Abstract
Background
Clinical trials of therapeutic angiogenesis with vascular endothelial growth factor (VEGF) have been disappointing, owing likely to endothelial dysfunction. We used a swine model of chronic ischemia and endothelial dysfunction to determine whether resveratrol coadministration would improve the angiogenic response to VEGF therapy.
Methods
Yorkshire swine fed a high-cholesterol diet underwent left circumflex ameroid constrictor placement, and were given either no drug (high cholesterol control [HCC], n = 8), perivascular VEGF (2 μg sustained release [high cholesterol VEGF-treated; HCV], n = 8), or VEGF plus oral resveratrol (10 mg/kg, [high cholesterol VEGF- and resveratrol-treated; HCVR], n = 8). After 7 weeks, myocardial contractility, perfusion, and microvessel reactivity in the ischemic territory were assessed. Tissue was analyzed for vessel density, oxidative stress, and protein expression.
Results
Myocardial perfusion was significantly improved in the HCV group compared with the HCC group; resveratrol coadministration abrogated this improvement. There were no differences in regional myocardial contractility between groups. Endothelium-dependent microvessel relaxation was improved in the HCVR group, and endothelium-independent relaxation response was similar between groups. Arteriolar density was greatest in the HCV group, whereas capillary density was similar between groups. Expression of Akt and phospho-endothelial nitric oxide synthase were increased in the HCVR group. Total protein oxidative stress and myeloperoxidase expression were reduced in the HCVR group, but so was the oxidative-stress dependent phosphorylation of vascular endothelial cadherin (VE-cadherin) and β-catenin.
Conclusion
Although resveratrol coadministration decreases oxidative stress and improves endothelial function, it abolishes improvements in myocardial perfusion and arteriolar density afforded by VEGF treatment alone. This effect is due likely to inhibition of the oxidative stress-dependent phosphorylation of VE-cadherin, an essential step in the initiation of arteriogenesis.
“Therapeutic angiogenesis” refers to the use of various agents to enhance native myocardial blood vessel growth and maturation in patients with coronary artery disease in whom complete percutaneous or surgical revascularization is impossible. Ideally, administration of pro-angiogenic proteins, cells, or genes would increase collateral vessel development in at-risk myocardium, thereby improving myocardial perfusion where percutaneous or operative techniques cannot. A variety of therapeutics and delivery methods have demonstrated impressive results in healthy animal models of myocardial ischemia, but results in large-scale clinical trials have been much less impressive.1
Vascular endothelial growth factor (VEGF), one of the most promising proteins used in trials of therapeutic angiogenesis, is involved in a wide range of processes that are essential for angiogenesis, including vascular permeability, endothelial cell migration and mitosis, recruitment of monocytes, and mobilization of endothelial progenitor cells.2,3 Earlier studies using swine models of chronic ischemia showed significant improvements in ischemic territory collateral formation, blood flow, and myocardial function with VEGF treatment, irrespective of the route of administration.4 Results of clinical trials, however, have been less impressive; the VIVA trial of 2003 demonstrated only modest improvements in frequency of angina and exercise tolerance compared with placebo at 120 days follow-up with the greatest dose of intracoronary VEGF, with no improvement at 60 days, and no improvement in myocardial perfusion or left ventricular function at either time point.5 This disparity between preclinical and clinical results, thought to be due to the endothelial dysfunction present in the majority of human cardiac patients who suffer from hypercholesterolemia and diabetes, is a major hurdle in the field of therapeutic angiogenesis.
Resveratrol is a polyphenol found in grape skins and red wine that has antioxidant, cholesterol-lowering, and anti-apoptotic properties,6,7 possibly mediated by activation of Sirt1.8 Resveratrol has been shown to decrease the oxidation of low-density lipoprotein cholesterol (LDL) by a variety of mechanisms, including free radical scavenging, chelating metal ions to reduce their oxidizing capacity, and sparing vitamin E and carotenoids in the LDL particle.9,10 We demonstrated previously that oral treatment with purified resveratrol improved myocardial perfusion and reversed endothelial dysfunction in the ischemic myocardium of hypercholesterolemic swine, associated with upregulation of the VEGF pathway.11 Based on these findings, we hypothesized that concomitant treatment with exogenous VEGF and resveratrol, targeting both pro-angiogenic and anti-oxidant pathways, might result in a more dramatic improvement in perfusion in the setting of endothelial dysfunction.
