Abstract
Background and Purpose
White matter hyperintensities (WMHs) found on brain MRI in elderly individuals are largely thought to be due to microvascular disease, and its progression has been associated with cognitive decline. The present study sought to determine patterns of cognitive decline associated with anterior and posterior WMH progression.
Methods
Subjects included 110 normal controls, ages ≥60 years, who were participants in the Duke Neurocognitive Outcomes of Depression in the Elderly study. All subjects had comprehensive cognitive evaluations and MRI scans at baseline and after 2 years. Cognitive composites were created in five domains: complex processing speed, working memory, general memory, visual-constructional skills, and language. Change in cognition was calculated utilizing standard regression-based models accounting for variables known to impact serial testing. A semi-automated segmentation method was utilized to measure WMH extent in anterior and posterior brain regions. Hierarchical multiple linear regression models were used to evaluate which of the five measured cognitive domains was most strongly associated with regional (anterior and posterior) and total WMH progression, after adjusting for demographics (age, sex and education).
Results
Decline in complex processing speed was independently associated with both anterior (r2 = 0.06, p = 0.02) and total WMH progression (r2 =0.05, p = 0.04). In contrast, decline in visual-constructional skills was uniquely associated with posterior progression (r2= 0.05, p <0.05).
Conclusions
Distinct cognitive profiles are associated with anterior and posterior WMH progression among normal elders. These differing profiles need to be considered when evaluating the cognitive correlates of WMH.
Keywords: Cognition, Neuropsychology, White matter disease
Cerebral white matter areas that appear hyperintense on T2-weighted magnetic resonance imaging (MRI) are frequently found on MRI scans of elderly individuals. Although the pathophysiological causes of white matter hyperintensities (WMHs, also called “white matter lesions”) are varied, in elders they are generally considered to be a marker of small vessel disease1. A small number of large-scale longitudinal studies have shown an association between cognitive decline and WMH progression in non-demented older adults.2–6 Although declines in processing speed are often associated with WMH progression2, 5–6, impairments in memory or other cognitive skills have also been implicated.3–4 Thus, the relation between WMH and the domains of cognitive decline remains uncertain.
One investigative approach has been to explore the differential relationship between cognition and periventricular compared with deep WMHs4–6 The method of measurement of WMHs vary among studies, and the appropriateness of this anatomic dichotomization remains controversial.7 Not surprisingly, results have been inconsistent across studies, with some finding progression of periventricular WMHs5–6 and others progression of deep WMHs4 to be more strongly associated with cognitive decline.
Regional progression of WMHs may differ, with the frontal lesions progressing most and occipital lesions least.8 The impact of anterior WMHs on cognitive decline is also greater than that of posterior lesions in patients with vascular cognitive impairment.9 Further, a cross-sectional diffusion tensor imaging (DTI) study of 52 healthy participants aged 19 to 81 found that white matter degradation in regions of interest in anterior brain areas was related to reduced processing speed and working memory, whereas decline in posterior regions was associated with decreased inhibition and task switching.10 Taken together, these findings suggest that the distinction between anterior and posterior white matter might provide additional characterization of the relationship between cognition and WMHs. The primary purpose of the study was to examine the cognitive correlates of anterior and posterior WMH progression.
Methods
Participants
Participants (n = 110) were community-dwelling older adults, who served as controls for the Duke University Medical Center Neurocognitive Outcomes of Depression in the Elderly (NCODE) study. The study was approved by the Duke institutional review board and all participants provided informed consent. Subjects were between 60 and 83 years of age at baseline, and had at least a high school education (see Table 1 for further demographic data). Other eligibility criteria included: nonfocal neurologic examination, no history of depression based on the Diagnostic Interview Schedule11 portion of the Duke Depression Evaluation Schedule12, and no self-report of neurologic or depressive illness. Participants selected for the present study underwent baseline and 2-year follow-up brain MRI scan and neuropsychological evaluations.
TABLE 1.
Participants’ Demographic and Health Data
| Variable | Baseline | 2-year follow-up |
|---|---|---|
| Age (years) * | 70.65 (5.55) | -- |
| Education (years) * | 15.98 (2.31) | -- |
| Female† | 80 (72.7) | -- |
| Race† | ||
| Caucasian | 97 (88.2) | -- |
| African American | 9 (8.2) | -- |
| Mixed | 4 (3.6) | -- |
| MMSE * | 28.85 (1.32) | 28.55 (1.56) |
| Hypertension† | 24 (21.8) | 28 (25.4)‡, |
| Diabetes† | 4 (3.6) | 4 (3.6)‡ |
| Heart Problem† | 8 (7.3) | 13 (11.8)‡ |
| Stroke† | 0 (0) | 2 (1.8)§ |
Note:
= M(SD);
= n (%);
= 2 missing cases;
= 3 missing cases;
MMSE = Mini Mental State Examination.
