Abstract
Significant effort has been focused on reducing neuronal damage from post-traumatic brain injury (TBI) inflammation and blood–brain barrier (BBB)-mediated edema. The orexigenic hormone ghrelin decreases inflammation in sepsis models, and has recently been shown to be neuroprotective following subarachnoid hemorrhage. We hypothesized that ghrelin modulates cerebral vascular permeability and mediates BBB breakdown following TBI. Using a weight-drop model, TBI was created in three groups of mice: sham, TBI, and TBI/ghrelin. The BBB was investigated by examining its permeability to FITC-dextran and through quantification of perivascualar aquaporin-4 (AQP-4). Finally, we immunoblotted for serum S100B as a marker of brain injury. Compared to sham, TBI caused significant histologic neuronal degeneration, increases in vascular permeability, perivascular expression of AQP-4, and serum levels of S100B. Treatment with ghrelin mitigated these effects; after TBI, ghrelin-treated mice had vascular permeability and perivascular AQP-4 and S100B levels that were similar to sham. Our data suggest that ghrelin prevents BBB disruption after TBI. This is evident by a decrease in vascular permeability that is linked to a decrease in AQP-4. This decrease in vascular permeability may diminish post-TBI brain tissue damage was evident by decreased S100B.
Key words: aquaporin-4, blood–brain barrier, ghrelin, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability, contributing to one-third of all injury-related deaths in the U.S., and results in significant lost productivity.(Faul 2010; Finkelstein et al., 2006). Despite the associated public health implications, there is a paucity of medical therapies for TBI (Narayan et al., 2002). Currently, the primary tenet of treating severe TBI is controlling intracranial pressure (ICP) and consequently minimizing secondary insults such as ischemia, hypoxia, or cerebral herniation (Unterberg et al., 2004). Unfortunately, present medical management of TBI, including sedation, avoidance of hypercapnia, intravenous hyperosmolar solutions, and decompressive craniectomy, has remained relatively unchanged for decades, and though widely practiced, irrefutable data supporting its efficacy are lacking (Saul and Ducker, 1982). Specific therapies targeting edema are limited, and large multicenter trials of various pharmacologic agents have failed to yield positive clinical results (Narayan et al., 2002; Unterberg et al., 2004).
Post-TBI brain tissue undergoes significant osmotic changes from both vasogenic and cytotoxic sources (Schilling and Wahl, 1999). Cytotoxic edema, involving intracellular accumulation of water, seems to be more prominent in astrocytes than neurons. In contrast, vasogenic edema, and extracellular water accumulation of water are likely directly related to blood–brain barrier (BBB) disruption (Nag et al., 2009; Unterberg et al., 2004), Ghrelin, a 28-amino acid peptide predominantly secreted by gastric mucosa, is a neuroendocrine hormone that acts as an endogenous ligand for growth hormone secretagogue receptor (Kojima et al., 1999). Beyond the known effects on hunger regulation, ghrelin is known to have potent anti-inflammatory properties, and has been shown to be protective in several models of neuronal injury (Ersahin et al., 2010; Liu et al., 2006; Miao et al., 2007; Sehirli et al., 2008; Xu et al., 2009). Recently, Kwan and associates have shown that ghrelin-treated animals had decreased intestinal vascular permeability following an LPS challenge. Therefore, given these experiments, we hypothesized that ghrelin will prevent neuronal injury and maintain BBB integrity following TBI.
Methods
Traumatic brain injury
Animal experiments, including anesthesia, TBI, and recovery, were approved by the University of California, San Diego Institutional Animal Care and Use Committee. Male BALB/c mice (20–24 g) were obtained (Jackson Laboratory, Sacramento, CA), and placed under a 12-h light/dark cycle.
A weight drop TBI model was used as previously described, to induce a well-defined cerebral contusion (Stahel et al., 2000; Habgood et al., 2007). Briefly, animals (n=4 per group) were anesthetized with 3% inhaled isoflurane by way of a veterinary vaporizer (Ohio Medical Products, Madison, WI). The flow of isoflurane was titrated to achieve appropriate anesthesia for each animal. Each animal was manually secured, a vertical incision was made over the cranium and using a surgical drill, a burr hole 4 mm in diameter, 1 mm lateral, and 1 mm posterior to the bregma was created to expose the dura mater. A 250-g metal rod was dropped from a height of 2 cm onto the exposed dura mater. The incision was closed with Vetbond (3M, St. Paul, MN), and buprenorphine in saline was injected subcutaneously for pain control in both the sham and TBI animals. Food and water were provided ad libitum. Sham animals underwent the identical procedure excluding the weight drop.
Ghrelin administration
Animals in the ghrelin group (n=4) received two doses (20 μg total) of IP ghrelin immediately prior to, and 1 h after, TBI. The dosing and timing of ghrelin administration was determined from previous experiments showing that IP ghrelin has a rapid onset of action, and its response is potentiated by a second IP injection within 4 h (Qiu et al., 2008; Wren et al., 2000).
