Abstract
Co-translational myristoylation of the N-terminal glycine residue of diverse signaling proteins is required for membrane attachment and proper function of these molecules. The transfer of myristate from myristoyl-coenzyme A (myr-CoA) is catalyzed by the enzyme N-myristoyltransferase (Nmt). Nmt has been implicated in a number of human diseases, including cancer and epilepsy, as well as pathogenic mechanisms such as fungal and virus infections, including HIV and Hepatitis B. Rational design has led to the development of potent competitive inhibitors, including several non-hydrolysable acyl-CoA substrate analogues. However, linear synthetic strategies, following the route of the original CoA synthesis, generate such analogues in very low over all yields that typically are not sufficient for in vivo studies. Here, we present a new, highly convergent synthesis of myristoyl-carba(dethia)-coenzyme A 1 that allows to obtain this substrate analogue in 11-fold increased yield compared to the reported linear synthesis. In addition, enzymatic cleavage of the adenosine-2',3'-cyclophosphate in the last step of the synthesis proved to be an efficient way to obtain the isomerically pure 3'-phosphate 1.
Keywords: N-myristoylation, Coenzyme A, Antifungal agents, Antiviral agents, Inhibitors
Introduction
Post- or co-translational lipidation is required for the proper functioning of many proteins involved in signal transduction and cellular growth control.[i] The co-translational transfer of a myristoyl group from myristoyl-coenzyme A (myr-CoA) to an N-terminal glycine residue of substrate proteins is catalyzed by the enzyme N-myristoyltransferase (Nmt; EC 2.1.3.97).[ii] Nmt recognizes the amino-terminal motif G(X)3(S/T/A/C/N) of the nascent peptide, after the initiating methionine has been removed by methionine amino peptidase during translation. Known human N-myristoylated proteins include kinases, e.g. the Src family kinases and the catalytic subunit of cAMP-dependent protein kinase, as well as phosphatases, e.g. calcineurin.[iii] Nmt has been implicated in human diseases such as colon cancer, where N-myristoylation of the oncogene product p60c-src is required for its malign effect,[iv] or epilepsy, where myristoylation of calcineurin is involved in neuronal apoptosis.[v] Myristoylated proteins play also important roles in several pathogens such as Cryptococcus neoformans, the cause of a fungal infection,[vi] or in viruses like HIV-I,[vii,viii] Polio virus,[ix] and Hepatitis B virus.[x] For instance, assembly of HIV infectious virions is dependent on the myristoylation of gag, gag-pol, and nef viral polyprotein precursors by cellular Nmt.[vii] Thus, Nmt is regarded as a potential drug target for anticancer, antiepilepsy, antifungal, and antiviral agents.
Inhibitors of Nmt have been reported (reviewed in ref. xi,xii,xiii) and include substrate analogues, such as peptides, peptidomimetics, and myr-CoA analogues, as well as several reported small molecules, including benzofurans and benzothiazole derivatives, which showed promising antifungal activity in vivo.[xiv,xv] Rational design has led to the development of several non-hydrolysable acyl-coenzyme A analogues as potent competitive inhibitors of Nmt, including S-(2-Oxo)pentadecyl-CoA,[xvi] S-(3-oxohexadecyl)-CoA, and myristoyl-carba(dethia)-CoA.[xvii] However, one major drawback is the limited amounts that such compounds are accessible at through existing synthetic strategies. For example, myristoyl-carba(dethia)-CoA 1 (Figure 1), in which the sulphur atom of the myr-CoA thioester bond is replaced by a methylene group, was generated via a linear synthesis[xvii] that applies the route of Moffatt and Khorana's original coenzyme A synthesis.[xviii] The low overall yield of 1.7%, (referred to myristic acid) does not offer the means to easily obtain 1 or similar molecules in quantities necessary for in vivo studies. Here, we present a novel and highly convergent strategy to easily synthesize myristoyl-carba(dethia)-CoA 1 and other carba(dethia)-CoA derivatives as single isomers in high overall yields.
