Abstract
Cooperation is needed for evolution to construct new levels of organization. The emergence of genomes, cells, multi-cellular organisms, social insects and human society are all based on cooperation. Cooperation means that selfish replicators forgo some of their reproductive potential to help one another. But natural selection implies competition and therefore opposes cooperation unless a specific mechanism is at work. Here I discuss five mechanisms for the evolution of cooperation: kin selection, direct reciprocity, indirect reciprocity, network reciprocity and group selection. For each mechanism, a simple rule is derived which specifies whether natural selection can lead to cooperation.
Evolution is based on a fierce competition between individuals and should therefore only reward selfish behavior. Every gene, every cell and every organism should be designed to promote its own evolutionary success at the expense of its competitors. Yet we observe cooperation on many levels of biological organization. Genes cooperate in genomes. Chromosomes cooperate in eukaryotic cells. Cells cooperate in multi-cellular organisms. There are many examples for cooperation among animals. Humans are the champions of cooperation: from hunter gatherer societies to nation states, cooperation is the decisive organizing principle of human society. No other life form on earth is engaged in the same complex games of cooperation and defection. The question how natural selection can lead to cooperative behavior has fascinated evolutionary biologists for several decades.
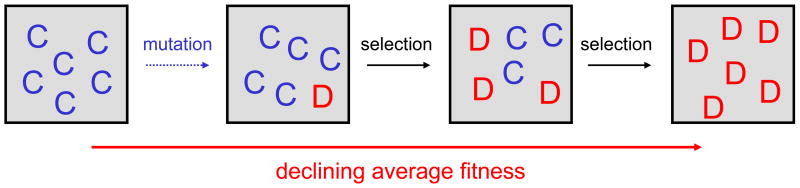
A cooperator is someone who pays a cost, c, for another individual to receive a benefit, b. A defector has no cost and does not deal out benefits. Cost and benefit are measured in terms of fitness. Reproduction can be genetic or cultural. In any mixed population, defectors have a higher average fitness than cooperators (Fig 1). Therefore, selection acts to increase the relative abundance of defectors. After some time cooperators vanish from the population. Remarkably, however, a population of only cooperators has the highest average fitness, while a population of only defectors has the lowest. Thus, natural selection constantly reduces the average fitness of the population. Fisher's fundamental theorem, which states that average fitness increases under constant selection, does not apply here because selection is frequency dependent: the fitness of individuals depends on the frequency (=relative abundance) of cooperators in the population. We see that natural selection in well-mixed populations needs help for establishing cooperation.
Figure 1.
Without any mechanism for the evolution of cooperation, natural selection favors defectors. In a mixed population defectors, D, have a higher payoff (=fitness) than cooperators, C. Therefore, natural selection continuously reduces the abundance, i, of cooperators until they are extinct. The average fitness of the population also declines under natural selection. The total population size is given by N. There are i cooperators and N − i defectors. The fitness of cooperators and defectors is respectively given by fC = b(i − 1)/(N − 1) − c and fD = bi/(N − 1). The average fitness of the population is given by f̅ = (b − c)i/N.
Kin selection
When JBS Haldane remarked ‘I will jump into the river to save two brothers or eight cousins’, he anticipated what became later known as Hamilton's rule (1). The ingenious idea is that natural selection can favor cooperation if the donor and the recipient of an altruistic act are genetic relatives. More precisely, Hamilton's rule states that the coefficient of relatedness, r, must exceed the cost-to-benefit ratio of the altruistic act:
| (1) |
Relatedness is defined as the probability of sharing a gene. The probability that two brothers share the same gene by descent is 1/2, while the same probability for cousins is 1/8. Hamilton's theory became widely known as ‘kin selection’ or ‘inclusive fitness’(2-7). When evaluating the fitness of the behavior induced by a certain gene it is important to include the behavior's effect on kin who might carry the same gene. Therefore, the ‘extended phenotype’ of cooperative behavior is the consequence of ‘selfish genes’ (8, 9).
Direct reciprocity
It is unsatisfactory to have a theory that can only explain cooperation among relatives. We also observe cooperation between unrelated individuals or even between members of different species. Such considerations led Trivers (10) to propose another mechanism for the evolution of cooperation, direct reciprocity. There are a repeated encounters between the same two individuals. In every round, each player has a choice between cooperation and defection. If I cooperate now, you may cooperate later. Hence, it might pay off to cooperate. This game theoretic framework is known as the repeated Prisoner's Dilemma.