Histopathological evaluation
Sections of the brain (n=4 animals per group) were cut 7-μm thick and stained with hematoxylin and eosin (Surgipath, Richmond, IL). Images were viewed with an Olympus FSX100 light microscope (Center Valley, PA), and examined with Olympus FSX-BSW software (Center Valley, PA). Histological brain injury in the neocortex and hippocampus was evaluated by a neuropathologist.
Determination of BBB vascular permeability by xenogen imaging
TBI or sham procedure was performed (n=4 per group). Five hours and 30 min later, the mice were injected with 70 kD FITC-dextran (Sigma-Aldrich, St. Louis, MO). Thirty minutes later the animals were subjected to systemic intracardiac perfusion with 1 USP U/mL of heparin in saline to flush the intravascular FITC-dextran out of the vasculature. The perfused brains were then harvested. Six consecutive 1-mm coronal sections from each brain were cut and imaged. Extravasated FITC (vascular permeability) was then measured using an IVIS Lumina (Xenogen IVIS Lumina; Caliper Life Sciences, Hopkinton, MA). A uniformly sized circular region of interest (ROI) was placed so that it bordered the midline and superior aspect of the injured hemisphere in each 1-mm coronal section. Using Living Image® 3.1 software (Caliper Life Sciences), radiated fluorescence was measured, quantified, and an average was calculated for each brain.
Immunohistochemistry of AQP4 and CD31
Several aquaporins have been identified in different regions of brain tissue. AQP-1, for example, is present on epithelial cells of the choroid plexus, while AQP-4, AQP-5, and AQP-9 are present on astrocytes and ependymal cells (zelenina). Our focus on AQP-4 relates to its polarized presence on astrocytic end-feet; this puts the AQP-4 water channel in contact with brain vessels, and suggests a role in BBB integrity (Simard et al., 2003).
Brain tissue was harvested (n=4 per group), embedded in optimal cutting temperature media, and stored at −80°C. Coronal sections of each brain were cut directly in the center of the injury. Two 10-μm sections separated by 200 μm were cut with a Reichert-Jung Cryocut 1800 (Reichert Microscopes, Depew, NY). Standard immunohistochemistry was performed using the following primary antibodies at the following dilutions: rat polyclonal anti-CD31 (553370, 1:100; BD Biosciences, San Jose, CA), rabbit polyclonal anti-AQP-4 (1:100, anti-AQ4; Millipore, Billerica, MA). Alexa-Fluor–conjugated secondary antibodies (1:200; Molecular Probes, Eugene, OR) were used to detect immunofluorescence signal, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Immunostaining of tissue sections were imaged with an Olympus FluoView 1000 (ASW 1.7b) laser scanning confocal microscope equipped with 10×/0.4 N.A. or 20×/0.7 N.A. dry objective lenses on a BX61 microscope (Olympus). Vessels in the area of neocortical injury were imaged. We restricted our analysis to this region because our permeability data suggest that this is the location of the greatest breach in the BBB. Once the images were obtained, a small circular ROI was chosen such that it would fit within any blood vessel. The ROI was then randomly placed over three blood vessels in the area of injury of each section and used to determine the integrated fluorescence of AQP-4 and CD31. The average was calculated and called arbitrary fluorescent units.
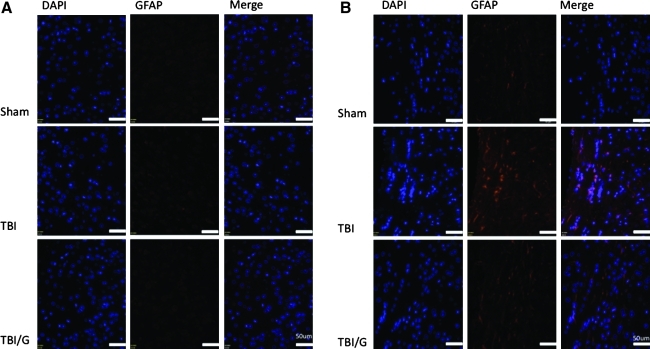
Immunohistochemistry of glial fibrillary acidic protein (GFAP)
Because AQP-4 is protein-expressed on astrocytes, we used GFAP staining to investigate concomitant patterns of astrocytic reactivity. Paraffin-embedded brain sections (7 μm) were deparaffinized (n=4 animals per group) and incubated in citrate buffer for antigen retrieval. Standard immunohistochemistry was then performed using chicken polyclonal anti-GFAP (1:100, AB5541; Millipore). Alexa-Fluor–conjugated secondary antibodies (1:200; Molecular Probes) were used to detect immunofluorescence signal, and nuclei were counterstained with DAPI. Immunostaining of tissue sections was imaged with an Olympus FSX100 (Center Valley, PA), and viewed with FSX-BSW software (Olympus).