Materials and Procedure
i) Demographic and Health Data
Demographic data were recorded at baseline. Subjects also completed a questionnaire at baseline and 2-year follow up to assess the presence or absence of several medical conditions, including hypertension, diabetes, heart problems, and stroke.
ii) MRI Protocol
Participants completed MRI scans at baseline and after approximately 2 years. Subjects were imaged with a 1.5-T whole-body MRI system (Signa, GE Medical Systems, Milwaukee, WI). A rapid sagittal localizer scan was acquired for alignment, and a dual-echo fast spin-echo acquisition was obtained in the axial plane for morphometry. Axial images had 3mm thickness without inter-slice gaps. Images were processed in Duke’s Neuropsychiatric Imaging Research Laboratory (NIRL) on SunOS workstations. Volume measurements were performed with a NIRL-modified version of MrX software (General Electric Corporate Research and Development, Schenectady, NY). The segmentation protocol, or process for converting image intensity to segmented tissue types, has been described previously13. Briefly, it is a semi-automated method that uses the multiple MR contrasts available to identify different tissue classifications through a “seeding” process, in which a trained analyst manually selects pixels in each tissue type to be identified (grey matter, white matter, cerebrospinal fluid, grey and white matter lesions or background). Infratentorial lesions were not included. WMH volumes were obtained in periventricular and deep white matter. Infarcts, which were present on some follow-up scans, were not included with these lesions. Cerebrum volume was defined as the sum of grey and white matter, and lesions.
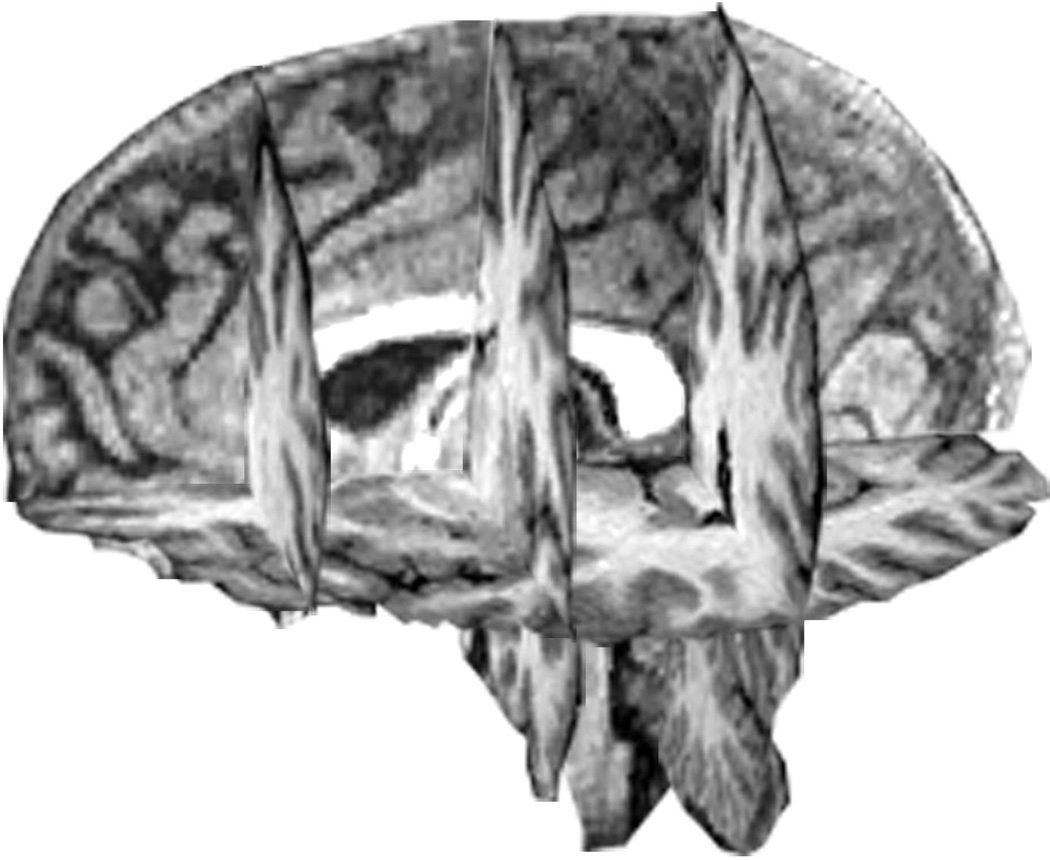
Additional procedures were required to obtain measures of WMH and volume of the cerebrum in anterior and posterior brain areas (Figure 1), and have been described previously14. First, an axial plane was created along the anterior -posterior commissural line, dividing superior regions from inferior. Next coronal planes were created perpendicular to the axial plane at the anterior and posterior extent of the corpus callosum. Finally, a third coronal plane was created at the midpoint between the first two coronal planes, which divided the brain into anterior and posterior halves. The middle coronal plane was used to separate anterior and posterior brain regions for white matter lesion volumes. WMH progression was calculated by subtracting WMH volume at baseline from WMH volume at the follow-up for each participant. This calculation was performed for WMHs in anterior and posterior halves of the brain, as well as overall WMHs. Similar computations were used to measure cerebrum volume change over time.