But what is a good strategy for playing this game? In two computer tournaments, Axelrod (11) discovered that the ‘winning strategy’ was the simplest of all, tit-for-tat. This strategy always starts with a cooperation, then it does whatever the other player has done in the previous round: a cooperation for a cooperation, a defection for a defection. This simple concept captured the fascination of all enthusiasts of the repeated Prisoner's Dilemma. Many empirical and theoretical studies were inspired by Axelrod's ground breaking work (12-14).
But soon an Achilles heel of the world champion was revealed: if there are erroneous moves, caused by ‘trembling hands’ or ‘fuzzy minds’, then the performance of tit-for-tat declines (15,16). Tit-for-tat cannot correct mistakes, because an accidental defection leads to a long sequence of retaliation. At first, tit-for-tat was replaced by generous-tit-for-tat (17), a strategy which cooperates whenever you cooperate, but sometimes cooperates although you have defected (with probability 1 − c/b). Natural selection can promote forgiveness.
Subsequently, tit-for-tat was replaced by win-stay, lose-shift, which is the even simpler idea of repeating your previous move whenever you are doing well, but changing otherwise (18). By various measures of success, win-stay, lose-shift is more robust than either tit-for-tat or generous-tit-for-tat (15,18). Tit-for-tat is an efficient catalyst of cooperation in a society where nearly everybody is a defector, but once cooperation is established win-stay, lose-shift is better able to maintain it.
The number of possible strategies for the repeated Prisoner's Dilemma is unlimited, but a simple, general rule can be shown without any difficulty. Direct reciprocity can only lead to the evolution of cooperation if the probability, w, of another encounter between the same two individuals exceeds the cost-to-benefit ratio of the altruistic act:
| (2) |
Indirect reciprocity
Direct reciprocity is a powerful mechanism for the evolution of cooperation, but leaves out certain aspects that are particularly important for humans. Direct reciprocity relies on repeated encounters between the same two individuals, and both individuals must be able to provide help, which is less costly for the donor than beneficial for the recipient. But often the interactions among humans are asymmetric and fleeting. One person is in a position to help another, but there is no possibility for a direct reciprocation. We help strangers who are in need. We donate to charities that do not donate to us. Direct reciprocity is like a barter economy based on the immediate exchange of goods, while indirect reciprocity resembles the invention of money. The money that fuels the engines of indirect reciprocity is reputation.
Helping someone establishes a good reputation, which will be rewarded by others. When deciding how to act we take into account the possible consequences for our reputation. We feel strongly about events that affect us directly, but also take a keen interest in the affairs of others, as demonstrated by the contents of gossip.
In the standard framework of indirect reciprocity, there are randomly chosen, pairwise encounters, where the same two individuals need not meet again. One individual acts as donor the other as recipient. The donor can decide whether or not to cooperate. The interaction is observed by a subset of the population who might inform others. Reputation allows evolution of cooperation by indirect reciprocity (19). Natural selection favors strategies that base the decision to help on the reputation of the recipient. Theoretical and empirical studies of indirect reciprocity show that people who are more helpful are more likely to receive help (20-28).
Although simple forms of indirect reciprocity can be found in animals (29), only humans seem to engage in the full complexity of the game. Indirect reciprocity has substantial cognitive demands. Not only do we have to remember our own interactions, but also monitor the ever-changing social network of the group. Language is needed to gain the information and spread the gossip associated with indirect reciprocity. Presumably, the selection for indirect reciprocity and human language has played a decisive role in the evolution of human intelligence (28). Indirect reciprocity also leads to the evolution of morality (30) and social norms (21,22).
The calculations of indirect reciprocity are complicated and only a tiny fraction of this universe has been uncovered, but again a simple rule has emerged (19). Indirect reciprocity can only promote cooperation if the probability, q, to know someone's reputation exceeds the cost-to-benefit ratio of the altruistic act:
| (3) |
Network reciprocity
The argument for natural selection of defection (Fig 1) is based on a well-mixed population, where everybody interacts equally likely with everybody else. This approximation is used by all standard approaches to evolutionary game dynamics (31-34). But real populations are not well-mixed. Spatial structures or social networks imply that some individuals interact more often than others. One approach of capturing this effect is evolutionary graph theory (35), which allows us to study how spatial structure affects evolutionary and ecological dynamics (36-39).