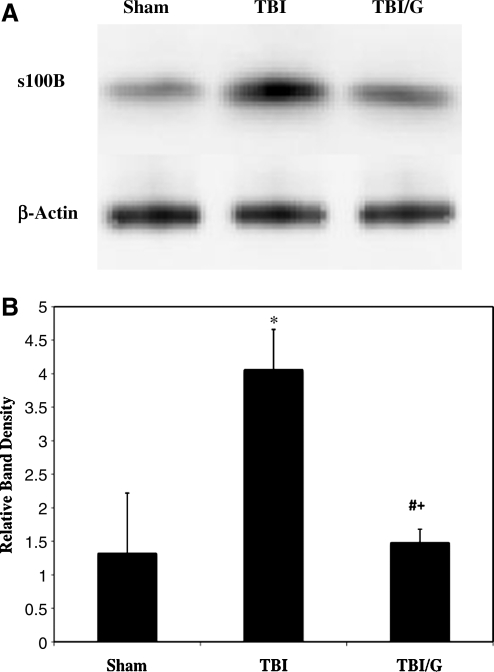
Western blotting of serum S100B
Serum was harvested (n=4 per group) 6 h following TBI and processed for immunoblotting. Membranes were blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich in TBS/Tween for 1 h at room temperature. After another wash, primary antibody specific to S100B (1:500 in 3% BSA; Dako Cytomation, Carpinteria, CA) was added and the membranes were incubated overnight at 4°C. The membranes were washed again, then incubated with anti-rabbit IgG, horseradish peroxidase (HRP)-linked antibody (1:2000 in 5% BSA; Cell Signaling, Danvers, MA) for 1 h at room temperature. Supersignal® west pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) was used for HRP detection. Images were produced with a Xenogen IVIS Lumina imaging machine using the program Living Image® 3.1 (Caliper Life Sciences). For each detected S100B band in the sham, TBI, and TBI/ghrelin experimental groups, pixel density was determined using UN-SCAN-IT Gel Digitizing software (Silk Scientific, Orem, UT). Data are expressed as relative band densities, which were calculated by dividing the corrected pixel density of each band by the mean pixel density of sham bands.
Statistical analysis
Values are expressed as mean±standard deviation. The statistical significance among groups was determined by analysis of variance (ANOVA) with Bonferroni correction where appropriate, and a p value<0.05 was considered statistically significant.
Results
Ghrelin improves post-TBI histologic changes
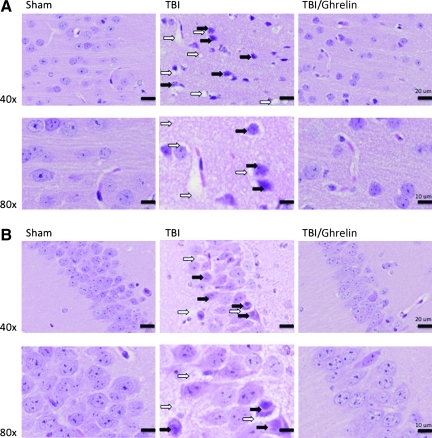
Sections of brain from the sham and the 6-h following TBI±IP ghrelin groups underwent histological examination 200 μm medial to the cortical impact site. Compared to sham animals, TBI caused significant neuronal degeneration in the neocortex, with increased vacuolization and axonopathy in the neuropil. Ghrelin-treated animals had decreased cortical degeneration and axonopathy (Fig. 1A). Histologic evaluation of hippocampal regions, CA1, and the dentate gyrus clearly depict TBI-induced darkened and degenerating neurons. Administration of ghrelin further blunted neuronal degeneration in these areas (Fig. 1B).
FIG. 1.
(A) Histological examination of tissue 200 μm medial to the cortical impact site reveals that compared to sham animals, traumatic brain injury (TBI) caused significant neuron degeneration as evidenced by neuronal contraction (solid arrows) in the neocortex with increased vacuolization (open arrows) and axonopathy in the neuropil. Ghrelin-treated animals (TBI/G) had decreased cortical degeneration and axonopathy. (B) Hippocampal regions, CA1, and dentate gyrus show TBI-induced degenerating neurons (solid arrows) and vacuolization (open arrows). Administration of ghrelin also blunted neuronal degeneration in these areas.
Ghrelin decreases TBI-induced BBB Vascular Permeability
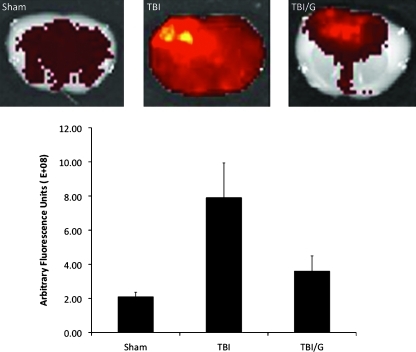
To determine BBB permeability, we examined extravasation of 70-kD FITC-dextran as a marker of vascular permeability. Vascular permeability to FITC-dextran at 6 h post-TBI is significantly reduced by IP ghrelin injection (Fig. 2C). The arbitrary fluorescence intensity (arbitrary fluorescence units [afu]×108) of the right cerebral hemisphere in the sham group was 2.09±0.27, compared with 7.90±0.52 afu in the TBI group, and 3.60±0.90 afu in the ghrelin-treated TBI group. There was a significant increase in the TBI group compared with the sham group (mean difference 5.81 afu; p<0.001). The ghrelin-treated TBI group had significantly reduced arbitrary fluorescent intensity compared to TBI alone (mean difference 4.30, p<0.01). There was no statistically significant difference between sham-treated mice and ghrelin-treated TBI mice (p=NS;. Fig. 2B)
FIG. 2.