METHODS
Animal model
Intact male Yorkshire swine (Parsons Research, Amherst, MA), were fed 500 g/d of a high-cholesterol diet consisting of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research Center, Columbia, MO) starting at 8 weeks of age and continuing for the duration of the experiment. All animals had unlimited access to water and were kept in a warm, non-stressful environment for the duration of the study. For all operative procedures, anesthesia was induced with telazol (4.4 mg/kg IM) and maintained with 2.0% isoflurane, and animals were intubated and mechanically ventilated at 16 breaths/min.
After 4 weeks of dietary modification, all animals were made chronically ischemic by left circumflex artery (LCx) ameroid constrictor placement (Research Instruments SW, Escondido, CA), and the ischemic territory was determined by injection of gold-labeled microspheres (Biophysics Assay Laboratory, Worcester, MA) into the left atrium via left mini-thoracotomy. Animals were then divided into 3 groups: High cholesterol control (HCC; n = 8), high cholesterol VEGF-treated (HCV; n = 8), and high cholesterol VEGF- and resveratrol-treated (HCVR; n = 8). HCVR animals were started on oral resveratrol (10 mg/kg per day; Irvine, CA) at the time of ameroid placement. Three weeks after ameroid placement, HCV and HCVR animals underwent repeat mini-thoracotomy and osmotic pump placement (Alzet Inc., Cupertino, CA) to deliver recombinant human VEGF solution (VEGF165, R&D systems, Minneapolis, MN) at a rate of 3 μL/h via a microcatheter implanted subepicardially in the ischemic territory, as described previously.12
Seven weeks after ameroid placement, all animals were once again anesthetized, and blood glucose measurements were taken 30 and 60 minutes after an intravenous dextrose challenge (0.5 mg/kg; Phoenix Pharmaceutical, St. Joseph, MO). X-ray coronary angiography was completed via a 5-French arterial sheath placed into the right common femoral artery via direct cutdown. Blood was withdrawn from the femoral artery sheath and analyzed for total cholesterol, HDL, calculated LDL, and triglycerides (Beckman DXC 800 chemistry analyzer, Brea, CA). The heart was exposed via midline sternotomy, hemodynamic and cardiac functional measurements were taken, and microspheres were injected at resting and paced conditions. The heart was then harvested and sectioned into two 1-cm thick transverse slices at the mid-ventricular level, which in turn were sectioned into 8 segments each. Samples were divided and rapidly frozen in liquid nitrogen (molecular and immunohistochemical studies), placed in 4°C Krebs solution (microvessel studies), or weighed and dried (microsphere perfusion analysis).
All experiments were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Rhode Island Hospital. Animals were cared for in accordance with the “Principles of Laboratory Animal Care” formulated by the national society for medical research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
X-ray coronary angiography
X-ray coronary angiography with iohexol (Omnipaque, GE Health-care, Princeton, NJ) was carried out via femoral artery approach to verify LCx occlusion and assess collateral formation at the time of sacrifice. A 5-French AR-1 catheter (Cordis Corporation, Bridgewater, NJ) was advanced into the right and left coronary artery ostia and 4 mL of contrast injected per side to visualize coronary vessels. Collateral formation was assessed by a blinded cardiologist according to the Rentrop grading system of 0–3 to assess the presence and extension of coronary collateral vessel filling, as well as a myocardial blush grading system of 0–3 to assess myocardial perfusion in the ischemic territory as previously described.13
Measurement of global and regional myocardial function
Heart rate, mean arterial pressure, developed left ventricular pressure, first derivative of LV pressure, and regional myocardial function in the ischemic area at risk (AAR) as measured by vertical and horizontal segmental shortening were recorded before cardiac harvest using single-sensor pressure catheters (Millar Instruments, Houston, TX) and the Sonometrics system (Sonometrics Corp. London, ON, Canada) as previously described.14
Myocardial perfusion analysis
