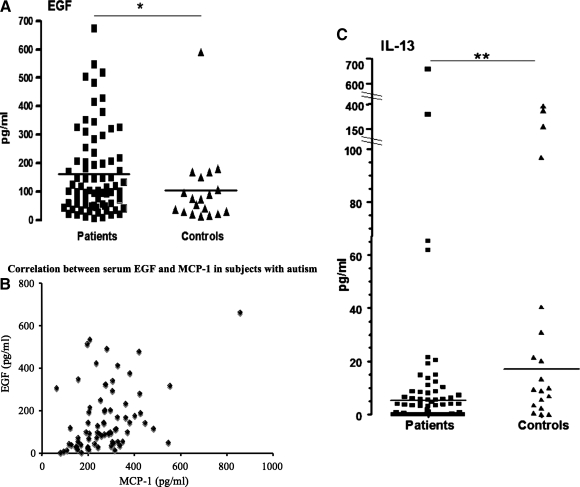
After correcting for multiple comparisons, we observed significantly higher serum levels of EGF in subjects with autism (n=21) compared to healthy controls (n=15; medians 87.7 pg/mL versus 16 pg/mL, subjects versus controls respectively, p=0.001). Serum levels of T-cell derived cytokines were below the detection threshold [IL-2, IFN-γ, IL-4, IL-5, tumor necrosis factor (TNF)-α, IL-1β] of R&D Systems Luminex kit or were detectable only in some subjects (IL-13). Several other cytokines not related to T cells were also undetectable (G-CSF, GM-CSF, and FGF basic; summarized in Table 2). Using another luminex kit, we compared an expanded group of healthy control subjects (n=19) to the larger group of other children with autism (n=77). Significantly elevated levels of EGF were confirmed in subjects with autism (median 103 pg/mL, n=75) in comparison to healthy controls (median 75 pg/mL, n=19; p<0.05) (Fig. 1A). According to the information from Millipore, the antibodies detecting EGF were specific and did not cross-react with EGF-like factors (personal communication of Dr. Tobiasova with Dr. Mayer of R&D systems and Dr. Hendrich of Millipore). In addition, significantly lower levels of IL-13 were found in affected children versus controls (medians 0.8 pg/mL vs. 9.8 pg/mL, n=77; Mann-Whitney test p=0.0003) (see Fig. 1C). To identify relationships among the cytokines, we performed Spearman correlation tests. After adjustment for multiple comparions, alpha <0.0024 was considered significant. We found a significant correlation between EGF and monocyte chemoattractant protein (MCP)-1 (r=0.40, p=0.0004) (Fig. 1B). None of the cytokines correlated with the age of the subjects. To determine a possible gender influence, we compared cytokine levels in female and male subjects and found a significant difference only in MCP-1 levels (304±136 pg/mL in boys, n=61, versus 192±77 pg/mL in girls, n=16, p=0.001).
FIG. 1. (A).
Serum levels of EGF in medication-free RUPP study subjects with autism (n=75) were significantly higher than in healthy control subjects (n=19) (* p<0.05), and (B) significantly correlated with MCP-1 serum levels in the subjects with autism (r=0.40, p=0.0004). (C) The levels of IL-13 were significantly lower in the subjects with autism than in healthy controls (p=0.0003 for IL-13). EGF, endothelial growth factor; RUPP, Research Units on Pediatric Psychopharmacology Autism Network; MCP, monocyte chemoattractant protein.
Examination of temporal stability of cytokine levels in serum of subjects with autism
To assess whether the cytokine levels are stable over time in subjects with autism, we compared the levels of cytokines in the group receiving placebo at baseline and at 8 weeks later. We employed paired t-tests and Spearman correlation tests. The paired t-tests revealed no significant differences between week 1 and week 8, and Spearman correlation tests confirmed the strong correlations between cytokines at the two time points (see Table 3B). Gender had no effect on the temporal stability of serum cytokine levels.