Mice were injected with 70-kDa FITC-dextran permeability tracer, and vascular permeability was measured. Representative images of a 1-mm-thick brain section are shown for sham, for TBI, TBI/ghrelin (TBI/G). Vascular permeability (VP) to 70-kD FITC-dextran was quantified. TBI increases VP compared to sham animals (mean difference 5.81E+08; *p<0.001), and ghrelin significantly reduced arbitrary fluorescent intensity compared to TBI alone (mean difference 4.30E+08, #p<0.01; +p=NS versus sham). Error bars represent standard deviation.
Ghrelin decreases perivascular AQP-4 expression
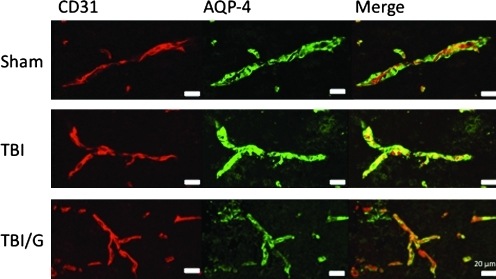
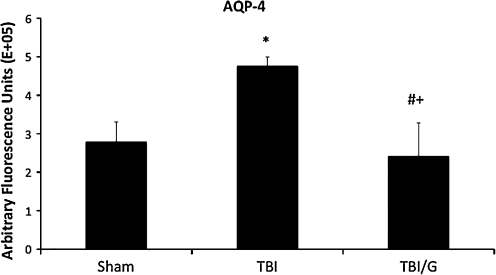
Perivascular AQP-4 is significantly increased 6 h after TBI; this increase is thought to have a role in brain edema. To investigate the effect of ghrelin on AQP-4 following TBI, we measured immunofluorescence of vessels in the area of contusion. As expected, TBI increased perivascular AQP-4 staining. In ghrelin-treated animals, this expression is attenuated. The fluorescence labeling of AQP-4 (afu×10 5) revealed that the fluorescence of the right cerebral hemisphere in the sham group was 2.79±0.52 afu, compared with 4.76±0.24 afu in the TBI group, and 2.41±0.87 afu in the ghrelin-treated TBI group. There was a significant increase in fluorescence in the TBI group compared with the sham group (mean difference 1.65E+05; p<0.05). The ghrelin-treated TBI group had significantly reduced fluorescence compared to TBI alone (mean difference 2.35E+05, p<0.01). There was no statistically significant difference between the fluorescence of sham-treated mice and ghrelin-treated TBI mice (p=NS; Figs. 3 and 4). There was no statistically significant difference in fluorescence of CD31 among the groups, indicating that changes in AQP-4 were not due to changes in microvascular density.
FIG. 3.
Immunohistochemical staining of injured sections using an anti-CD31 antibody (red) for endothelial cells, and anti-aquaporin-4 (AQP-4) antibody (green) to localize vessel-associated astrocytic end-feet is shown. There is increased AQ4 immunoreactivity in traumatic brain injury (TBI) mice compared to exposure-matched sham images. This increase is mitigated by treatment with ghrelin (TBI/G).
FIG. 4.
Quantification of aquaporin-4 (AQP-4) immunoreactivity in injured area of cortex is shown. AQP-4 immunoreactivity is significantly increased in traumatic brain injury (TBI) mice compared to sham animals (mean difference 1.65; *p<0.05). The ghrelin-treated TBI (TBI/G) group had significantly reduced fluorescence compared to TBI alone (mean difference 2.35, #p<0.005; +p=NS versus sham). Error bars represent standard deviation.
Ghrelin decreases astrocyte reactivity in white matter, but not cortical grey matter 6 h after TBI
To determine whether decreased AQP-4 after ghrelin administration was associated with decreased astrocyte reactivity, we examined the pattern of GFAP immunohistochemistry. GFAP staining was markedly increased in the corpus callosum after TBI (Fig. 5B). This effect was attenuated by treatment with ghrelin. However, there was no notable difference in the pattern of GFAP staining in the neocortex at 6 h post-TBI (Fig. 5A), suggesting that the differences in AQP-4 that we see in the site of cortical injury are not attributable to astrogliosis.
FIG. 5.
(A) Immunohistochemical staining of injured hemisphere using DAPI (blue) to locate cell nuclei, and GFAP (red) to identify reactive astrocytes. At 6 h following TBI astrocytes in the neocortex of the ipsilateral hemisphere show equivocal increases in GFAP staining such that GFAP in the neocortex of all three groups are similar. (B) Immunohistochemical staining of injured hemisphere using DAPI (blue) to locate cell nuclei, and GFAP (red) to identify reactive astrocytes. At 6 h following TBI astrocytes in the corpus callosum of the ipsilateral hemisphere display a marked increase in GFAP staining. This reactive astrogliosis is attenuated by treatment with ghrelin (TBI/G, TBI with ghrelin; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino-2-phenylindole).