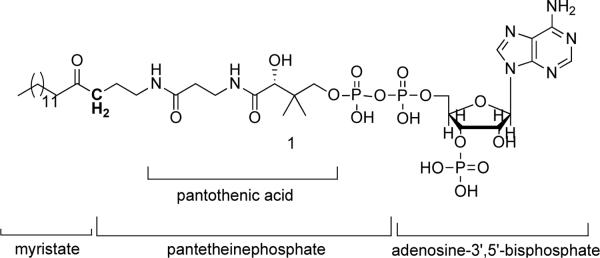
Figure 1.
Myristoyl-carba(dethia)-CoA 1. The methylene group that replaces the sulphur atom of the thioester bond in myr-CoA is highlighted in bold.
Results and Discussion
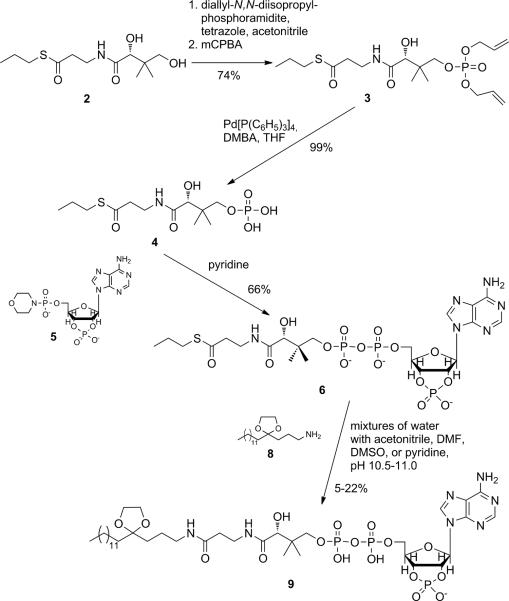
Our goal to efficiently generate myristoyl-carba(dethia)-CoA 1 prompted us to explore more convergent synthetic opportunities to access this non-hydrolysable substrate analogue and potent inhibitor of Nmt. Because the modest overall yield via the linear synthesis[xvii] is primarily due to the very low conversion during the phosphorylation reaction of myristoyl-carba(dethia)-pantetheine in the penultimate step (~30% reported,[xvii] 10% in our hands), we sought a strategy to introduce the phosphate group earlier in the synthesis. Taking a look at Mother Nature, the biosynthesis of coenzyme A starts with the phosphorylation of pantothenic acid (vitamin B5).[xix] Accordingly, Martin et al.[xx] reported a method for the generation of acetyl-carba(dethia)-CoA, in which the pantothenic acid moiety is first phosphorylated, then coupled to adenosinephosphate, and in the last step coupled with the acetate moiety via an aminolysis of the thioester-activated pantothenic acid. Inspired by the convergent strategy, we tested whether this route would also be suitable for effectively generating myristoyl-carba(dethia)-CoA 1.
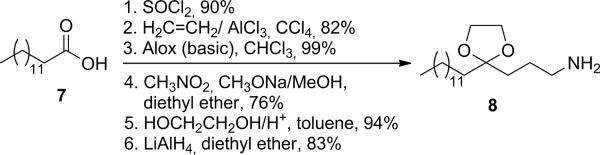
The pantothenic acid thiopropylester 2 (Scheme 1) was synthesized as reported by Martin et al.[xx] Phosphorylation of 2 was rather accomplished by applying a phosphoramidite method based on P(III)-chemistry.[xxi] Deprotection of the allylphosphate 3 yielded phosphate 4 with an overall yield of 74% in the phosphorylation step, accounting for a significant improvement over the reported 17% using dimethyl phosphorochloridate.[xx] 4 then reacted in dry pyridine under formation of a pyrophosphate bond with morpholidate 5,[xxii] yielding thioester 6. Amine 8 was generated from myristic acid 7 as described,[xvii,xxiii] with slight variations and yield improvements (Scheme 2 and Supplemental Material). The subsequent aminolysis of 6 with 8 constituted the key reaction of this convergent strategy. Martin et al.[xx] reported a 90% conversion to the amide within 20 h in phosphate buffer at pH 10.5 to generate the acetyl-CoA analogue. In our case, however, finding a common solvent or solvent mixture for 6 and 8 turned out to be problematic. We used different mixtures of water/buffer with acetonitrile, DMF, DMSO, or pyridine, at room temperature or 50°C, using NaOH or DMAP to adjust the pH between 10.5 and 11. Although product 9 was always formed, yields were found to be extremely low (between 5% and 22%).