Examination of risperidone effects on serum cytokine levels in subjects with autism
To determine whether treatment with risperidone was associated with effects on serum cytokine levels, we compared the levels of serum cytokines in medication-free subjects at baseline with their levels after 8 weeks of treatment with risperidone. Paired t-tests revealed no differences between cytokine levels before and after risperidone treatment, and Spearman correlations between cytokines at week 1 and week 8 were significant for all the cytokines (see Table 3C), similarly to the placebo group. This indicates that risperidone had no effect on serum cytokine levels in subjects with autism. There were no differences in risperidone effects between female and male subjects.
Association between immune markers and clinical characteristics
To evaluate whether the subjects with higher or lower cytokine levels differed in their clinical characteristics, we compared subjects above and below the median for EGF and IL-13. As shown in Tables 4A and 4B, there were no significant differences in clinical characteristics between groups stratified by EGF and IL-13 levels.
Table 4.
Characteristics of Autism in Subgroups at Baseline in Subjects with Autism
| A. Defined by Median Split of Serum Epithelial Growth Factor (EGF) Levels | |||||
|---|---|---|---|---|---|
| Clinical tests for autism | EGF median split | Mean | SD | n | p value* |
| ADI-R | Below | 25.4 | 3.5 | 37 | 0.08 |
| Above | 26.8 | 3.6 | 38 | ||
| ABC | |||||
| Irritablility | Below | 27.1 | 6.3 | 37 | 0.59 |
| Above | 28.0 | 6.9 | 38 | ||
| Social withdrawal | Below | 17.4 | 7.5 | 37 | 0.36 |
| Above | 19.1 | 8.6 | 38 | ||
| Stereotypy | Below | 10.9 | 4.9 | 37 | 0.48 |
| Above | 10.1 | 4.9 | 37 | ||
| Hyperactivity | Below | 31.8 | 9.3 | 37 | 0.32 |
| Above | 33.8 | 8.1 | 38 | ||
| Inappropriate speech | Below | 6.3 | 4.1 | 37 | 0.23 |
| Above | 5.2 | 4.0 | 37 | ||
| VABS | |||||
| Communication domain | Below | 46.1 | 15.4 | 37 | 0.45 |
| Above | 43.2 | 17.1 | 38 | ||
| Social domain | Below | 50.6 | 14.2 | 37 | 0.52 |
| Above | 48.5 | 13.7 | 38 | ||
| Daily living | Below | 40.0 | 20.5 | 37 | 0.91 |
| Above | 39.4 | 18.8 | 38 | ||
| B. Defined by Medium Split of Serum Interleukin-13 Levels | |||||
|---|---|---|---|---|---|
| Clinical tests for autism | IL-13 median split | Mean | SD | n | p value* |
| ADI-R | Below | 25.7 | 3.7 | 38 | 0.31 |
| Above | 26.6 | 3.3 | 39 | ||
| ABC | |||||
| Irritablility | Below | 26.6 | 7.0 | 38 | 0.3 |
| Above | 28.2 | 6.1 | 39 | ||
| Social withdrawal | Below | 19.1 | 7.9 | 38 | 0.25 |
| Above | 16.9 | 8.2 | 36 | ||
| Stereotypy | Below | 10.4 | 5.3 | 38 | 0.91 |
| Above | 10.5 | 4.5 | 38 | ||
| Hyperactivity | Below | 32.7 | 8.4 | 38 | 0.9 |
| Above | 32.9 | 9.1 | 39 | ||
| Inappropriate speech | Below | 5.1 | 4.0 | 38 | 0.17 |
| Above | 6.4 | 4.0 | 38 | ||
| VABS | |||||
| Communication domain | Below | 44.8 | 17.6 | 38 | 0.88 |
| Above | 44.2 | 14.5 | 39 | ||
| Social domain | Below | 49.4 | 14.4 | 38 | 0.93 |
| Above | 49.6 | 13.2 | 39 | ||
| Daily living | Below | 39.2 | 20.0 | 38 | 0.92 |
| Above | 39.7 | 19.1 | 39 | ||
Student's t-test, alpha=0.05.