Serum S100B decreases in ghrelin-treated TBI
To evaluate the degree of generalized brain injury, we immunoblotted serum for S100B. Serum S100B is known to increase, as injured brain tissue releases this protein into the systemic circulation. Western blotting showed that 6 h post-TBI, brain tissue in ghrelin-treated TBI mice had nearly a fourfold decrease in S100B after TBI compared those with TBI alone (Fig. 6A). S100B relative band densities for ghrelin-treated TBI mice (1.48±0.2; n=5) were significantly less (p<0.05) than those of TBI mice (4.06±0.6; n=5), and were similar to sham animals (1.32±0.9; n=5, p=NS; Fig. 6B).
FIG. 6.
(A) Immunoblotting of injured hemisphere for S100B, a marker of astrocyte injury, with β-actin control. (B) Quantification of relative band density of S100B. There is a fourfold increase in brain S100B expression following TBI (*p<0.05). Animals treated with ghrelin (TBI/G) exhibited S100B levels similar to sham animals (#p<0.05 versus TBI; +p=NS versus sham). Error bars represent standard deviation.
Discussion
Post-TBI edema, and the complications associated with increased ICP, account for a significant portion of trauma deaths (Marmarou, 2003). Medically managing intracranial hypertension utilizes a “reactive strategy” combining sedation and osmotic agents such as mannitol and hypertonic saline, generally after clinical evidence of intracranial hypertension develops (Donkin and Vink, 2010). Despite these therapeutic agents, a pharmacologic agent targeting the specific pathogenesis of TBI-induced edema remains elusive (Unterberg et al., 2004). Recently, Ersahin associates have demonstrated that exogenous ghrelin, in a rat model of subarachnoid hemorrhage (SAH), decreased post-injury brain edema and BBB breakdown (Ersahin et al., 2010). Prevention of post-SAH-induced edema, BBB breakdown, and neuronal injury was associated with decreased levels of cytokines and reactive oxidative species, suggesting that post-SAH edema is modulated by attenuation of the inflammatory response. Furthermore, Kwan and colleagues showed, in a rat model of endotoxemia, that ghrelin decreased mesenteric vascular permeability (Kwan et al., 2010). Similarly, knowing that ghrelin has potent anti-inflammatory effects, we have shown that ghrelin prevents intestinal dysfunction and systemic inflammation following TBI (Bansal et al., 2010). The mechanism of these observations are unknown, but it may be, at least in part, a result of decreasing transcription of inflammatory cytokines (i.e., tumor necrosis-α) from macrophages and monocytes, which may affect vascular permeability and tissue edema. Given these studies, we hypothesized that exogenous ghrelin would prevent post-TBI vascular permeability and reduce the degree of brain tissue injury. In this regard, manipulating expression of the aquaporin protein family has recently garnered interest, since increased expression and upregulation of these proteins, particularly AQP-4, has been associated with modulating brain edema in models of TBI and ischemic stroke (Okuno et al., 2008; Taya et al., 2008). AQP-4 is unique since it has polarized expression at the BBB. This property leads to an important water transport regulatory role in brain parenchymal tissue, most likely by enhancing transmembrane water flux in astrocytes (Simard et al., 2003). Therefore, finding an agent that may regulate and modulate the BBB following TBI would have definite clinical implications.
To investigate post-TBI BBB vascular permeability we measured, as previously described, fluorescence of extravasated FITC-dextran (Lee et al., 2010a). Ghrelin-treated TBI animals had a significant decrease in brain fluorescence correlating with decreased vascular permeability. While both vasogenic and cytotoxic elements are known components of post-TBI brain edema, our use of the vascular tracer FITC-dextran specifically captures vasogenic edema, which is thought to occur within hours following TBI (Barzo et al., 1996; O'Connor et al., 2006). This method of examining the integrity of the BBB has also been used to determine increased vascular permeability in brain tissue surrounding glioma (Lee et al., 2010a).
Furthermore, we show that ghrelin prevents post-TBI upregulation of astrocytic AQP-4. Because ghrelin has potent anti-inflammatory effects, it was important to determine whether the ability of ghrelin to inhibit post-TBI upregulation of AQP-4 was dependent on attenuation of reactive astrogliosis following TBI. We used immunohistochemical staining of GFAP, an intermediate filament protein involved in maintenance of the BBB, as a marker of reactive astrogliosis. We found that TBI induced very minimal GFAP immunoreactivity in the ipsilateral cortex, but significantly increased GFAP immunoreactivity in the ipsilateral corpus callosum. This finding is in accord with other studies of GFAP in TBI at early time points (Adelson et al., 2001; Dietrich et al., 1999). While we found that ghrelin can mitigate reactive astrogliosis in the corpus callosum, this pathology is not a dramatic component of neocortical injury at the 6-h time point. The finding that reactive astrogliosis is not significant in the area of neocortical injury at this time point allows us to extrapolate that the increase in ipsilateral cortical AQP-4 expression we see after TBI, and its decrease with ghrelin administration, are independent of the ability of ghrelin to mitigate astrocyte reactivity.