Scheme 1.
Convergent strategy applied from Martin et al.'s acetyl-carba(dethia)-CoA synthesis,[xx] involving an aminolysis reaction of amine 8 and thioester 6 in the penultimate step.
Scheme 2.
Synthesis of amine 8, starting from myristic acid 7.
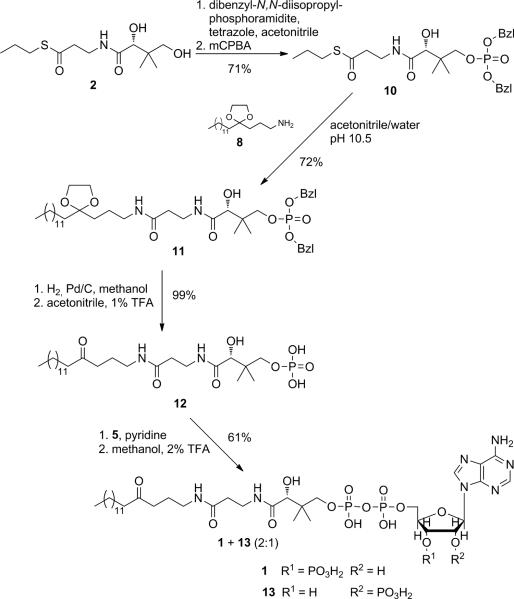
To overcome the solubility problems, we changed the synthetic strategy to that effect that the aminolysis reaction was carried out before formation of the pyrophosphate (Scheme 3). Therefore, thioester 10 was generated and then used in the aminolysis reaction. In acetonitrile/water at pH 10.5, both 8 and 10 could be readily dissolved. The reaction was followed by HPLC, while the pH was kept at 10.5. After 4 days, dibenzylphosphate 11 was isolated in 72% yield. After cleavage of the benzyl groups and acidic hydrolysis of the ketal, 12 could be coupled with morpholidate 5. Subsequent acidic hydrolysis of the cyclophosphate yielded myristoyl-carba(dethia)-CoA 1 and its 2'-phosphate isomer myristoyl-carba(dethia)-isoCoA 13 in a ratio of 2:1, according to 1H- and 31P-NMR-spectra (Figures S1/2). Therefore, the overall yield of the final product 1 was 13%, referred to myristic acid. However, attempts to separate the two isomers using ion-exchange chromatopraphy with a DEAE-cellulose-column as described for the coenzyme A synthesis,[xviii] or using Dowex® 1×8 (Cl−-form), were not successful, most likely because of the high surface activity of these compounds. Also using HPLC on a C18 reversed phase column as described in [xvii] was not able to separate the isomers, prompting us to search for an alternative strategy.
Scheme 3.
Improved strategy applies aminolysis reaction earlier in the synthesis. Acidic hydrolysis of the adenosine-2',3'-cyclophosphate results in a mixture of the phosphate isomers 1 and 13.