Myocardial perfusion was measured via isotope-labeled microspheres (Biophysics Assay Laboratories) as described previously.11 Briefly, 1.5 × 107 gold-labeled microspheres were injected during temporary LCx occlusion at to identify the AAR. Lutetium-labeled (resting heart rate) and Europium-labeled (ventricular pacing to 150 beats/min) microspheres were injected at the final procedure while simultaneously withdrawing arterial blood from a femoral artery catheter. LV samples were dried in a 60°C oven for ≥48 hours, then exposed to neutron beams and microsphere densities measured using a gamma counter (Biophysics Assay Laboratories). Myocardial blood flow in the AAR was determined using the following equation:
Microvessel studies
Coronary arterioles (80–180 μm diameter) from the AAR were isolated as described previously.12 Vessels were preconstricted with thromboxane-A2 analog U46619 (0.1–1.0 μmol/L), then treated with endothelium-independent vasodilator sodium nitroprusside (10−9–10−4 mol/L) or endothelium-dependent vasodilator adenosine 5′-diphosphate (ADP, 10−9–10−4 mol/L). Responses were defined as percent relaxation of the preconstricted diameter. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
Immunohistochemistry
12-μm-thick frozen sections of myocardium from the AAR were formalin fixed, then incubated with goat antibody against endothelium-specific CD-31 (R&D Systems) followed by DyLight-conjugated anti-goat secondary antibody (Jackson ImmunoResearch, West Grove, PA), or mouse antibody against smooth muscle actin (Abcam, Cambridge, MA) followed by Dylight-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch). Sections were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) under 20× magnification. Capillaries (defined as CD31-positive structures between 5 and 25 μm2 in cross-sectional area) and arterioles (defined as smooth muscle actin-positive structures that co-stained with CD31) from 3 random, high-power fields per section were counted using Image J software (National Institutes of Health, Bethesda, MD) and averaged. Data are presented as vessels per square millimeters.
Immunoblotting and oxidative stress assay
Sixty micrograms of total protein from AAR homogenates were fractionated by SDS-PAGE (Invitrogen, San Diego, CA) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were incubated with antibodies against the pro-angiogenic proteins pan-Akt and phospho-endothelial nitric oxide synthase (eNOS; Ser1177; Cell Signaling Technology, Danvers, MA), the oxidative stress marker myeloperoxidase (Athens Research and Technology, Athens, GA), and cell–cell adhesion proteins VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-VE-cadherin (Abcam), and β-catenin (BD Biosciences, San Jose, CA) at dilutions recommended by the manufacturer, followed by the appropriate horse-radish peroxidase-linked secondary antibodies (Jackson ImmunoResearch). Blotting for oxidative stress was carried out via dinitrophenylhydrazine derivatization of tissue homogenates using the Oxyblot kit (Millipore). Immune complexes were visualized via chemiluminescence (Amersham, Piscataway, NJ) and photographed using GeneSnap software (Syngene, Cambridge, England). Densitometry was performed using Image J software (National Institutes of Health). Ponceau staining was used to ensure equal protein loading.
Statistical analysis
All results are presented as mean values ± SEM. Microvessel responses are expressed as percent relaxation of the preconstricted diameter, and were analyzed using 2-way, repeated-measures analysis of variance with a post hoc Bonferroni test. All other comparisons were carried out using 1-way analysis of variance a post hoc Bonferroni test using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA).
RESULTS
Animal model
Nine animals were assigned originally to each group at the start of the experiment. All 9 HCC animals survived to the end of the experiment, but 1 demonstrated incomplete LCx occlusion at the time of humane killing and was, therefore, excluded from analysis. Two HCV animals died before completion of the experiment—the first 10 days after ameroid placement, and the second on the day of drug pump placement, both presumably owing to acute arrhythmia leading to sudden cardiac death. Of the original 9 HCVR animals, 3 died from acute arrhythmia during induction of anesthesia directly before VEGF pump placement. Two additional animals were added to bring the HCVR group to a total of 8. Body mass index at the time of humane killing, calculated as body weight in kilograms divided by the square of the pigs length from the end of the snout to the base of the tail in meters, was not significantly different between groups (35.5 ± 1.24 kg/m2 in HCC, 37.7 ± 1.0 kg/m2 in HCV, and 35.2 ± 1.6 kg/m2 in HCVR; P = .13).