The individuals of a population occupy the vertices of a graph. The edges determine who interacts with whom. Let us consider plain cooperators and defectors without any strategic complexity. A cooperator pays a cost, c, for each neighbor to receive a benefit, b. Defectors have no costs, and their neighbors receive no benefits. In this setting, cooperators can prevail by forming network clusters, where they help each other. The resulting ‘network reciprocity’ is a generalization of ‘spatial reciprocity’ (40).
Games on graphs are easy to study by computer simulation, but difficult to analyze mathematically because of the enormous number of possible configurations that can arise. Nevertheless, a surprisingly simple rule determines if network reciprocity can favor cooperation (41). The benefit-to-cost ratio must exceed the average number of neighbors, k, per individual:
| (4) |
Group selection
Selection does not only act on individuals but also on groups. A group of cooperators might be more successful than a group of defectors. There have been many theoretical and empirical studies of group selection with some controversy, and most recently there is a renaissance of such ideas under the heading of ‘multi-level selection’ (42-50).
A simple model of group selection works as follows (51). A population is subdivided into groups. Cooperators help others in their own group. Defectors do not help. Individuals reproduce proportional to their payoff. Offspring are added to the same group. If a group reaches a certain size it can split into two. In this case, another group becomes extinct in order to constrain the total population size. Note that only individuals reproduce, but selection emerges on two levels. There is competition between groups because some groups grow faster and split more often. In particular, pure cooperator groups grow faster than pure defector groups, while in any mixed group defectors reproduce faster than cooperators. Therefore, selection on the lower level (within groups) favors defectors, while selection on the higher level (between groups) favors cooperators. This model is based on ‘group fecundity selection’, which means groups of cooperators have a higher rate of splitting in two. We can also imagine a model based on ‘group viability selection’, where groups of cooperators are less likely to go extinct.
In the mathematically convenient limit of weak selection and rare group splitting, we obtain a simple result (51): if n is the maximum group size and m the number of groups, then group selection allows evolution of cooperation provided
| (5) |
Evolutionary success
Before proceeding to a comparative analysis of the five mechanisms, let me introduce some measures of evolutionary success. Suppose a game between two strategies, cooperators C and defectors D, is given by the payoff matrix
The entries denote the payoff for the row player. Without any mechanism for the evolution of cooperation, defectors dominate cooperators, which means α < γ and β < δ. A mechanism for the evolution of cooperation can change these inequalities.
If α > γ then cooperation is an evolutionarily stable strategy (ESS). An infinitely large population of cooperators cannot be invaded by defectors under deterministic selection dynamics (32).
If α + β > γ + δ then cooperators are risk-dominant (RD). If both strategies are ESS, then the risk dominant strategy has the bigger basin of attraction.
If α + 2β > γ + 2δ then cooperators are advantageous (AD). This concept is important for stochastic game dynamics in finite populations. Here, the crucial quantity is the fixation probability of a strategy, defined as the probability that the lineage arising from a single mutant of that strategy will take over the entire population consisting of the other strategy. An advantageous strategy has a fixation probability greater than the inverse of the population size, 1/N. The condition can also be expressed as a 1/3 rule: if the fitness of the invading strategy at a frequency of 1/3 is greater than the fitness of the resident, then the fixation probability of the invader is greater than 1/N. This condition holds in the limit of weak selection (52).
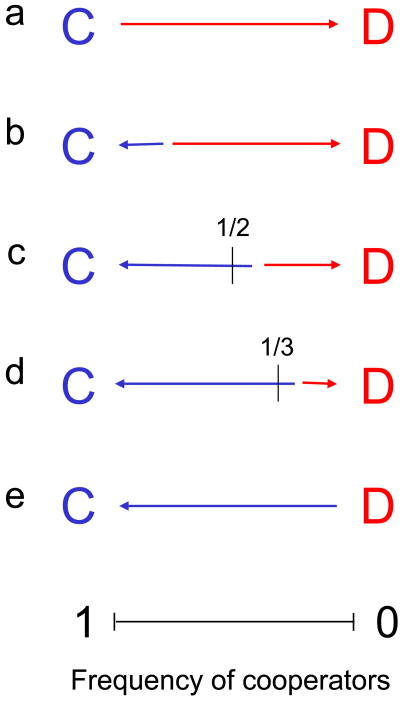
A mechanism for the evolution of cooperation can ensure that cooperators become ESS, RD or AD (Fig 2). Some mechanisms even allow cooperators to dominate defectors, which means α > γ and β > δ.