Figure 1.
Parcellation diagram provided by the Duke Neuropsychiatric Imaging Research Laboratory
iii) Neuropsychological Testing
Cognitive data were obtained from the neuropsychological testing date closest to MRI acquisition (days between MRI and testing: at baseline M = 4.44, SD = 21.37; at follow-up M = 2.96, SD = 19.92). The test battery was comprised of the Consortium to Establish a Registry in Alzheimer’s Disease (CERAD) neuropsychological battery15 and supplemental cognitive tests.
The CERAD measures included (1) the Mini Mental State Exam (MMSE)16; (2) language tasks consisting of category fluency (Animal Naming) and object naming (15-item Boston Naming Test); (3) visual constructional praxis and visual memory, requiring copy of 4 geometric designs, delayed recall and delayed recognition procedures; and (4) verbal learning and memory, consisting of immediate recall of 3 learning trials of a 10-item word list, delayed recall, and recognition of target words from non-target foils. Additional measures included the Logical Memory subtest of the Wechsler Memory Scale–Revised (WMS-R)17, the Benton Visual Retention Test (BVRT)18, the Controlled Oral Word Association Test (COWAT) from the Multilingual Aphasia Examination19, Trail Making Test20, Symbol Digit Modalites Test21, Digit Span subtest of the Wechsler Adult Intelligence Scale–Revised (WAIS-R)22, and a separate Ascending Digit Span task modeled after the Digit Ordering Test23.
Five cognitive composites (Table 2) were created based on known relationships among the tests and studies supporting construct validity (e.g. Larrabe et al.24, Wechsler17). Measures were grouped by speeded (complex processing speed) and non-speeded (working memory) executive tasks, memory, language, and visual-constructional skills.
TABLE 2.
Cognitive Composites
| Composite | Cognitive tests |
|---|---|
| Executive function | |
| Complex processing speed | COWAT, Trail Making Test A and B, Symbol Digit Modalities Test |
| Working memory | WAIS-R Digit Span backwards and Ascending Digit Span |
| General memory | CERAD list-learning, delay and recognition; WMS-R Logical Memory I and II; CERAD praxis delayed |
| Language | CERAD category fluency (animal naming) and 15-item Boston Naming Test |
| Visual-constructional skills | CERAD visual constructional praxis copy, BVRT (total correct), BVRT (total errors) |
BVRT = Benton Visual Retention Test; CERAD = Consortium to Establish a Registry in Alzheimer’s Disease; COWAT = Controlled Oral Word Association Test; WAIS-R = Wechsler Adult Intelligence Scale–Revised; WMS-R= Wechsler Memory Scale–Revised
Baseline composite scores were created using two steps. First, individual raw test scores were transformed into z scores using the standard formula: z = (χi −M)/SD; where χi is the raw test score to be standardized, M is the sample mean for a given test, and SD is the sample standard deviation on that same test. Next, the individual z scores for the tests comprising each composite were averaged to compute standardized scores for each composite.
Standardized regression based models were used to quantify cognitive progression25. Specifically, linear regression of the difference scores on the initial scores was used to generate a formula for predicting a difference score from any baseline score for each test. Using this formula, the deviation from expected change was calculated for each patient on each test. Standardized z difference scores were calculated on all tests using the formula: (observed difference score − predicted difference score)/standard error of estimate derived from the regression equation for a given subtest. Composite difference scores were computed by calculating the average z difference score for tests comprising each composite.