SD=standard deviation; ADI-R=Autism Diagnostic Interview-Revised; ABC=Aberrant Behavior Checklist; VABS=Vineland Adaptive Behavior Scales.
Effects of anti-inflammatory medications on immune and growth factors in serum
The healthy subjects did not use any medication at the time of blood draw. The children with autism used several medications that could affect cytokines levels: antihistamines (n=11), acetaminophen (n=9), antibiotics (n=7) and local steroids (n=1). Comparison of cytokines in the subjects who did use versus those who did not use a drug of one of the three medication groups revealed no significant differences.
Discussion
Our data indicate that the levels of inflammatory serum markers of subjects with autism remain stable over a period of eight weeks, and that risperidone-induced clinical improvement in aberrant and maladaptive behavior in these subjects (McCracken et al. 2002; McDougle et al. 2005) is not associated with any changes in serum levels of seven inflammatory markers (EGF, IFN-γ, IL-13, IL-17, MCP-1, IL-1 and IL-1-RA). Thus, these data do not support our hypothesis that risperidone-induced improvement in irritability of children with autism is associated with altered activity of immune cells in peripheral blood.
Risperidone is an atypical antipsychotic drug that acts as an antagonist of dopamine D2 receptors and several subtypes of receptors for serotonin (5-HT) (Moller 2005). These receptors are present not only in the brain, but also on immune cells in peripheral blood (Levite 2008). In patients with schizophrenia, risperidone was reported to reduce levels of inflammatory markers in their serum (Cazzullo et al. 2002; Crespo-Facorro et al. 2008; Erbagci et al. 2001; Song et al. 2009; Zhang et al. 2005; Zhang et al. 2009). In our subjects with autism, however, we did not observe such effects. Our study is the first to investigate risperidone-induced effects on inflammatory markers in a placebo-controlled trial and in medication-free subjects, avoiding the influence of confounding effects of environmental factors over time or other medications. The lack of risperidone effects on immune parameters suggests several possibilities. First, the immune system may not play any role in the clinical improvement induced by risperidone. Second, risperidone may not be affecting the levels of immune factors, but these factors could mediate or facilitate effects of neurotransmitters, such as dopamine or serotonin, that are then antagonized by risperidone. Third, risperidone may inhibit receptors for neurotransmitters on immune cells, but such effects may act only locally in the brain, and not be sufficiently pronounced in the systemic circulation.
The exploratory comparison of inflammatory markers in medication-free subjects with autism at the baseline visit to healthy controls revealed significantly increased serum levels of EGF (a growth factor released at multiple sites) and decreased levels of IL-13 (a cytokine derived predominantly from the T helper 2 lymphocytes) in subjects with autism. To assess whether these levels correlated with any of the clinical characteristics of autism, we stratified the subjects with autism above and below the median levels of EGF and IL-13 at the baseline visit, and asked whether patients with higher versus lower values of cytokines differ with regard to a number of clinical measures. No relationships between EGF or IL-13 levels with any of the clinical parameters were found, implying that these two markers are not related to the severity or associated behavioral characteristics of autism. In contrast, a recent study by Ashwood et al. (2010) suggested that levels of IL-12p40, IL-6 and IL-1beta may be related to some domains of diagnostic scales (Ashwood et al. 2011). Our risperidone trial selectively recruited subjects with severe behavioral problems, which might have hindered the detection of differences between groups dichotomized by cytokine levels. Future studies could examine the relationship between cytokine levels and clinical characteristics in a larger, more heterogeneous sample of subjects with autism spectrum disorders. Existing data, particularly in relation to EGF, warrant further investigation.