Figure 2.
Evolutionary dynamics of cooperators and defectors. The red and blue arrows indicate selection favoring defectors and cooperators, respectively. (a) Without any mechanism for the evolution of cooperation, defectors dominate. A mechanism for evolution of cooperation can allow cooperators to be the evolutionarily stable strategy (ESS), risk dominant (RD) or advantageous (AD) in comparison with defectors. (b) Cooperators are ESS if they can resist invasion by defectors. (c) Cooperators are RD if the basin of attraction of defectors is less than 1/2. (d) Cooperators are AD if the basin of attraction of defectors is less that 1/3. In this case, the fixation probability of a single cooperator in a finite population of defectors is greater than the inverse of the population size (for weak selection). (e) Some mechanisms allow cooperators to dominate defectors.
Comparative analysis
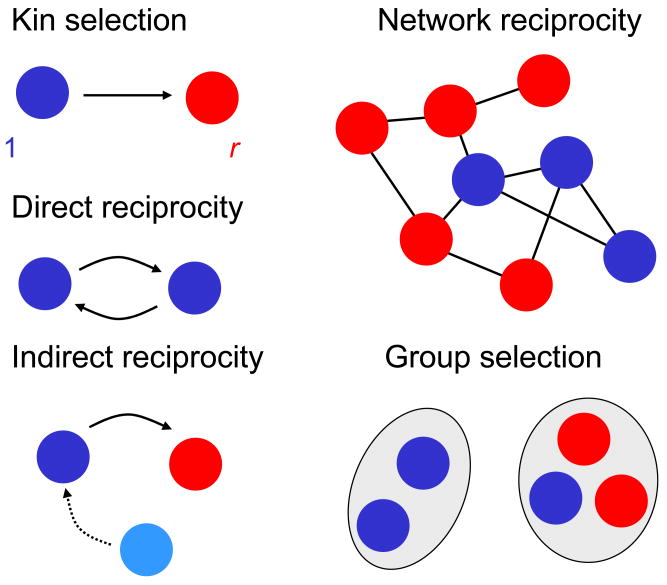
We have encountered five mechanisms for the evolution of cooperation (Fig 3). Although the mathematical formalisms underlying the five mechanisms are very different, at the center of each theory is a simple rule. I will now present a coherent mathematical framework that allows the derivation of all five rules. The crucial idea is that each mechanism can be presented as a game between two strategies given by a 2 × 2 payoff matrix (Table 1). From this matrix, we can derive the relevant condition for evolution of cooperation.
Figure 3.
Five mechanisms for the evolution of cooperation. Kin selection operates when the donor and the recipient of an altruistic act are genetic relatives. Direct reciprocity requires repeated encounters between the same two individuals. Indirect reciprocity is based on reputation; a helpful individual is more likely to receive help. Network reciprocity means that clusters of cooperators outcompete defectors. Group selection is the idea that competition is not only between individuals but also between groups.
Table 1.
Each mechanism can be described by a simple 2 × 2 payoff matrix, which specifies the interaction between cooperators and defectors. From these matrices we can directly derive the necessary conditions for evolution of cooperation. The parameters c and b denote, respectively, the cost for the donor and the benefit for the recipient. All conditions can be expressed as the benefit-to-cost ratio exceeding a critical value. The concepts of evolutionarily stable (ESS), risk-dominant (RD) and advantageous strategies (AD) are defined in the text. Further explanations of the underlying calculations can be found in the Supporting Online Material (53).