Statistical Analyses
Given prior findings showing more prominent anterior than posterior WMH progression8, we evaluated regional WMH load at baseline by computing independent sample t-tests on WMH volumes in anterior vs. posterior regions at baseline. Paired sample t-tests on WMH volumes at baseline and follow-up were conducted to determine whether lesions had progressed. To determine whether rate of progression of WMHs varied by region, changes in regional lesion volumes were calculated by subtracting individual values at baseline from follow-up scores in anterior and posterior regions with a two-sample t-test on these difference scores.
To determine which cognitive domain/s would be most strongly associated with total and regional WMH progression, three hierarchical multiple linear regressions (p < 0.05 to enter, and p > 0.10 to remove) were calculated, one on each WMH progression score (i.e. total, anterior, and posterior). Age, education, and sex were entered in the first step of these models to adjust for their effect. Next, cognitive composite difference scores were entered stepwise as predictors. This approach might have underestimated the independent contribution of individual cognitive domains due to shared variance among cognitive composites; however, it allowed the identification of the cognitive domains that were most impacted by regional WMH progression, while avoiding over estimation of associations between cognition and WMH based on common variance with other domains. Similar analyses were performed on WMH load and cognitive composite scores at baseline to evaluate the relation between these variables. For those models not meeting regression assumptions, models were re-run using data converted into ranks to assess the robustness of the findings.
The sample size provided an approximate 80% power to detect, for any particular predictor, an increase in the model r2 of 0.04 corresponding to a moderate effect size26. SPSS for Windows (version 12, SPSS Inc) was used for all analyses.
Results
Regional WMH progression
Table 3 shows descriptive statistics on WMH volumes (in mL) at baseline and follow up (mean follow-up interval was 737.49 days, SD = 26.21 days). Mean volumes did not significantly differ in anterior and posterior lesions on baseline scans (p = .06). WMHs progressed in both anterior and posterior regions (Table 3), but there was no difference in progression by region (p = .40).
TABLE 3.
Statistics on White Matter Hyperintensity Volume (in mL)
| Region | Initial Scan M (SD) |
Follow-up Scan M (SD) |
Difference M (SD) |
r2 | p |
|---|---|---|---|---|---|
| Anterior | 2.71(2.96) | 3.26 (3.55) | 0.56 (1.05) | 0.93 | <.001 |
| Posterior | 2.26 (4.93) | 2.96 (5.81) | 0.69 (1.36) | 0.96 | <.001 |
| Total | 4.92 (7.58) | 6.13(9.02) | 1.21 (2.3) | 0.95 | <.001 |
Relationship between cognition and regional WMH load at baseline
Hierarchical multiple regressions of WMH load at baseline showed that age was associated with total (p <0.01), anterior (p <0.01), and posterior (p =0.02) WMH load. Although initial analyses showed that general memory was associated with anterior (F = 4.56, p < 0.01, R2 = 0.18) and total (F = 3.26, p =0.02, R2 =0.13) WMH load after controlling for demographics, secondary analysis utilizing ranked data did not find an independent relationship.
Cognitive correlates of regional WMH progression
Results from hierarchical multiple regression analyses indicated that change in complex processing speed was uniquely associated with anterior WMH progression, whereas change in visual-constructional skills was solely associated with posterior WMH progression (Table 4), after adjusting for demographics. Demographic variables did not predict regional or total WMH progression (all ps > 0.20), but note that our sample had a restricted age range. The results were similar when the analyses were repeated with data transformed into ranks and after adjusting for change in cerebrum volumes.
TABLE 4.
Cognitive Correlates of Regional (Anterior vs. Posterior) and Total White Matter Hyperintensity Progression
| WMH Progression | Demographics | Added Variable Cognitive Domain |
|||||
|---|---|---|---|---|---|---|---|
| r2 | p | β | SE | r2 | p | ||
| Anterior | .04 | 0.33 | Complex Processing Speed | −0.29 | .23 | .06 | .02 |
| Posterior | .05 | 0.24 | Visual Construction | −0.22 | .23 | .05 | <.05 |
| Total | .04 | 0.30 | Complex Processing Speed | −0.25 | .51 | .05 | .04 |
Note. Hierarchical multiple regression analyses consisted of two steps: 1) demographic variables (age, sex and education) were entered as a block, and 2) five cognitive composite difference scores (complex processing speed, working memory, general memory, visual-constructional skills, and language) were entered in a stepwise fashion.