The association of EGF with autism was first suggested by an association of EGF-related haplotypes with autism (Toyoda et al. 2007) and by decreased levels of EGF in the serum of 17 adult males with high-functioning autism (Suzuki et al. 2007). In contrast, we found elevated levels in children with autism which did not correlate with age or clinical characteristics. Our findings are consistent with the recent report of Iseri at al. (2011) who also found increased EGF in serum of younger children with autism (5.1±2.05 years old), where the levels did not correlate with scores on the Children Autism Rating Scale, age, birth weight, or length of breast feeding (Iseri et al. 2011). It will be important to establish whether EGF and other growth factors cause morphological changes in brains of young and adult subjects with autism. These may include the size of the brains, as well as the high number of abnormally enlarged neurons in the vertical limb of the diagonal band of Broca, and the cerebellar nuclei and inferior olive in young subjects in comparison to reduced numbers of small and pale neurons in adult subjects (Bauman and Kemper 2005).
The source of elevated serum EGF in the serum of the subjects remains unknown. It does not appear to be from the central nervous system (Vargas et al. 2005). The correlation with MCP-1 that we noted in the present study, suggests an inflammatory site of currently unknown location. EGF was first discovered to be produced by salivary and serous glands of the gastrointestinal tract (Kasselberg et al. 1985; Van Noorden et al. 1977). While the relationship between gastrointestinal symptoms and autism has not been fully established (Buie et al. 2010; Erickson et al. 2005), and remains controversial, MET gene variants were found to be significantly associated in families with at least one child that has comorbidity for autism and gastrointestinal symptoms (Campbell et al. 2009). Hepatocyte growth factor acts via cMET, and activation of EGF receptors and cMET induces independent but interacting pathways (Puri and Salgia 2008). In the clinical setting, mutations in EGF receptors are associated with amplification of MET in cancer patients (Toschi and Cappuzzo 2010). In subjects with autism, altered permeability of the intestinal barrier has been reported (D'eufemia et al. 1996; De Magistris et al. 2010). EGF is essential for epithelial impermeability of the gastrointestinal tract, and elevated levels of EGF in serum and saliva occur in patients with inflammatory bowel disease (Jahanshahi et al. 2004). EGF appears to be released as a protective compensatory mechanism, since animals deficient in EGF receptors develop necrotizing colitis (Miettinen 1997) and exogenous administration of EGF suppresses inflammatory bowel disease both in animals and humans (Clark et al. 2006; Nair et al. 2008).
Regardless of the source of EGF, once it is in the systemic circulation, EGF can cross the blood-brain barrier rapidly (Pan and Kastin 1999) and then activate EGF receptors in neuronal cells. EGF is critical for the growth and survival of Purkinje cells in the cerebellum (Yamada et al. 1997), which were previously implicated in autism (Bauman and Kemper 2005; Vargas et al. 2005). In the cerebellum, EGF receptors colocalize with GAD67 (glutamate decarboxylase, 67kDa), an enzyme recently shown to be decreased in interneurons and increased in Purkinje cells in subjects with autism (Yip et al. 2007). These observations together warrant further investigation of the role of EGF in autism.
The decreased serum levels of IL-13 in our subjects may reflect a decreased function of Th2 lymphocytes (Kasaian and Miller 2008) which could be related to enhanced activity of Th1 cells in autism, as has been suggested by several reports (Ashwood et al. 2010; Croonenberghs et al. 2002; Jyonouchi et al. 2005; Jyonouchi et al. 2002; Jyonouchi et al. 2001; Singh 1996) These data support the notion that establishing consequences of enhanced Th1 immunity may be useful for elucidation of the pathogenesis of autism.