Serum chemistries
There was no difference between groups with respect to total cholesterol, although there was a strong trend toward greater cholesterol levels in the HCVR group (460.0 ± 68.2 mg/dL in HCC, 401.0 ± 28.9 mg/dL in HCV, and 574.9 ± 31.4 mg/dL in HCVR; P = .06). The ratio of HDL:LDL (0.33 ± 0.04 in HCC, 0.26 ± 0.04 in HCV, and 0.30 ± 0.02 in HCVR; P = .3), and serum triglycerides (24.4 ± 4.4 mg/dL in HCC, 17.7 ± 3.8 mg/dL in HCV, and 18.9 ± 3.1 mg/dL in HCVR; P = .4) were not different between groups. Blood glucose responses to dextrose challenge were also similar between groups at 30 minutes (177 ± 5 mg/dL in HCC, 175 ± 10 mg/dL in HCV, and 180 ± 18 mg/dL in HCVR) and at 60 minutes (104 ± 7 mg/dL in HCC, 92 ± 13 mg/dL in HCV, and 88 ± 26 mg/dL in HCVR; P = .98).
Angiographic assessment of collateral formation
There was no significant difference in the mean Rentrop score (1.13 ± 0.35 in HCC, 0.71 ± 0.18 in HCV, and 0.78 ± 0.28 in HCVR; P = .6) or blush score (0.25 ± 0.16 in HCC, 0.86 ± 0.14 in HCV, and 1.00 ± 0.33 in HCVR; P = .1) between the groups.
Global and regional left ventricular function
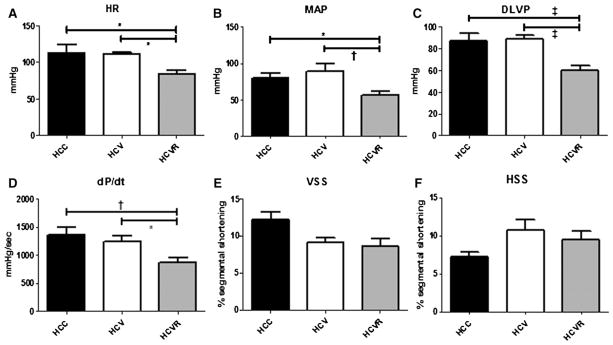
Heart rate, mean arterial pressure, developed left ventricular pressure, and first derivative of LV pressure were all significantly decreased in the HCVR group compared with the HCC and HCV groups (Fig 1, A–D). There was no difference between groups with respect to myocardial function in the AAR as measured by vertical or horizontal segmental shortening (Fig 1, E and F).
Fig 1.
Measurements of global and regional function. Shown are heart rate (HR), mean arterial pressure (MAP), developed left ventricular pressure (DLVP), derivative of LVP over time (dP/dt), and vertical segmental shortening (VSS) and horizontal segmental shortening (HSS) in the AAR. Measurements of global LV function were decreased in the HCVR group (A–D), whereas there was no difference in regional function in the AAR between groups (E, F).
*P < .05; †P < .01; ‡P < .001. Data are presented as mean values ± SEM.
Myocardial blood flow
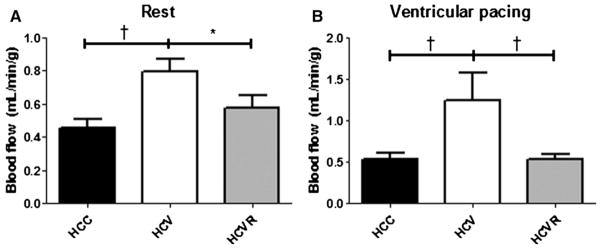
Blood flow in the AAR was increased in the HCV group compared with both the HCC and HCVR groups both at rest (P < .05) and under ventricular pacing (P < .05; Fig 2, A and B).
Fig 2.
Myocardial perfusion. Myocardial perfusion was significantly higher in the HCV group compared with both other groups at rest (A) and under ventricular pacing to 150 beats/min (B). *P < .05; †P < .01.