| Payoff matrix | Cooperation is … | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ESS | RD | AD | |||||||
| Kin selection |
|
|
|
|
r…genetic relatedness | ||||
|
| |||||||||
| Direct reciprocity |
|
|
|
|
w…probability of next round | ||||
|
| |||||||||
| Indirect reciprocity |
|
|
|
|
q…social acquaintanceship | ||||
|
| |||||||||
| Network reciprocity |
|
|
|
|
k…number of neighbors |
||||
|
| |||||||||
| Group selection |
|
|
|
|
n…group size m…number of groups |
||||
For kin selection, I use the approach of inclusive fitness proposed by Maynard Smith. The relatedness between two players is r. Therefore, your payoff multiplied by r is added to mine. A second method, shown in the Supporting Online Material (53), leads to a different matrix but the same result. For direct reciprocity, the cooperators use tit-for-tat while the defectors use ‘always-defect’. The expected number of rounds is 1/(1 − w). Two tit-for-tat players cooperate all the time. Tit-for-tat versus always-defect cooperates only in the first move and then defects. For indirect reciprocity, the probability to know someone's reputation is given by q. A cooperator helps unless the reputation of the other person indicates a defector. A defector never helps. For network reciprocity, it can be shown that the expected frequency of cooperators is described by a standard replicator equation using a transformed payoff matrix (54). For group selection, the payoff matrices of the two games - within and between groups - can be added up. The details of all these arguments and their limitations are given in the Supporting Online Material (53).
For kin selection, the calculation shows that Hamilton's rule, r > c/b, is the decisive criterion for all three measures of evolutionary success: ESS, RD and AD. Similarly for network reciprocity and group selection we obtain the same condition for all three evaluations, namely b/c > k and b/c > 1 + n/m, respectively. The reason is the following: if these conditions hold then cooperators dominate defectors. For direct and indirect reciprocity, we find that the ESS conditions lead to w > c/b and q > c/b, respectively. Slightly more stringent conditions have to hold for cooperation to be risk-dominant (RD) or advantageous (AD).
Conclusion
I have discussed five mechanisms for the evolution of cooperation: kin selection, direct reciprocity, indirect reciprocity, network reciprocity and group selection. Each mechanism can be described by a characteristic 2×2 payoff matrix, from which we can directly derive the fundamental rules that specify if cooperation can evolve (Table 1). Each rule can be expressed as the benefit-to-cost ratio of the altruistic act being greater than some critical value. The payoff matrices can be imported into standard frameworks of evolutionary game dynamics. For example, we can study replicator equations for games on graphs (54), for group selection and for kin selection. This creates interesting new possibilities for the theory of evolutionary dynamics (55).
I have not discussed all potential mechanisms for the evolution of cooperation. An interesting possibility is offered by ‘green beard’ models where cooperators recognize each other via arbitrary labels (56-58). Another option to obtain cooperation is making the game voluntary rather than obligatory: if players can choose between cooperation, defection or not playing at all, then some level of cooperation usually prevails in dynamic oscillations (59). Punishment is an important factor which can promote cooperation (60-64). It is unclear, however, if punishment alone constitutes a mechanism for the evolution of cooperation. All evolutionary models of punishment so far are based on underlying mechanisms such as indirect reciprocity (65), group selection (66, 67) or network reciprocity (68). Punishment can enhance the level of cooperation that is achieved in such models.
Kin selection has led to mathematical theories (based on the Price equation) which are more general than just analyzing interactions between genetic relatives (4,5). The interacting individuals can have any form of phenotypic correlation. Therefore, kin selection theory also provides an approach to compare different mechanisms for the evolution of cooperation (69, 70). I have not taken this approach in the present paper.
The two fundamental principles of evolution are mutation and natural selection. But evolution is constructive because of cooperation. New levels of organization evolve when the competing units on the lower level begin to cooperate. Cooperation allows specialization and thereby promotes biological diversity. Cooperation is the secret behind the open endedness of the evolutionary process. Perhaps the most remarkable aspect of evolution is its ability to generate cooperation in a competitive world. Thus, we might add ‘natural cooperation’ as a third fundamental principle of evolution beside mutation and natural selection.
Supplementary Material
Acknowledgments
Support from the John Templeton Foundation and the NSF/NIH joint program in mathematical biology (NIH grant 1R01GM078986-01) is gratefully acknowledged. The Program for Evolutionary Dynamics at Harvard University is sponsored by Jeffrey Epstein.