The results of this study need to be considered in the light of two sources of limitations. First, blood samples of subjects with autism versus healthy controls were collected at different sites and shipped over night to the Semel center where they were processed and then stored for a long period of time. It is possible that the transport and the long storage might have led to degradation of some of the markers, although our observation of elevated and stable EGF concentrations in our subjects with autism cannot be explained by differential degradation. Future studies involving freshly collected and promptly processed sera samples may reveal differences in additional inflammatory markers. The second source of limitation is related to the group of our healthy control subjects. The group of control samples (n=19) was substantially smaller than the patient sample (n=77), possibly affecting the outcome of the statistical evaluation. Also, the control samples were obtained at a later time point, independent of the risperidone clinical trial. Clearly, future studies need to recruit subjects with autism and healthy subjects in parallel. Further, next studies should be extended to a period of a longer time to determine if alterations in inflammatory mediators persist and whether these are related to any physical symptoms in children with autism, which could help identify the source of the inflammatory markers. In addition, the future collection of samples should also involve evaluation of cortisol levels which would help to determine whether the observed effects are related to chronic stress.
Conclusions
The novel contribution of this study is in demonstrating that risperidone-induced improvement in subjects with autism is not associated with changes in serum concentrations of selected inflammatory markers, that the levels of selected inflammatory markers remain unchanged over a period of eight weeks, that levels of EGF and IL-13 in subjects with autism appear elevated and decreased, respectively, in comparison to healthy controls and that EGF levels correlate with a marker of chronic inflammation, MCP-1. These findings provide a rationale for addressing the role of EGF and IL-13 in autism experimentally, for example by employing EGF receptor knockout mice and anti-EGF antibodies (Vinter-Jensen 1999; Wong 2003), and by IL-13 or IL-13 receptor transgenic and knock-out mice or antibodies against IL-13 (Wynn 2003).
Clinicial Significance
This study suggests that irritability in children with autism is not related to immune aberrations, because risperidone significantly decreased irritability without changing the immune markers that were assayed. However, the elevation of EGF, and its correlation with MCP-1, in subjects with autism indicates a need for further investigation of possible EGF involvement in pathophysiology of autism.
Disclosures
This study was supported by a grant from Autism Speaks (to Dr. Kawikova). Dr. Tobiasova was partially supported by Czech Ministry of Education (MSM 0021620812) and Klaas van der Lingen by Groningen University Fund. The analysis was performed on archived samples collected during previous studies performed by the Research Units on Pediatric Psychopharmacology (RUPP) Autism Network: Dr. McCracken at University of California Los Angeles; Dr. Aman at Ohio State University; Dr. McDougle at Indiana University; Dr. Scahill at Yale University; Dr. Tierney at Kennedy Krieger Institute. The RUPP network was funded by NIMH: N01MH70001 to IU, N01MH80011 to OSU, N01MH70010 and MH01805 to UCLA, N01MH70009 to Yale. Collection of serum samples of healthy controls was supported by The Brian and Linda Richmand Foundation at Yale University (to Dr. Leckman). Christopher J. McDougle is a consultant to Bristol-Myers Squibb Co., F. Hoffmann-LaRoche, Ltd., and Forest Research Institute; has received research grants from Bristol-Myers Squibb Co., and is a member of the speaker's bureau for Bristol-Myers Squibb Co. Pieter Hoekstra has been paid consultant for Shire, Eli Lilly, and Desitin. James McCracken has had research contracts with Seaside, Bristol-Myers Squibb, has been consultant for Shionogi, BioMarin and receives honoraria from CME Outfitters, Veritas, Tourette Syndrome Association and APSARD. Drs. Zhang, Chae, Vitiello, Arnold, Volkmar, and Bothwell and Ms. Katsovich have no conflicts of interest or financial ties to disclose.
Risperidone and placebo treatment were supplied by Janssen Pharmaceuticals.
Acknowledgments
We thank Mrs. Heidi Grantz, M.S.W. for coordination of sample collection from healthy control subjects. Dr. Kawikova would also like to thank Drs. James C. Lai of Idaho State University and Vaclav Spicka of the Czech Academy of Sciences for valuable discussions during preparation of the manuscript.