Microvessel reactivity studies
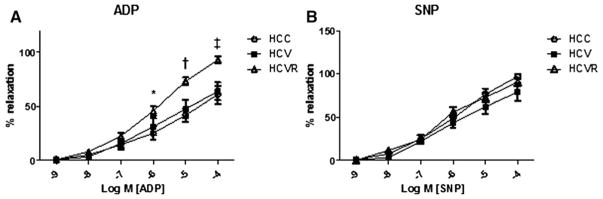
Microvessel relaxation response to endothelium-dependent vasodilator ADP was significantly greater in the HCVR group than either the HCC or HCV groups (P <.001), and relaxation response to endothelium-independent vasodilator sodium nitroprusside was not different between groups (Fig 3, A and B).
Fig 3.
Microvessel function: Dose–response curves to endothelium-dependent vasodilator ADP (A) and endothelium-independent vasodilator sodium nitroprusside (B). Vessel relaxation response to each drug was measured and expressed as a percentage of the preconstricted vessel diameter. The relaxation response to ADP was significantly better in the HCVR group compared with both other groups. *P < .05; †P < .01; ‡P < .001.
Arteriolar and capillary density
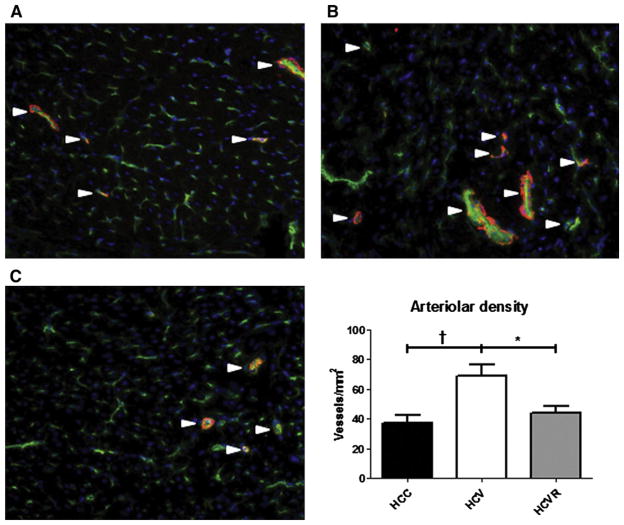
Arteriolar density was significantly higher in the HCV group compared with both the HCC and HCVR groups (P <.05; Fig 4, A), although there was no significant difference in capillary density between groups (833 ± 71 vessels/mm2 in HCC, 637 ± 41 vessels/mm2 in HCV, and 867 ± 73 vessels/mm2 in HCVR; P = .07).
Fig 4.
Arteriolar density. Sections from the AAR were stained for endothelium-specific CD31 (green), smooth muscle actin (red), and nucleus-specific DAPI (blue). Shown are representative sections from (A) HCC, (B) HCV, and (C) HCVR animals. Arterioles were defined as structures co-staining for both CD-31 and smooth muscle actin (white arrows). Arteriolar density was significantly greater in the HCV group compared with both other groups. *P < .05; †P < .01.
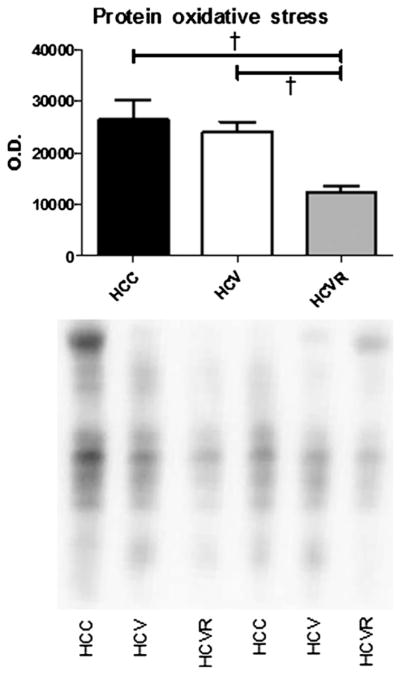
Protein oxidative stress
Total protein oxidative stress was decreased in the HCVR group compared with both the HCC and the HCV groups (P < .01; Fig 5).
Fig 5.
Protein oxidative stress. Immunoblotting was used to quantify total protein oxidative stress in the AAR. Representative lanes are shown. Densitometry was used to measure signal intensity of the entire lane, which is expressed as arbitrary units of optical density (OD). The HCVR group demonstrated significantly lower oxidative stress than both other groups. †P < .01.