References and Notes
- 1.Hamilton WD. J Theor Biol. 1964;7:1. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 2.Grafen A. In: Oxford Surveys in Evolutionary Biology. Dawkins R, Ridley M, editors. Vol. 2. Oxford University Press; Oxford: 1985. pp. 28–89. [Google Scholar]
- 3.Taylor PD. Evol Ecol. 1992;6:352. [Google Scholar]
- 4.Queller DC. Am Nat. 1992;139:540. [Google Scholar]
- 5.Frank SA. Foundations of Social Evolution. Princeton Univ. Press; Princeton, NJ: 1998. [Google Scholar]
- 6.West SA, Pen I, Griffin AS. Science. 2002;296:72. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 7.Foster KR, Wenseleers T, Ratnieks FLW. Trends Ecol Evol. 2006;21:57. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Dawkins R. The Selfish Gene. Oxford Univ. Press; Oxford: 1976. [Google Scholar]
- 9.Wilson EO. Sociobiology. Harvard Univ. Press; Cambridge, MA: 1975. [Google Scholar]
- 10.Trivers R. Q Rev Biol. 1971;46:35. [Google Scholar]
- 11.Axelrod R. The Evolution of Cooperation. Basic Books; New York: 1984. [Google Scholar]
- 12.Axelrod R, Hamilton WD. Science. 1981;211:1390. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 13.Milinski M. Nature. 1987;325:434. doi: 10.1038/325433a0. [DOI] [PubMed] [Google Scholar]
- 14.Dugatkin LA. Cooperation Among Animals. Oxford Univ. Press; Oxford: 1997. [Google Scholar]
- 15.Fudenberg D, Maskin E. Am Econ Rev. 1990;80:274. [Google Scholar]
- 16.Selten R, Hammerstein P. Behav Brain Sci. 1984;7:115. [Google Scholar]
- 17.Nowak MA, Sigmund K. Nature. 1992;355:250. [Google Scholar]
- 18.Nowak MA, Sigmund K. Nature. 1993;364:56. doi: 10.1038/364056a0. [DOI] [PubMed] [Google Scholar]
- 19.Nowak MA, Sigmund K. Nature. 1998;393:573. doi: 10.1038/31225. [DOI] [PubMed] [Google Scholar]
- 20.Wedekind C, Milinski M. Science. 2000;288:850. doi: 10.1126/science.288.5467.850. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsuki H, Iwasa Y. J Theor Biol. 2004;231:107. doi: 10.1016/j.jtbi.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Brandt H, Sigmund K. J Theor Biol. 2004;231:475. doi: 10.1016/j.jtbi.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Leimar O, Hammerstein P. Proc R Soc London Ser B. 2001;268:745. doi: 10.1098/rspb.2000.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milinski M, Semmann D, Krambeck HJ. Nature. 2002;415:424. doi: 10.1038/415424a. [DOI] [PubMed] [Google Scholar]
- 25.Fishman MA. J Theor Biol. 2003;225:285. doi: 10.1016/s0022-5193(03)00246-7. [DOI] [PubMed] [Google Scholar]
- 26.Hauser MD, Chen MK, Chen F, Chuang E. Proc R Soc London Ser B. 2003;270:2363. doi: 10.1098/rspb.2003.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchanathan K, Boyd R. Nature. 2004;432:499. doi: 10.1038/nature02978. [DOI] [PubMed] [Google Scholar]
- 28.Nowak MA, Sigmund K. Nature. 2005;437:1291. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- 29.Bshary R, Grutter AS. Nature. 2006;441:975. doi: 10.1038/nature04755. [DOI] [PubMed] [Google Scholar]
- 30.Alexander RD. The Biology of Moral Systems. Aldine de Gruyter; New York: 1987. [Google Scholar]
- 31.Smith J Maynard. Evolution and the Theory of Games. Cambridge Univ. Press; Cambridge, UK: 1982. [Google Scholar]
- 32.Hofbauer J, Sigmund K. the book. 1998 [Google Scholar]
- 33.Hofbauer J, Sigmund K. B Am Math Soc. 2003;40:479. [Google Scholar]
- 34.Nowak MA, Sigmund K. Science. 2004;303:793. doi: 10.1126/science.1093411. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman E, Hauert C, Nowak MA. Nature. 2005;433:312. doi: 10.1038/nature03204. [DOI] [PubMed] [Google Scholar]
- 36.Durrett R, Levin SA. Theor Popul Biol. 1994;46:363. [Google Scholar]