References
- Ashwood P. Enstrom A. Krakowiak P. Hertz-Picciotto I. Hansen RL. Croen LA. Ozonoff S. Pessah IN. Van de Water J. Decreased transforming growth factor beta1 in autism: A potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P. Krakowiak P. Hertz-Picciotto I. Hansen R. Pessah I. Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P. Krakowiak P. Hertz-Picciotto I. Hansen R. Pessah IN. Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2011b;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML. Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Brown EC. Aman MG. Havercamp SM. Factor analysis and norms for parent ratings on the aberrant behavior checklist-community for young people in special education. Res Dev Disabil. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Buie T. Campbell DB. Fuchs GJ., 3rd Furuta GT. Levy J. Vandewater J. Whitaker AH. Atkins D. Bauman ML. Beaudet AL. Carr EG. Gershon MD. Hyman SL. Jirapinyo P. Jyonouchi H. Kooros K. Kushak R. Levitt P. Levy SE. Lewis JD. Murray KF. Natowicz MR. Sabra A. Wershil BK. Weston SC. Zeltzer L. Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with asds: A consensus report. Pediatrics 125 Suppl. 2010;1:S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Campbell DB. Buie TM. Winter H. Bauman M. Sutcliffe JS. Perrin JM. Levitt P. Distinct genetic risk based on association of met in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- Cazzullo CL. Sacchetti E. Galluzzo A. Panariello A. Adorni A. Pegoraro M. Bosis S. Colombo F. Trabattoni D. Zagliani A. Clerici M. Cytokine profiles in schizophrenic patients treated with risperidone: A 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:33–39. doi: 10.1016/s0278-5846(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Chez MG. Dowling T. Patel PB. Khanna P. Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Clark JA. Doelle SM. Halpern MD. Saunders TA. Holubec H. Dvorak K. Boitano SA. Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of egf treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- Corbett BA. Schupp CW. Levine S. Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Res. 2009;2:39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Campbell K. Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B. Carrasco-Marin E. Perez-Iglesias R. Pelayo-Teran JM. Fernandez-Prieto L. Leyva-Cobian F. Vazquez-Barquero JL. Interleukin-12 plasma levels in drug-naive patients with a first episode of psychosis: Effects of antipsychotic drugs. Psychiatry Res. 2008;158:206–216. doi: 10.1016/j.psychres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J. Bosmans E. Deboutte D. Kenis G. Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- D'Eufemia P. Celli M. Finocchiaro R. Pacifico L. Viozzi L. Zaccagnini M. Cardi E. Giardini O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85:1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- de Magistris L. Familiari V. Pascotto A. Sapone A. Frolli A. Iardino P. Carteni M. De Rosa M. Francavilla R. Riegler G. Militerni R. Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- Emanuele E. Orsi P. Barale F. di Nemi SU. Bertona M. Politi P. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin Biochem. 2010;43:317–319. doi: 10.1016/j.clinbiochem.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Enstrom AM. Lit L. Onore CE. Gregg JP. Hansen RL. Pessah IN. Hertz-Picciotto I. Van de Water JA. Sharp FR. Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbagci AB. Herken H. Koyluoglu O. Yilmaz N. Tarakcioglu M. Serum il-1beta, sil-2r, il-6, il-8 and tnf-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10:109–115. doi: 10.1080/09629350123895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA. Stigler KA. Corkins MR. Posey DJ. Fitzgerald JF. McDougle CJ. Gastrointestinal factors in autistic disorder: A critical review. J Autism Dev Disord. 2005;35:713–727. doi: 10.1007/s10803-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Autism: Many genes, common pathways? Cell. 2008;135:391–395. doi: 10.1016/j.cell.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P. Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseri E. Guney E. Ceylan MF. Yucel A. Aral A. Bodur S. Sener S. Increased serum levels of epidermal growth factor in children with autism. J Autism Dev Disord. 2011;41:237–241. doi: 10.1007/s10803-010-1046-3. [DOI] [PubMed] [Google Scholar]