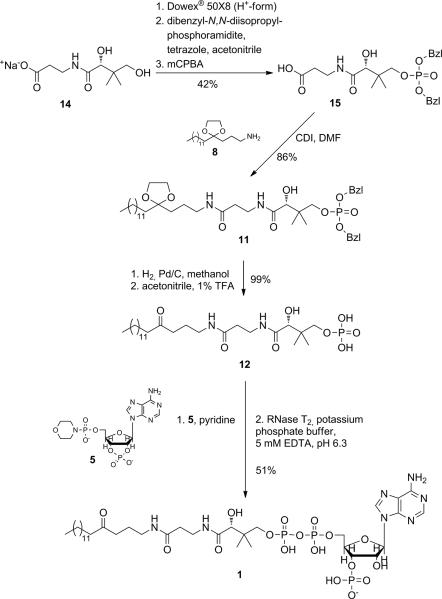
Additionally to the failed separation of the two phosphate isomers, we realized room for improvement and simplification of the synthesis. The aminolysis conditions, favoured by Martin et al., allowed forming the amide bond in the presence of the unprotected phosphate group. Since we already modified the synthetic route to that effect that a side reaction with the free phosphate group of the adenosine moiety was no longer a concern, we could also use peptide chemistry to more efficiently couple amine 8 with the pantothenic acid phosphate entity (Scheme 4). In addition to the expected higher yields, compared to the aminolysis reaction, there was also no need for the two extra steps to generate the thioester. We first generated D-pantothenic acid-4'-dibenzylphosphate 15 by applying the phosphoramidite method developed by Bannwarth and Trzeciak,[xxi] which had been proven superior over other phosphorylation strategies. Free D-pantothenic acid was obtained from its sodium salt 14 upon eluting it from a Dowex® 50×8 (H+-form) column, and removing the water by lyophilisation, followed by drying in high vacuum. Under water-free conditions, in the presence of the weak base tetrazole, the primary hydroxyl group of D-pantothenic acid was selectively phosphinylated with dibenzyl-N,N-diisopropylphosphoramidite. Subsequent oxidation with m-chloroperbenzoic acid (mCPBA) led to the benzylester-protected phosphate 15. In the key reaction of this new synthetic strategy, the carboxyl group of 15 was first activated with 1,1′-carbonyldiimidazole (CDI), forming the corresponding N,N-dimethylformamide. Amine 8 was then added, and the ketal-protected dibenzylphosphate 11 was obtained in 86% yield (compared to 72% via aminolysis). Cleavage of the benzyl groups with hydrogen in the presence of palladium on charcoal, and acidic hydrolysis of the ketal were achieved quantitatively to yield phosphate 12. Adenosine-2',3'-cyclophosphate-5'-phosphomorpholidate 5 was generated as reported[xxii] and was coupled with 12 in dry pyridine as described above. Since attempts to separate the two phosphate isomers myristoyl-carba(dethia)-CoA 1 and myristoyl-carba(dethia)-isoCoA 13 were not successful before, we sought a way to only generate the desired product 1. Utilization of the enzyme ribonuclease T2 (RNase T2) turned out to be an elegant solution to obtain the pure isomer 1 via a regioselective opening of the cyclophosphate. First used by Keep et al.,[xxiv] RNase T2 cleaves nucleoside-2',3'-cyclophosphate specifically to the 3'-phosphate. The enzymatic reaction was set up in potassium phosphate buffer at 30°C, and the pH was optimized to 6.3. Lower pH values as described in the originally report[xxiv] led to increased acidic hydrolysis, and consequently to formation of the 2'-phosphate isomer. After 24 h, over 90% (according to analytical HPLC) of the adenosine-2',3'-cyclophosphate was converted. The product was purified by preparative HPLC using a C18 reversed phase column and an acetonitrile/potassium phosphate gradient. The desired myristoyl-carba(dethia)-CoA 1 was isolated in 51% yield, referring to phosphate 12, and 19% referring to myristic acid 7. More importantly, 1 was generated isomerically pure without any further need for separation from its 2'-phosphate isomer. 1H- and 31P-NMR-spectra (Figures S3/4) clearly demonstrated the presence of only one isomer.
Scheme 4.
Final route to generate isomerically pure myristoyl-carba(dethia)-CoA 1. Key intermediate 12 was obtained using peptide chemistry to couple amine 8 with pantothenic acid phosphate 15. The final step of the synthesis involves an enzymatic cleavage of the adenosine-2',3'-cyclophosphate, using RNase T2, which exclusively generates desired product 1.