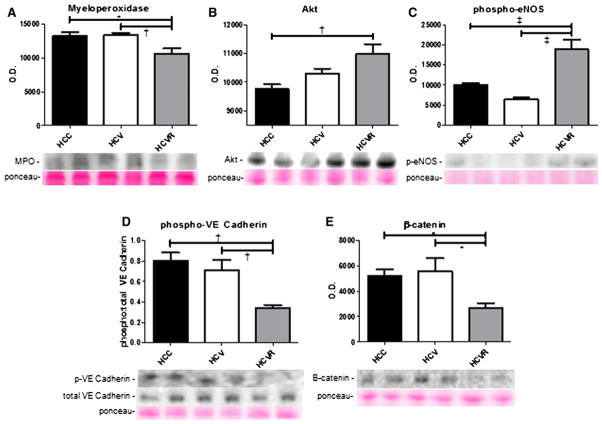
Immunoblotting
Immunoblotting results are summarized in Fig 6. Expression of the oxidative stress marker myeloperoxidase in the AAR was significantly lower in the HCVR group compared with the HCC and HCV groups (P <.05). Akt expression was greater in the HCVR group compared with the HCC group (P <.01), and tended to be greater compared with the HVR group as well, although this did not attain significance. Phospho-eNOS expression was greater in the HCVR group compared with both other groups (P < .001). Normalized expression of phospho-VE cadherin was lower in the HVR group compared with both the HCC and HCV groups (P < .01), as was the expression of β-catenin (P <.05).
Fig 6.
Protein expression. Shown are representative bands and quantification of protein expression in the AAR expressed in arbitrary units of optical density (OD). Expression of myeloperoxidase was significantly lower in the HCVR group (A), whereas pro-angiogenic and vasodilatory proteins Akt and phospho-eNOS were more highly expressed in the HCVR group (B, C). Expression of phospho-VE cadherin and β-catenin was significantly lower in the HCVR group (D, E).
DISCUSSION
Previous studies in animal models of chronic ischemia showed a robust improvement in collateral vessel formation in response to locally delivered VEGF, fibroblast growth factor-2, and other pro-angiogenic growth factors.4,15 Despite these initial favorable preclinical studies, clinical trials using these same growth factors have been disappointing.5,16 This difference between preclinical and clinical efficacy is thought to be due to the presence of advanced atherosclerosis and endothelial dysfunction in cardiac patients compared with relatively normal, healthy animals.17 Subsequent experiments from our laboratory18,19 and that of others20 using hypercholesterolemic or diabetic animal models have found a marked reduction in both endogenous, nonstimulated angiogenesis and growth factor-stimulated collateral formation.12,21
Resveratrol is a component of red wine that has been found to have potential therapeutic effects, in part owing to a reduction in tissue oxidative stress in addition to activating the SIRT family of signaling pathways. Previous studies have found that resveratrol improves collateral dependent perfusion in the ischemic myocardium of hyper-cholesterolemic swine, but failed to increased collateral vessel density.11 We postulated that the antioxidant properties of resveratrol would improve endothelial dysfunction and increase the VEGF-stimulated formation of collateral vessels. What we found was quite the opposite, that myocardial perfusion and collateral vessel density were decreased with the coadministration of resveratrol. Because angiogenesis is dependent on a certain level of oxidative stress,22 the antioxidant effects of resveratrol may actually inhibit the angiogenic effects of VEGF.
All 3 groups of swine were successfully made hypercholesterolemic, and there was no difference in the degree of hypercholesterolemia or response to dextrose challenge between groups. Although we found previously that high-dose oral resveratrol decreased serum cholesterol levels by about 30%,11 this study did not demonstrate a similar effect; in fact, cholesterol tended to be greater in the HCRV group. This could be because we chose to use a 10-fold lesser dose of the drug in this experiment, first to more closely reflect a clinically relevant dosage, and second because high doses of resveratrol have since been suggested to activate anti-angiogenic proteins such as angiostatin and thrombospondin.23
All animals included in the study demonstrated successful occlusion of the LCx, but there were no differences in angiographic measures of collateralization between groups. This is not altogether surprising; correlation between angiography and myocardial perfusion is somewhat limited by the resolution of the study and by vessel remodeling.24
Resveratrol has not been reported to alter global myocardial function in other studies, nor have we seen such an effect in our previous study investigating high-dose resveratrol in a swine model. Although it is possible that the alterations in hemodynamic parameters seen in this study may be related to the decreased arteriogenesis and myocardial perfusion associated with resveratrol treatment, many additional factors contribute to global left ventricular function, and it is difficult to ascribe this effect to the drug treatment alone.