Discussion
In the present longitudinal study, we investigated the relationship between regional WMH progression (anterior vs. posterior) and decline in cognition (i.e., complex processing speed, working memory, general memory, language and visual-constructional skills) in a sample of community-dwelling elderly individuals without a history of stroke or dementia. We found that decline in complex processing speed was the most important cognitive correlate of anterior WMH progression, whereas decline in visual-constructional skills was the most important correlate of posterior WMH progression.
These results are consistent with previous studies showing an association between WMH progression and cognitive decline,2–6 but extend these findings by showing a differential pattern of cognitive decline related to anterior and posterior WMH progression among normal elders. Consistent with other large scale longitudinal studies, decline in complex processing speed was independently associated with both overall and anterior WMH progression.2, 5–6 The only study of this kind that assessed processing speed and did not find an association was the Austrian Stroke Prevention Study. In that study, non-speeded attentional/working memory tasks were included along with speeded tasks as part of an attention/speed composite metric, possibly explaining the difference.3
We also found that that decline in visual-constructional skills was uniquely associated with posterior WMH progression. The Austrian Stroke Prevention Study3 also reported an association between WMH changes and visual-motor skills, as measured by the Purdue Pegboard. However, this is a speeded motor task substantially different from the non-speeded visual-constructional tasks included in our study. Further, neither the other large scale studies with repeated MRI and cognitive testing, nor the one DTI study exploring anterior vs. posterior white matter integrity, assessed this cognitive domain.10 Visual constructional skills, as measured by the Block Design subtest of the WAIS, have been found to be associated with WMH in patients with stroke27 and healthy elders28, and our finding linking it to changes in posterior brain areas is consistent with established knowledge on functional neuroanatomy29. Harmonization standards identified visuospatial skills as one of four domains to be assessed by the 60-minute neuropsychological test protocol.30 Results from validation studies of this protocol will offer more data regarding the significance of visual-spatial deficits in posterior vascular brain changes.
Because both total and anterior WMH progression were associated with the same cognitive composite (i.e. complex processing speed), but posterior WMH progression was associated with visual-constructional skills, anterior WMH progression best reflects the effects of total WMH progression on cognition. One possible explanation for this finding is that anterior WMH progression places a greater burden on cognition than posterior WMH progression, which is consistent with findings on patients with vascular cognitive impairment.9
Whether the loss of brain volume that is associated with progression of lesion load might account for the relationship between WMH load and cognition has been controversial. In the Austrian Stroke Prevention Study3 the relationship between WMH and cognition was lost when brain atrophy was included in the same model. In our study, however, the relationship between WMH and cognition remained even when change in cerebrum volume was included. This is consistent with other studies4–5 and suggests that brain atrophy is not a mediator of the relationship between WMH progression and cognitive decline.
Cognitive variables were not predictive of WMH load at baseline after adjusting for demographics suggesting that certain variables can have differing effects on baseline versus longitudinal performance.31 Longitudinal studies may be more sensitive in identifying the relationship between cognition and WMH, in part because demographic variables may influence the change association less than they do the baseline relationships.
Our study has several strengths. It used repeated MRI scanning and comprehensive cognitive testing to explore anterior vs. posterior WMH progression and its association with cognitive decline. WMH load and cognition were assessed using valid and sensitive measures. WMH load was assessed using a semi-automated method with proven reliability.13 A comprehensive neuropsychological testing battery allowed evaluation of cognitive domains using composite measures, which tend to be more robust and valid than single test scores. Most longitudinal studies exploring the relationship between WMH and cognition use a single cognitive test and measure a limited number of cognitive domains, typically those domains that have been found by early studies to be related to WMHs, possibly resulting in oversight of subtle effects of WMHs on other cognitive domains. Additionally, the present study used standard based regression models to assess cognitive change. This approach controls for variables known to impact serial cognitive testing, such as regression to the mean and practice effects, which are of critical importance to consider in any longitudinal study of cognition25. This methodology is a more precise measure of cognitive change than the subtractions of raw or standardized scores, and also offers the advantage of yielding difference scores that are on a standardized scale across tests and domains, allowing for meaningful comparisons on the degree of change across measures despite their having different raw scores scales.