Conclusions
We have developed a new convergent synthesis that allows to generate the potent Nmt inhibitor myristoyl-carba(dethia)-CoA 1 in a total of 14 steps and a very good overall yield of 19% (referring to myristic acid). Compared to the linear synthesis that follows the original method for generating coenzyme A by Moffatt and Khorana,[xvii,xviii] this constitutes a 11-fold increase in yield, while the number of synthetic steps was reduced by 3. An effective new route to obtain key intermediate myristoyl-carba(dethia)-pantethein-4'-phosphate 12, using peptide chemistry to link the readily accessible amine 8 and pantothenic acid phosphate 15, as well as the specific enzymatic cleavage of the adenosine-2',3'-cyclophosphate with RNase T2, warranted for significant improvement and simplification over existing methods. Compared to the modified route via aminolysis reaction,[xx] the use of peptide chemistry saved two synthetic steps and should be easily applicable to the synthesis of any acyl-carba(dethia)-CoA analogue, regardless of the nature of the acyl group. Thereby, syntheses of analogous with hydrophobic acyl groups should in particular benefit from our new strategy. Conveniently, key reactant pantothenic acid phosphate can be easily obtained in good yield by applying the phosphoramidite method, which proved to be far superior over the reported phosphorylation of pantothenic acid with methyl phosphorochloridate.[xx] Most importantly, the use of RNase T2 in the last step of the synthesis exclusively generates the desired 3'-phosphate isomer, eliminating the need (and challenge) to separate the final product from its 2'-phosphate isomer.
Experimental Section
3-[4-(bis-allyloxy-phosphoryloxy)-2(R)-hydroxy-3,3-dimethyl-butyrylamino]-thiopropionic acid-S-propylester 3
In a 100mL-round flask with septum, 540 mg (1.95 mmol) 2 and 514 μl (1.95 mmol) diallyl-N,N-diisopropyl-phosphoramidite was dissolved in 5 mL dry acetonitrile under inert gas. 4.33 mL (1.95 mmol) of a 0.45 M tetrazole solution in acetonitrile was added, and the mixture was stirred for 5 min at room temperature. Then, 505 mg (2.92 mmol) mCPBA (722 mg of a mixture of 70% mCPBA, 10% 3-chlorobenzoic acid and 20% water) was added, and the solution was stirred for 2 h at room temperature. After evaporating the solvent and drying in high vacuum, the product was purified by silica gel flash chromatography (cyclohexane/ethyl acetate = 10/1, 2/1, 1/1, 1/5, 0/1, Rf (cyclohexane/ethyl acetate = 1/1) = 0.06). 3 was obtained as colourless oil.
Yield: 629 mg (74%); 1H-NMR (CD3OD, 500 MHz): 0.95 (s, 3H, -C-CH3), 0.97 (t, 3H, J = 7.4, CH3-CH2-), 1.01 (s, 3H, -C-CH3), 1.59 (m, 2H, CH3-CH2-), 2.82 (t, 2H, J = 6.7, -SCO-CH2-), 2.87 (m, 2H, -CH2-SCO-), 3.50 (m, 2H, CH2-NHCO-), 3.86 (s, 1H, -CHOH-), 3.96 (ABX, 2H, J1 = 9.3, J2 = 4.5, -CH2-OH), 4.58 (m, 4H, 2x -CH2-CH=CH2), 5.34 (ABX, 4H, 2x -CH2-CH=CH2), 5.99 (m, 2H, 2x -CH2-CH=CH2); 31P-NMR (CD3OD, 500 MHz): 0.05 (1P); HRMS (70eV), calcd: 437.1637, found: 437.1632.
3-(2(R)-hydroxy-3,3-dimethyl-4-phosphonooxy-butyrylamino)-thiopropionic acid-S-propylester 4
In a 50mL-round flask under inert gas, 630 mg (1.44 mmol) 3, 167 mg (5 mol% per allyl group) Pd[P(C6H5)3]4, and 225 mg (1.46 mmol) dimethylbarbituric acid were dissolved in 7 mL dry THF. After stirring the mixture over night at room temperature, the solvent was evaporated and the residue dried in high vacuum, before the product was purified by preparative HPLC on a C18 reversed phase column with a water/acetonitrile gradient (A: H2O + 0.1% TFA, B: acetonitrile + 0.1% TFA). 4 eluted between 25% and 35% B.