We found that resveratrol coadministration increased expression of Akt and phospho-eNOS and a decrease in both total oxidative stress and myeloperoxidase, a marker of oxidation. eNOS, when phosphorylated by Akt, synthesizes the potent vasodilator nitric oxide.25 Antioxidants such as resveratrol also enhance the bioavailability of nitric oxide by decreasing circulating free radicals that can inactivate it.26 These 2 factors likely contribute to the improved endothelium-dependent microvessel relaxation we observed in the resveratrol-treated animals.
Despite these beneficial effects, our findings suggest that arteriogenesis is a more important determinant of myocardial perfusion than microvessel function. Arteriogenesis refers specifically to the maturation of blood vessels into smooth-muscle–lined arterioles. Recent evidence shows that resveratrol may inhibit smooth muscle cell proliferation, as well as downregulate the VEGF pathway.27,28 Although these effects are hypothesized to be the basis for the drug’s antiatherosclerotic and anticancer effects, they may also confer anti-arteriogenic effects.
VE-cadherin is an adhesion protein that maintains vascular endothelial cell–cell junctions, while β-catenin stabilizes VE-cadherin. VEGF induces the phosphorylation and subsequent endocytosis of VE-cadherin, as well as the nitrosylation and disassociation of β-catenin, leading to endothelial permeability.29,30 Others have shown that a degree of oxidative stress is required for vascular permeability, and that the antioxidant resveratrol inhibits the phosphorylation of VE-cadherin and decreases tube formation by human umbilical endothelial cells.31 Thus, inhibition of the oxidative stress–dependent phosphorylation of VE-cadherin may be the mechanism by which resveratrol inhibits arteriogenesis.
In this study, we attempted to improve the post-ischemic angiogenic efficacy of perivascular VEGF in the setting of endothelial dysfunction by co-treating swine with the antioxidant resveratrol, but found that resveratrol actually inhibited the arteriogenic effects of VEGF. Recent evidence shows that angiogenesis requires oxidative stress; therefore, removing oxidative stress with resveratrol treatment likely limits angiogenesis. Nonetheless, resveratrol did demonstrate beneficial effects with regards to decreasing oxidative stress and improving endothelium-dependent vasorelaxation. A future study using a greater dose of resveratrol that is more effective in decreasing serum cholesterol would potentially be very interesting; however, our current study demonstrates that the use of antioxidants in conjunction with VEGF therapy may have unexpectedly undesirable effects.
Some limitations to this study should be mentioned. First, we noted that during the final operation, the HCVR group tended to have decreased global cardiac function compared with the other groups. Although this should not significantly affect our results, it is a potential limitation of the study. In addition, we specifically chose a 10-fold lesser dose of resveratrol than that used in our previous study to reflect a more clinically applicable dose. The lesser dose did not decrease serum cholesterol, as have the greater doses have in previous experiments11; in fact, there was an unexpectedly strong trend toward increased cholesterol in the resveratrol-treated group. Different doses of the drug may have different effects on cholesterol metabolism and the angiogenic response. Finally, as with many large animal studies, the relatively small numbers used per group does reduce the precision of this study.
Acknowledgments
Funded by grants from the National Heart, Lung, and Blood Institute (RO1HL46716, RO1HL69024, and RO1HL85647, Dr. Sellke), NIH training grant T32-HL094300 (Dr. Chu), the Irving Bard Memorial Fellowship (Drs. Chu and Robich), NIH training grants 5T32-HL0074 and T32-HL007734 (Dr. Robich), and the DeBakey foundation at Balamand University (Dr. Laham).
Dr. Frank W. Sellke has research support from Ikaria (Clinton, NJ) and Orthologic (Tempe, AZ), and is a consultant for Novo Nordisk (Princeton, NJ), and Cubist Pharmaceuticals (Lexington, MA) and Pfizer (Princeton, NJ).