- Jahanshahi G. Motavasel V. Rezaie A. Hashtroudi AA. Daryani NE. Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752–1757. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H. Geng L. Ruby A. Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: Their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51:77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H. Sun S. Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46:76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H. Sun S. Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kasaian MT. Miller DK. Il-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008;76:147–155. doi: 10.1016/j.bcp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kasselberg AG. Orth DN. Gray ME. Stahlman MT. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem. 1985;33:315–322. doi: 10.1177/33.4.3884705. [DOI] [PubMed] [Google Scholar]
- Levite M. Neurotransmitters activate t-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol. 2008;8:460–471. doi: 10.1016/j.coph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Levitt P. Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–754. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Chauhan A. Sheikh AM. Patil S. Chauhan V. Li XM. Ji L. Brown T. Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE. Sweeten TL. McMahon WM. Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Lord C. Rutter M. Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McCracken JT. McGough J. Shah B. Cronin P. Hong D. Aman MG. Arnold LE. Lindsay R. Nash P. Hollway J. McDougle CJ. Posey D. Swiezy N. Kohn A. Scahill L. Martin A. Koenig K. Volkmar F. Carroll D. Lancor A. Tierney E. Ghuman J. Gonzalez NM. Grados M. Vitiello B. Ritz L. Davies M. Robinson J. McMahon D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle CJ. Scahill L. Aman MG. McCracken JT. Tierney E. Davies M. Arnold LE. Posey DJ. Martin A. Ghuman JK. Shah B. Chuang SZ. Swiezy NB. Gonzalez NM. Hollway J. Koenig K. McGough JJ. Ritz L. Vitiello B. Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162:1142–1148. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ. Epidermal growth factor receptor in mice and men–any applications to clinical practice? Ann Med. 1997;29:531–534. doi: 10.3109/07853899709007477. [DOI] [PubMed] [Google Scholar]
- Miyara M. Sakaguchi S. Natural regulatory t cells: Mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Moller HJ. Risperidone: A review. Expert Opin Pharmacother. 2005;6:803–818. doi: 10.1517/14656566.6.5.803. [DOI] [PubMed] [Google Scholar]
- Mostafa GA. Al Shehab A. Fouad NR. Frequency of cd4+ cd25high regulatory t cells in the peripheral blood of egyptian children with autism. J Child Neurol. 2009;25:328–335. doi: 10.1177/0883073809339393. [DOI] [PubMed] [Google Scholar]
- Nair RR. Warner BB. Warner BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Pan W. Kastin AJ. Entry of egf into brain is rapid and saturable. Peptides. 1999;20:1091–1098. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Pardo CA. Vargas DL. Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Puri N. Salgia R. Synergism of egfr, c-met pathways, cross-talk, inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Romero LM. Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Singer HS. Morris CM. Gause CD. Gillin PK. Crawford S. Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Singer HS. Morris CM. Williams PN. Yoon DY. Hong JJ. Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:43–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Singh VK. Warren RP. Odell JD. Cole P. Changes of soluble interleukin-2, interleukin-2 receptor, t8 antigen, and interleukin-1 in the serum of autistic children. Clin Immunol Immunopathol. 1991;61:448–455. doi: 10.1016/s0090-1229(05)80015-7. [DOI] [PubMed] [Google Scholar]