Yield: 505 mg (98%); 1H-NMR (CD3OD, 500 MHz): 0.94 (s, 3H, -C-CH3), 0.96 (t, 3H, J = 7.4, CH3-CH2-), 1.00 (s, 3H, -C-CH3), 1.59 (m, 2H, CH3-CH2-), 2.82 (t, 2H, J = 6.7, -SCO-CH2-), 2.87 (m, 2H, -CH2-SCO-), 3.50 (m, 2H, CH2-NHCO-), 3.84 (ABX, 2H, J1 = 9.5, J2 = 5.1, -CH2-OH), 3.91 (s, 1H, -CHOH-); 31P-NMR (CD3OD, 500 MHz): 1.58 (1P); MS-FAB (glycerol) (m/z (%)): 358.2 (92) [M++H], 282.1 (2.5) [M+-C3H7S], 260.2 (100) [M+-H2PO4].
Pantothenic acid-4'-(adenosine-2',3'-cyclophosphate-5'-pyrophosphate)-thiopropylester 6
Each, 462 mg (0.373 mmol) bis-N,N'-dicyclohexyl-4-morpholinecarboxamidium salt of adenosine-2',3'-cyclophosphate-5'-phosphomorpholidate 5, and 134 mg (0.373 mmol) 4 were repeatedly dissolved in 5 mL dry pyridine, concentrated through evaporation, and dried in high vacuum. The reactants were then transferred into a 100mL-round flask and dissolved in 5 mL dry pyridine, before stirred under inert gas for 2 d at room temperature. After evaporating the solvent, the residue was washed with diethyl ether and dried in high vacuum. The product was then purified by preparative HPLC on a C18 reversed phase column with a potassium phosphate buffer/acetonitrile-gradient (A: 10 mM KPH pH 6.0, B: acetonitrile/10 mM KPH pH 6.0 = 80/20). 6 eluted between 20% and 40% B. After concentrating the eluate by lyophilisation, buffer salts were removed via HPLC on a C18 reversed phase column with a water/acetonitrile gradient. 6 was obtained as triple potassium salt.
Yield: 213 mg (66%); 1H-NMR (D2O, 500 MHz): 0.63 (s, 3H, -C-CH3), 0.74 (s, 3H, -C-CH3), 0.77 (t, 3H, J = 7.4, CH3-CH2-), 1.39 (m, 2H, CH3-CH2-), 2.69 (m, 4H, -CH2-SCO-CH2-), 3.38 (m, 2H, CH2-NHCO-), 3.56 (ABX, 2H, J1 = 9.8, J2 = 4.8, -CH2-OH), 3.88 (s, 1H, -CHOH-), 4.16 (m, 2H, 5'-position ribose), 4.53 (m, 1H, 4'-position ribose), 5.14 (m, 1H, 3'-position ribose), 5.33 (m, 1H, 2'-position ribose), 6.24 (d, 1H, J = 4.1, 1'-position ribose), 8.16 (s, 1H, adenine ring C2-position), 8.34 (s, 1H, adenine ring C8-position); 31P-NMR (D2O, 500 MHz): −10.67 – −9.90 (2P, pyrophosphate), 20.41 (1P, cyclophosphate); MS-FAB (glycerol) (m/z (%)): 862.9 (44) [M++3K-2H], 825.0 (100), [M++2K-H], 787.0 (33) [M++K], 749.3 (0.2) [M++H], 748.0 (0.08) [M+].
Adenosine-2',3'-cyclophosphate of 2-methyl-2-tridecyl-1,3-dioxolan-dethio-CoA 9
In a 25mL-round flask, 66 mg (0.076 mmol) 6 and 24 mg (0.076 mmol) 8 were heated in 2 mL pyridine to 50°C and dissolved completely by brief sonication using a sonicator bath. Then, the pH was adjusted to 11.0 with 100 mM NaOH solution, and the mixture was stirred at room temperature for 4 d. During this time, the pH value was checked several times and adjusted to 11.0. The solution was then neutralized with 1 M HCl, and the solvent was removed by lyophilisation. The product was purified by preparative HPLC on a C18 reversed phase column with a potassium phosphate buffer/acetonitrile-gradient (A: 10 mM KPH pH 6.0, B: acetonitrile/10 mM KPH pH 6.0 = 80/20). 9 eluted between 60% and 70% B. After concentrating the eluate by lyophilisation, buffer salts were removed via HPLC on a C18 reversed phase column with a water/acetonitrile gradient, and 9 was obtained as potassium salt.