Moreover, the ability of ghrelin to prevent post-TBI upregulation of AQP-4 is critical, as others have shown that inhibition of AQP-4 is protective. Higashida and associates showed that post-TBI brain edema formation was mitigated by pharmacologic inhibition of a pathway involving AQP-4 (Higashida et al., 2010). This suggests an integral role for AQP-4 in the formation of TBI-induced edema. A study in a septic encephalopathy model further supports the importance of AQP-4 in generating brain edema formation, since higher levels of AQP-4 were directly correlated with an increase in the degree of brain edema (Davies, 2002). Fazzina and associates have shown that downregulation of AQP4 by an exogenously-treated protein kinase C activator significantly reduced brain water content and edema following an ischemic stroke model in rats (Fazzina et al., 2010). In addition, AQP-4 deficiency prevents brain edema in models of cytotoxic edema models such as water intoxication, ischemic stroke, and acute bacterial meningitis (Manley et al., 2000; Papadopoulos and Verkman, 2005). In these models, it is thought that a lack of AQP-4 reduces astrocytic swelling, thereby preventing brain edema. Conversely, in experimental models of vasogenic edema, such as a cortical freeze and a brain abscess model, AQP-4 deficiency can increase brain edema by impairing water clearance from brain tissues (Bloch et al., 2005; Papadopoulos et al., 2004). The binary role of aquaporin, as evidenced by its roles in both increasing cytotoxic swelling of astrocytes and clearing vasogenic edema, highlights the importance of AQP-4 as a bidirectional channel, and emphasizes the importance of understanding the timing of these functions. Importantly, in our ghrelin-treated mice, AQP4 levels are not depleted, but are conserved at levels equivocal to sham animals. This is worthy of mention, since while AQP-4 deficiency reduces cytotoxic edema, it may actually increase vasogenic edema in some models, further implicating the link between brain water regulation and AQP-4 expression.
Additionally AQP-4, via its role in cytotoxic edema in astrocyte end-feet, may further affect the BBB at the level of endothelial tight junctions. Tight junction proteins such as zona occludens-1 are essential to maintaining the integrity of the BBB. Though we did not examine the effect of ghrelin on brain endothelial tight junction proteins, this would be an interesting study in light of the findings of Qi and associates, who showed that swelling of astrocyte end-feet induces a protein kinase–mediated cascade, causing tight junction breakdown of the BBB endothelium (Qi et al., 2008). Further studies in a ghrelin-treated TBI model would be interesting to examine whether tight junction integrity of the BBB can be altered, as we have similarly demonstrated in the post-TBI intestine (Bansal et al., 2010).
Given our findings, combined with previous literature, ghrelin may decrease brain edema by modulating and decreasing TBI-induced AQP-4 expression. However, the fact that many inflammatory processes have been shown to increase BBB permeability (Huber et al., 2006; Jacob et al., 2010; Patel et al., 2008; Tsao et al., 2001), in combination with the known anti-inflammatory properties of ghrelin (Iseri et al., 2008), suggests that ghrelin may also decrease BBB breakdown by impeding the inflammatory response. TBI causes a complex inflammatory response, consisting of leukocyte migration, microglial activation, and the upregulation of small molecules involved in these processes, such as intercellular adhesion molecule 1, and matrix metalloproteinases, all of which lead to increased BBB permeability. By impeding this inflammatory cascade, and improving the structural integrity of the post-TBI BBB, ghrelin may be able to prevent secondary neuronal injury following TBI. It is intriguing to hypothesize that ghrelin-mediated immune modulation may alter AQP4 expression. Indeed, the autoimmune inflammatory disease neuromyelitis optica specifically targets astrocytic AQP4; however, the inflammatory cytokine profile of this process has not been clearly elucidated (Hinson et al., 2010). Further studies, perhaps with AQP-4 knockout animals, in combination with chemical ghrelin-receptor antagonists selectively targeting brain parenchyma, may help elucidate these salient questions.
Finally, we found that ghrelin reduces post-TBI neuronal injury, as was evident by preservation of normal brain histology, coupled with decreased levels of serum S100B. Serum concentration of the astrocytic protein S100B has been proposed as a measure of BBB damage and brain tissue injury following various brain injury models (Kapural et al., 2002; Marchi et al., 2003; Mrak and Griffinbc, 2001). We recognize that the clinical utility of this biomarker has been questioned; however, increased release of S100B has been correlated with severity of brain tissue injury (Pelinka et al., 2003). Whether ghrelin may be protective by directly preventing cellular damage following TBI or by decreasing secondary injury is unknown. In a recent study, Lee and associates demonstrated that exogenous ghrelin prevented neuronal and oligodendrocyte apoptosis in a rat spinal cord injury model (Lee et al., 2010b). It would be important to study whether ghrelin has similar effects on cerebral astrocytes and neurons following TBI.