- Song XQ. Lv LX. Li WQ. Hao YH. Zhao JP. The interaction of nuclear factor-kappa b and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65:481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sparrow SS. Cicchetti DV. The behavior inventory for rating development (bird): Assessments of reliability and factorial validity. Appl Res Ment Retard. 1984;5:219–231. doi: 10.1016/s0270-3092(84)80003-x. [DOI] [PubMed] [Google Scholar]
- Sugihara G. Hashimoto K. Iwata Y. Nakamura K. Tsujii M. Tsuchiya KJ. Sekine Y. Suzuki K. Suda S. Matsuzaki H. Kawai M. Minabe Y. Yagi A. Takei N. Sugiyama T. Mori N. Decreased serum levels of hepatocyte growth factor in male adults with high-functioning autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:412–415. doi: 10.1016/j.pnpbp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Hashimoto K. Iwata Y. Nakamura K. Tsujii M. Tsuchiya KJ. Sekine Y. Suda S. Sugihara G. Matsuzaki H. Sugiyama T. Kawai M. Minabe Y. Takei N. Mori N. Decreased serum levels of epidermal growth factor in adult subjects with high-functioning autism. Biol Psychiatry. 2007;62:267–269. doi: 10.1016/j.biopsych.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Sweeten TL. Posey DJ. Shankar S. McDougle CJ. High nitric oxide production in autistic disorder: A possible role for interferon-gamma. Biol Psychiatry. 2004;55:434–437. doi: 10.1016/j.biopsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Toschi L. Cappuzzo F. Clinical implications of met gene copy number in lung cancer. Future Oncol. 2010;6:239–247. doi: 10.2217/fon.09.164. [DOI] [PubMed] [Google Scholar]
- Toyoda T. Nakamura K. Yamada K. Thanseem I. Anitha A. Suda S. Tsujii M. Iwayama Y. Hattori E. Toyota T. Miyachi T. Iwata Y. Suzuki K. Matsuzaki H. Kawai M. Sekine Y. Tsuchiya K. Sugihara G. Ouchi Y. Sugiyama T. Takei N. Yoshikawa T. Mori N. SNP analyses of growth factor genes egf, TGFbeta-1, and HGF reveal haplotypic association of EGF with autism. Biochem Biophys Res Commun. 2007;360:715–720. doi: 10.1016/j.bbrc.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Van Noorden S. Heitz P. Kasper M. Pearse AG. Mouse epidermal growth factor: Light and electron microscopical localisation by immunocytochemical staining. Histochemistry. 1977;52:329–340. doi: 10.1007/BF00508405. [DOI] [PubMed] [Google Scholar]
- Vargas DL. Nascimbene C. Krishnan C. Zimmerman AW. Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vinter-Jensen L. Pharmacological effects of epidermal growth factor (egf) with focus on the urinary and gastrointestinal tracts. APMIS Suppl. 1999;93:1–42. [PubMed] [Google Scholar]
- Volkmar F. Chawarska K. Klin A. Autism in infancy and early childhood. Annu Rev Psychol. 2005;56:15–336. doi: 10.1146/annurev.psych.56.091103.070159. [DOI] [PubMed] [Google Scholar]
- Wong RW. Transgenic and knock-out mice for deciphering the roles of egfr ligands. Cell Mol Life Sci. 2003;60:113–118. doi: 10.1007/s000180300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Il-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- Yamada M. Ikeuchi T. Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51:19–37. doi: 10.1016/s0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Yip J. Soghomonian JJ. Blatt GJ. Decreased gad67 mrna levels in cerebellar purkinje cells in autism: Pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zhang XY. Zhou DF. Cao LY. Wu GY. Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: Association with psychopathology and response to antipsychotics. Neuropsychopharmacology. 2005;30:1532–1538. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]
- Zhang XY. Zhou DF. Qi LY. Chen S. Cao LY. Chen da C. Xiu MH. Wang F. Wu GY. Lu L. Kosten TA. Kosten TR. Superoxide dismutase and cytokines in chronic patients with schizophrenia: Association with psychopathology and response to antipsychotics. Psychopharmacology (Berl) 2009;204:177–184. doi: 10.1007/s00213-008-1447-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW. Connors SL. Matteson KJ. Lee LC. Singer HS. Castaneda JA. Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW. Jyonouchi H. Comi AM. Connors SL. Milstien S. Varsou A. Heyes MP. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]