Yield: 18 mg (22%); 1H-NMR (D2O, 500 MHz): 0.74 (s, 3H, -C-CH3), 0.83 (t, 3H, J = 6.9, CH3-CH2-), 0.88 (s, 3H, -C-CH3), 1.19 (s, 22H, -(CH2)11-), 1.48 (m, 2H, -C(OCH2)2-CH2-CH2-), 1.59 (m, 4H, -CH2-C(OCH2)2-CH2-), 2.44 (m, 2H, -NHCO-CH2-), 3.12 (m, 2H, CH2-NHCO-), 3.45 (m, 2H, -NHCO-CH2-CH2-), 3.67 (ABX, 2H, J1 = 9.4, J2 = 4.9, -CH2-OH), 3.96 (s, 4H, -C(OCH2)2-), 4.00 (s, 1H, -CHOH-), 4.25 (m, 2H, 5'-position ribose), 4.62 (m, 1H, 4'-position ribose), 5.23 (m, 1H, 3'-position ribose), 5.40 (m, 1H, 2'-position ribose), 6.33 (d, 1H, J = 4.1, 1'-position ribose), 8.25 (s, 1H, adenine ring C2-position), 8.45 (s, 1H, adenine ring C8-position); 31P-NMR (D2O, 500 MHz): −11.18 – −10.41 (2P, pyrophosphate), 20.13 (1P, cyclophosphate); MS-FAB (glycerol) (m/z (%)): 1068.9 (91), 1051,1 (20) [M++3Na-3H], 1025.3 (100) [M++K+H], 1007.4 (26) [M++Na-H], 986.4 (1.5) [M++H], 985.4 (4) [M+], 981.4 (94), 937.4 (77).
3-[4-(bis-benzyloxy-phosphoryloxy)-2(R)-hydroxy-3,3-dimethyl-butyrylamino]-thiopropionic acid-S-propylester 10
In a 100mL-round flask with septum and inert gas, 540 mg (1.95 mmol) 2 and 641 μl (1.95 mmol) dibenzyl-N,N-diisopropyl-phosphoramidite were dissolved in 5 mL dry acetonitrile. 4.33 mL (1.95 mmol) of a 0.45 M tetrazole solution in acetonitrile was added, and the mixture was stirred for 5 min at room temperature. Then, 505 mg (2.92 mmol) mCPBA (722 mg of a mixture of 70% mCPBA, 10% 3-chlorobenzoic acid and 20% water) was added, and the solution was stirred for 2 h at room temperature. After evaporating the solvent and drying in vacuum, the product was purified by silica gel flash chromatography (cyclohexane/ethyl acetate = 10/1, 2/1, 1/1, 1/5, 0/1, Rf (cyclohexane/ethyl acetate = 1/1) = 0.015). 10 was obtained as colourless oil.
Yield: 748 mg (71%); 1H-NMR (CDCl3, 250 MHz): 0.77 (s, 3H, -C-CH3), 0.95 (t, 3H, J = 7.4, CH3-CH2-), 1.06 (s, 3H, -C-CH3), 1.58 (m, 2H, CH3-CH2-), 2.78 (t, 2H, J = 6.3, -SCO-CH2-), 2.84 (m, 2H, -CH2-SCO-), 3.54 (m, 2H, -CH2-NHCO-), 3.80 (ABX, J1 = 8.1, J2 = 10.1, 2H, -CH2-O-), 3.91 (s, 1H, -CHOH-), 5.03 (m, 4H, 2x -CH2-C6H5), 7.33 (m, 10H, 2x -C6H5); 31P-NMR (CDCl3, 500 MHz): 1.04 (1P); MS-FAB (3-NBA) (m/z (%)): 560.2 (6) [M++Na], 538.2 (28) [M++H], 260.2 (56) [M+-dibenzylphosphate], 180.3 (100) [M+-dibenzylphosphate-(C(O)SCH2CH2CH3)+Na].