In conclusion, we have demonstrated that in a mouse model of TBI, exogenous ghrelin prevents increased cerebral vascular permeability, decreases TBI-induced expression of AQP-4, preserves neuronal histology, and decreases levels of serum S100B. In order to proceed with the development of ghrelin as a possible therapeutic agent, further studies are needed to delineate its mechanistic properties.
Acknowledgment
Funding for this work was supported partly by American Surgical Association Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P.D. Jenkins L.W. Hamilton R.L. Robichaud P. Tran M.P. Kochanek P.M. Histopathologic response of the immature rat to diffuse traumatic brain injury. J. Neurotrauma. 2001;18:967–976. doi: 10.1089/08977150152693674. [DOI] [PubMed] [Google Scholar]
- Bansal V. Ryu S.Y. Blow C. Costantini T. Loomis W. Eliceiri B. Baird A. Wolf P. Coimbra R. The hormone ghrelin prevents traumatic brain injury induced intestinal dysfunction. J. Neurotrauma. 2010 doi: 10.1089/neu.2010.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzo P. Marmarou A. Fatouros P. Corwin F. Dunbar J. Magnetic resonance imaging-monitored acute blood-brain barrier changes in experimental traumatic brain injury. J. Neurosurg. 1996;85:1113–1121. doi: 10.3171/jns.1996.85.6.1113. [DOI] [PubMed] [Google Scholar]
- Bloch O. Papadopoulos M.C. Manley G.T. Verkman A.S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005;95:254–262. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- Davies D.C. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002;200:639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W.D. Truettner J. Zhao W. Alonso O.F. Busto R. Ginsberg M.D. Sequential changes in glial fibrillary acidic protein and gene expression following parasagittal fluid-percussion brain injury in rats. J. Neurotrauma. 1999;16:567–581. doi: 10.1089/neu.1999.16.567. [DOI] [PubMed] [Google Scholar]
- Donkin J.J. Vink R. Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr. Opin. Neurol. 2010;23:293–299. doi: 10.1097/WCO.0b013e328337f451. [DOI] [PubMed] [Google Scholar]
- Ersahin M. Toklu H.Z. Erzik C. Cetinel S. Akakin D. Velioglu-Ogunc A. Tetik S. Ozdemir Z.N. Sener G. Yegen B.C. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J. Neurotrauma. 2010;27:1143–1155. doi: 10.1089/neu.2009.1210. [DOI] [PubMed] [Google Scholar]
- Faul M. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. 2010. http://www.cdc.gov/traumaticbraininjury/tbi_ed.html http://www.cdc.gov/traumaticbraininjury/tbi_ed.html
- Fazzina G. Amorini A.M. Marmarou C.R. Fukui S. Okuno K. Dunbar J.G. Glisson R. Marmarou A. Kleindienst A. The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J. Neurotrauma. 2010;27:453–461. doi: 10.1089/neu.2008.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E. Corso P.S. Miller T.R. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; Oxford, New York: 2006. [Google Scholar]
- Habgood M.D. Bye N. Dziegielewska K.M. Ek C.J. Lane M.A. Potter A. Morganti-Kossmann C. Saunders N.R. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur. J. Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- Higashida T. Kreipke C.W. Rafols J.A. Peng C. Schafer S. Schafer P. Ding J.Y. Dornbos D., 3rd Li X. Guthikonda M. Rossi N.F. Ding Y. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J. Neurosurg. 2011;114:92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- Hinson S.R. McKeon A. Lennon V.A. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010;168:1009–1018. doi: 10.1016/j.neuroscience.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Huber J.D. Campos C.R. Mark K.S. Davis T.P. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseri S.O. Sener G. Saglam B. Ercan F. Gedik N. Yegen B.C. Ghrelin alleviates biliary obstruction-induced chronic hepatic injury in rats. Regulatory Peptides. 2008;146:73–79. doi: 10.1016/j.regpep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Jacob A. Hack B. Chiang E. Garcia J.G. Quigg R.J. Alexander J.J. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010;24:1682–1688. doi: 10.1096/fj.09-138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapural M. Krizanac-Bengez L. Barnett G. Perl J. Masaryk T. Apollo D. Rasmussen P. Mayberg M.R. Janigro D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- Kojima M. Hosoda H. Date Y. Nakazato M. Matsuo H. Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kwan R.O. Cureton E. Dozier K. Curran B. Sadjadi J. Victorino G.P. Ghrelin decreases microvascular leak during inflammation. J. Trauma. 2010;68:1186–1191. doi: 10.1097/TA.0b013e3181bb9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Borboa A.K. Chun H.B. Baird A. Eliceiri B.P. Conditional deletion of the focal adhesion kinase FAK alters remodeling of the blood-brain barrier in glioma. Cancer Res. 2010a;70:10131–10140. doi: 10.1158/0008-5472.CAN-10-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y. Chung H. Yoo Y.S. Oh YJ. Oh TH. Park S. Yune TY. Inhibition of apoptotic cell death by ghrelin improves functional recovery after spinal cord injury. Endocrinology. 2010b;151:3815–3826. doi: 10.1210/en.2009-1416. [DOI] [PubMed] [Google Scholar]