- 37.Hassell MP, Comins HN, May RM. Nature. 1994;370:290. [Google Scholar]
- 38.Hauert C, Doebeli M. Nature. 2004;428:643. doi: 10.1038/nature02360. [DOI] [PubMed] [Google Scholar]
- 39.May RM. Trends Ecol Evol. 2006;21:394. doi: 10.1016/j.tree.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Nowak MA, May RM. Nature. 1992;359:826. [Google Scholar]
- 41.Ohtsuki H, Hauert C, Lieberman E, Nowak MA. Nature. 2006;441 doi: 10.1038/nature04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams GC, Williams DC. Evolution. 1957;11:32. [Google Scholar]
- 43.Wilson DS. Proc Natl Acad Sci USA. 1975;72:143. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor PD, Wilson DS. Evolution. 1988;42:193. doi: 10.1111/j.1558-5646.1988.tb04119.x. [DOI] [PubMed] [Google Scholar]
- 45.Rogers AR. Am Nat. 1990;135:398. [Google Scholar]
- 46.Michod RE. Darwinian Dynamics. Princeton Univ. Press; Princeton, NJ: 1999. [Google Scholar]
- 47.Keller L, editor. Levels of Selection in Evolution. Princeton Univ. Press; Princeton, NJ: 1999. [Google Scholar]
- 48.Paulsson J. Genetics. 2002;161:1373. doi: 10.1093/genetics/161.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rainey PB, Rainey K. Nature. 2003;425:72. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 50.Wilson EO, Hölldobler B. Proc Natl Acad Sci USA. 2005;102:13367. doi: 10.1073/pnas.0505858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traulsen A, Nowak MA. Proc Natl Acad Sci USA. 2006;103:10952. doi: 10.1073/pnas.0602530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nowak MA, Sasaki A, Taylor C, Fudenberg D. Nature. 2004;428:646. doi: 10.1038/nature02414. [DOI] [PubMed] [Google Scholar]
- 53.see Supporting Material at Science Online.
- 54.Ohtsuki H, Nowak MA. J Theor Biol. 2006 doi: 10.1016/j.jtbi.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowak MA. Evolutionary Dynamics. Harvard Univ. Press; Cambridge: 2006. [Google Scholar]
- 56.Riolo RL, Cohen MD, Axelrod R. Nature. 2001;414:441. doi: 10.1038/35106555. [DOI] [PubMed] [Google Scholar]
- 57.Traulsen A, Schuster HG. Phys Rev E. 2003;68:046129. doi: 10.1103/PhysRevE.68.046129. [DOI] [PubMed] [Google Scholar]
- 58.Jansen VA, van Baalen M. Nature. 2006;440:663. doi: 10.1038/nature04387. [DOI] [PubMed] [Google Scholar]
- 59.Hauert C, De Monte S, Hofbauer J, Sigmund K. Science. 2002;296:1129. doi: 10.1126/science.1070582. [DOI] [PubMed] [Google Scholar]
- 60.Clutton-Brock TH, Parker GA. Nature. 1995;373:209. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- 61.Fehr E, Gaechter S. Nature. 2002;415:137. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- 62.Fehr E, Fischbacher U. Nature. 2003;425:785. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 63.Camerer CF, Fehr E. Science. 2006;311:47. doi: 10.1126/science.1110600. [DOI] [PubMed] [Google Scholar]
- 64.Gürerk Ö, Irlenbusch B, Rockenbach B. Science. 2006;312:108. doi: 10.1126/science.1123633. [DOI] [PubMed] [Google Scholar]
- 65.Sigmund K, Hauert C, Nowak MA. Proc Natl Acad Sci USA. 2001;98:10757. doi: 10.1073/pnas.161155698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyd R, Gintis H, Bowles S, Richerson PJ. Proc Natl Acad Sci USA. 2003;100:3531. doi: 10.1073/pnas.0630443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowles S, Gintis H. Theor Popul Biol. 2004;65:17. doi: 10.1016/j.tpb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Nakamaru M, Iwasa Y. Evol Ecol Res. 2005;7:853. [Google Scholar]
- 69.Lehmann L, Keller L. J Evol Biol. 2006;19:1365. doi: 10.1111/j.1420-9101.2006.01119.x. [DOI] [PubMed] [Google Scholar]
- 70.Fletcher JA, Zwick M. Am Nat. 2006;168:252. doi: 10.1086/506529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.