The authors thank the Beth Israel Deaconess Animal Research Facility and the Rhode Island Hospital Central Research Facility for their excellent care of the animals used in this study.
Footnotes
Presented at the 6th annual Academic Surgical Congress, February 3, 2011, Huntington Beach, CA.
References
- 1.Sodha NR, Chu LM, Boodhwani M, Sellke FW. Pharmacotherapy for end-stage coronary artery disease. Expert Opin Pharmacother. 2010;11:207–13. doi: 10.1517/14656560903439737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon YS, Johnson IA, Park JS, Diaz L, Losordo DW. Therapeutic myocardial angiogenesis with vascular endothelial growth factors. Mol Cell Biochem. 2004;264:63–74. doi: 10.1023/b:mcbi.0000044375.33928.62. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez JJ, Laham RJ, Stamler A, Pearlman JD, Bunting S, Kaplan A, et al. VEGF administration in chronic myocardial ischemia in pigs. Cardiovasc Res. 1998;40:272–81. doi: 10.1016/s0008-6363(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 5.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 6.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 7.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CP, Odewale I, Alcendor RR, Sadoshima J. Sirt1 protects the heart from aging and stress. Biol Chem. 2008;389:221–31. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 9.Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54:268–76. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- 10.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–39. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122(11 Suppl):S142–9. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voisine P, Bianchi C, Ruel M, Malk T, Rosinberg A, Feng J, et al. Inhibition of the cardiac angiogenic response to exogenous vascular endothelial growth factor. Surgery. 2004;136:407–15. doi: 10.1016/j.surg.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Henriques JP, Zijlstra F, van’t Hof AW, de Boer MJ, Dambrink JH, Gosselink M, et al. Angiographic assessment of re-perfusion in acute myocardial infarction by myocardial grade. Circulation. 2003;107:2115–9. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 14.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–13. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther. 2000;292:795–802. [PubMed] [Google Scholar]
- 16.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double blind, randomized, controlled clinical trial. Circulation. 2002;105:788–93. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 17.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 18.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic ischemia in diabetes. Circulation. 2007;16(11 Suppl):131–7. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–41. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 20.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, et al. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–8. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 21.Ruel M, Wu GF, Khan TA, Voisine P, Bianchi C, Li J, et al. Inhibition of the cardiac angiogenic response to surgical FGF-2 therapy in a swine endothelial dysfunction model. Circulation. 2003;108(Suppl 1):1335–40. doi: 10.1161/01.cir.0000087903.75204.ad. [DOI] [PubMed] [Google Scholar]
- 22.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–6. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robich MP, Chu LM, Chaudray M, Nezafat R, Han Y, Clements RT, et al. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 2010;148:453–62. doi: 10.1016/j.surg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoefer IE, van Royen N, Jost MM. Experimental models of arteriogenesis: differences and implications. Lab Anim (NY) 2006;35:36–44. doi: 10.1038/laban0206-36. [DOI] [PubMed] [Google Scholar]
- 25.Duran WN, Breslin JW, Sanchez FA. The NO cascade, eNOS location and microvascular permeability. Cardiovasc Res. 2010;87:254–61. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, Lin FY, et al. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol. 2010;30:869–77. doi: 10.1161/ATVBAHA.109.200618. [DOI] [PubMed] [Google Scholar]
- 27.Poussier B, Cordova AC, Becquemin JP, Sumpio BE. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005;42:1190–7. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Uchiyama T, Toda K, Takahashi T. Resveratrol inhibits angiogenic response of cultured endothelial F-2 cells to vascular endothelial growth factor, but not to basic fibroblast growth factor. Biol Pharm Bull. 2010;33:1095–100. doi: 10.1248/bpb.33.1095. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki N. Vascular integrity mediated by vascular endothelial cadherin and regulated by sphingosine 1-phosphate and angiopoietin-1. Circ J. 2009;73:2183–91. doi: 10.1253/circj.cj-09-0666. [DOI] [PubMed] [Google Scholar]
- 30.Thibeault S, Rautureau Y, Oubah M, Faubert D, Wilkes BC, Delisle C, et al. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell. 2010;39:468–76. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–36. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]