- Liu Y. Wang P.S. Xie D. Liu K. Chen L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/reperfusion. Chin. J. Physiol. 2006;49:244–250. [PubMed] [Google Scholar]
- Manley G.T. Fujimura M. Ma T. Noshita N. Filiz F. Bollen A.W. Chan P. Verkman A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Marchi N. Rasmussen P. Kapural M. Fazio V. Kight K. Mayberg M.R. Kanner A. Ayumar B. Albensi B. Cavaglia M. Janigro D. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- Marmarou A. Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir. Suppl. 2003;86:7–10. doi: 10.1007/978-3-7091-0651-8_2. [DOI] [PubMed] [Google Scholar]
- Miao Y. Xia Q. Hou Z. Zheng Y. Pan H. Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem. Biophys. Res. Comm. 2007;359:795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- Mrak R.E. Griffinbc W.S. The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease. Neurobiol. Aging. 2001;22:915–922. doi: 10.1016/s0197-4580(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Nag S. Manias J.L. Stewart D.J. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118:197–217. doi: 10.1007/s00401-009-0541-0. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrichm M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C.A. Cernakn I. Vink R. The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir. Suppl. 2006;96:121–124. doi: 10.1007/3-211-30714-1_27. [DOI] [PubMed] [Google Scholar]
- Okuno K. Taya K. Marmarou C.R. Ozisik P. Fazzina G. Kleindienst A. Gulsen S. Marmarou A. The modulation of aquaporin-4 by using PKC-activator (phorbol myristate acetate) and V1a receptor antagonist (SR49059) following middle cerebral artery occlusion/reperfusion in the rat. Acta Neurochir. Suppl. 2008;102:431–436. doi: 10.1007/978-3-211-85578-2_84. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M.C. Manley G.T. Krishna S. Verkman A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M.C. Verkman A.S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005;280:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- Patel T.H. Sprague S. Lai Q. Jimenez D.F. Barone C.M. Ding Y. Blood brain barrier (BBB) dysfunction associated with increased expression of tissue and urokinase plasminogen activators following peripheral thermal injury. Neurosci. Lett. 2008;444:222–226. doi: 10.1016/j.neulet.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Pelinka L.E. Toegel E. Mauritz W. Redl H. Serum S 100B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock. 2003;19:195–200. doi: 10.1097/00024382-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Qiu W.C. Wang Z.G. Lv R. Wang W.G. Han X.D. Yan J. Wang Y. Zheng Q. Ai K.X. Ghrelin improves delayed gastrointestinal transit in alloxan-induced diabetic mice. World J. Gastroenterol. 2008;14:2572–2577. doi: 10.3748/wjg.14.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X. Inagaki K. Sobel R.A. Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J. Clin. Invest. 2008;118:173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul T.G. Ducker T.B. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J. Neurosurg. 1982;56:498–503. doi: 10.3171/jns.1982.56.4.0498. [DOI] [PubMed] [Google Scholar]
- Schilling L. Wahl M. Mediators of cerebral edema. Adv. Exp. Med. Biol. 1999;474:123–141. doi: 10.1007/978-1-4615-4711-2_11. [DOI] [PubMed] [Google Scholar]
- Sehirli O. Sener E. Sener G. Cetinel S. Erzik C. Yegen B.C. Ghrelin improves burn-induced multiple organ injury by depressing neutrophil infiltration and the release of pro-inflammatory cytokines. Peptides. 2008;29:1231–1240. doi: 10.1016/j.peptides.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Simard M. Arcuino G. Takano T. Liu Q.S. Nedergaard M. Signaling at the gliovascular interface. J. Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel P.F. Shohami E. Younis F.M. Kariya K. Oho V.I. Lenzlinger P.M. Grosjean M.B. Eugster H.P. Trentz O. Kossman T. Morganti-Kossman M.C. Experimental closed head injury: analysis of neurological outcome blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for proinflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Taya K. Gulsen S. Okuno K. Prieto R. Marmarou C.R. Marmarou A. Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir. Suppl. 2008;102:425–429. doi: 10.1007/978-3-211-85578-2_83. [DOI] [PubMed] [Google Scholar]
- Tsao N. Hsu H.P. Wu C.M. Liu C.C. Lei H.Y. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- Unterberg A.W. Stover J. Kress B. Kiening K.L. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Wren A.M. Small C.J. Ward H.L. Murphy K.G. Dakin C.L. Taheri S. Kennedy A.R. Roberts G.H. Morgan D.G. Ghatei M.A. Bloom S.R. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Xu J. Wang S. Lin Y. Cao L. Wang R. Chi Z. Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci. Lett. 2009;453:58–61. doi: 10.1016/j.neulet.2009.01.067. [DOI] [PubMed] [Google Scholar]
- Zelenina M. Regulation of brain aquaporins. Neurochem. Int. 2010;57:468–488. doi: 10.1016/j.neuint.2010.03.022. [DOI] [PubMed] [Google Scholar]