The study also has several limitations. The subjects were relatively healthier than the general population, and thus, the findings may not be applicable to all community-dwelling elders. The sample size permitted the detection of only a moderate effect; a study with a larger number of subjects is required to identify more subtle differences. Replication of these findings in other populations with known risk factors and documented cerebrovascular disease would help support the generalizability of the results. Although our method of quantifying WMH was reliable, the cause and pathological correlates of WMHs as identified are unknown as is the etiology of concomitant WMHs and atrophy. Therefore, additional work is needed to determine the clinical significance of the findings.
The results of this longitudinal study in non-demented older adults suggests differential patterns of cognitive decline associated with regional (anterior vs. posterior) WMH variation. The findings may have implications in choosing a neurocognitive marker for the evaluation of regional WMH, and exploring the effects of candidate therapies.
Acknowledgments
This study was supported by an American Stroke Association/Bugher Foundation Center for Stroke Prevention Research award (Grant Number 0775001N), and National Institutes of Health grants (P50 MH60451, R01 MH54846). Dr. Goldstein is also supported by the Department for Veterans Affairs. The authors also acknowledge Dr. Robert Rybczynski and Mr. Kulpreet Singh of the Duke Neuropsychiatric Imaging Research Laboratory for figure preparation.
Footnotes
Conflicts of Interest/Disclosures: None
References
- 1.Pantoni L, Garcia JH. Pathogenesis of Leukoaraiosis : A Review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, Manifestations, and Predictors of Worsening White Matter on Serial Cranial Magnetic Resonance Imaging in the Elderly: The Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, Matthews PM, Fazekas F. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 4.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Heuvel DMJ, ten Dam VH, de Craen AJM, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J. Neurol. Neurosurg. Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 7.Kim KW, MacFall JR, Payne ME. Classification of White Matter Lesions on Magnetic Resonance Imaging in Elderly Persons. Biological Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- 9.Williamson JB, Nyenhuis DL, Pedelty L, Byrd S, Jhaveri M, Wang C, deToledo-Morrell L, Sripathirathan K, Gorelick P. Baseline differences between vascular cognitive impairment no dementia reverters and non-reverters. J Neurol Neurosurg Psychiatry. 2008;79:1208–1217. doi: 10.1136/jnnp.2007.137554. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its History, Characteristics, and Validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 12.Landerman R, George LK, Campbell RT, Blazer DG. Alternative models of the stress buffering hypothesis. American Journal of Community Psychology. 1989;17:625–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- 13.Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KRR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Research: Neuroimaging. 2002;115:63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 14.Potter GG, Blackwell AD, McQuoid DR, Payne ME, Steffens DC, Sahakian BJ, Welsh-Bohmer KA, Ranga Krishnan KR. Prefrontal white matter lesions and prefrontal task impersistence in depressed and nondepressed elders. Neuropsychopharmacology. 2007;32:2135–2142. doi: 10.1038/sj.npp.1301339. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state" : A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 18.Benton A. Revised Visual Retention Test. 4.ed. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 19.Benton A, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 20.Reitan R. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 21.Smith A. Symbol Digit Modalities Test-Manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 22.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 23.Hoppe C, Müller U, Werheid K, Thöne A, vonCramon YD. Digit Ordering Test: clinical, psychometric, and experimental evaluation of a verbal working memory test. Clinical Neuropsychologist. 2000;14:38–55. doi: 10.1076/1385-4046(200002)14:1;1-8;FT038. [DOI] [PubMed] [Google Scholar]
- 24.Larrabee GJ, Kane RL, Schuck JR, Francis DJ. Construct validity of various memory testing procedures. Journal of Clinical and Experimental Neuropsychology. 1985;7:239–250. doi: 10.1080/01688638508401257. [DOI] [PubMed] [Google Scholar]
- 25.Temkin NR, Heaton RK, Grant I, Dikmen SS. Detecting significant change in neuropsychological test performance: A comparison of four models. Journal of the International Neuropsychological Society. 1999;5:357–369. doi: 10.1017/s1355617799544068. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- 27.Jokinen H, Kalska H, Mäntylä R, Ylikoski R, Hietanen M, Pohjasvaara T, Kaste M, Erkinjuntti T. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:1229–1233. doi: 10.1136/jnnp.2004.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HBW. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. The Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- 29.Nolte J. The human brain : an introduction to its functional anatomy. St. Louis: Mosby; c2002. [Google Scholar]
- 30.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 31.Attix DK, Story TJ, Chelune GJ, Ball JD, Stutts ML, Hart RP, Barth JT. The prediction of change: normative neuropsychological trajectories. Clinical Neuropsychologist. 2009;23:21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]