(R)-dibenzyl-3-hydroxy-2,2-dimethyl-4-oxo-4-(3-oxo-3-(3-(2-tridecyl-1,3-dioxolan-2-yl)propylamino)propylamino)butyl phosphate 11 (via aminolysis)
In a 25mL-round flask, 100 mg (0.186 mmol) 10 and 58 mg (0.186 mmol) 8 were dissolved in 6 mL acetonitrile/water = 1/1. The pH was adjusted to 11.0 with 100 mM NaOH solution, and the mixture was stirred at room temperature for 4 d. During this time, the pH value was checked several times and adjusted to 11.0. The solution was then neutralized with 1 M HCl, and the solvent was removed by lyophilisation. The product was purified by preparative HPLC on a C18 reversed phase column with a water/acetonitrile-gradient (A: H2O, B: acetonitrile). 11 eluted between 94% and 100% B. After concentrating the eluate by lyophilisation, 11 was obtained as white solid.
Yield: 103 mg (72%); 1H-NMR (CDCl3, 500 MHz): 0.80 (s, 3H, CH3- pantetheine), 0.87 (t, 3H, J = 7.0, CH3-CH2-), 1.05 (s, 3H, CH3- pantetheine), 1.24 (s, 20H, -(CH2)10-), 1.29 (m, 2H, -CH2-CH2-C(OCH2)2-), 1.55 (m, 4H, -CH2-C(OCH2)2-CH2-CH2-), 1.62 (m, 2H, -C(OCH2)2-CH2-), 2.41 (m, 2H, -NHCO-CH2-), 3.21 (m, 2H, -NHCO-CH2-CH2-), 3.55 (m, 2H, -C(OCH2)2-CH2-CH2-CH2-), 3.78 (ABX, 2H, J1 = 10.1, J2 = 7.4, -CH2-O-), 3.88 (s, 1H, -CHOH-), 3.90 (s, 4H, -C(OCH2)2-), 5.03 (m, 4H, 2x -CH2-C6H5) 6.21 (m, 1H, -NH-), 7.28 (m, 1H, -NH-), 7.34 (m, 10H, 2x -C6H5); 31PNMR (CDCl3, 500 MHz): 0.72 (1P); MS-FAB (3-NBA) (m/z (%)): 813.4 (2.5) [M++K], 799.4 (11), 798.4 (45), 797.4 (100) [M++Na], 776.4 (25), 775.4 (55) [M++H], 774.4 (3) [M+].
(R)-3-hydroxy-2,2-dimethyl-4-oxo-4-(3-oxo-3-(4-oxoheptadecylamino)propylamino)butyl dihydrogen phosphate 12
In a 50mL-two neck flask with septum and stopcock, 100 mg (0.129 mmol) 11 and two spatula tips of palladium black (10%) were dissolved in 5 mL dry methanol. After purging the flask for several times with argon and then with hydrogen, the reaction mixture was stirred over night under hydrogen atmosphere at room temperature. The catalyst was then removed by filtration and the solvent was evaporated. Remaining benzylalcohol was removed in high vacuum. For hydrolysis of the ketal protecting group, the colourless oil was dissolved in 80% ethanol and applied to a column (25×300 mm) with Dowex® 50×8 (H+-Form). The product was eluted with 80% ethanol. The eluate was then concentrated, and the residue dissolved in 10 mL ethanol and evaporated, after addition of 2 mL toluene. After drying in high vacuum, 12 was obtained as colourless oil.
Yield: 76 mg (99%); 1H-NMR (DMSO-d6, 500 MHz): 0.78 (s, 3H, CH3- pantetheine), 0.83 (t, 3H, J = 6.9, CH3-), 0.86 (s, 3H, CH3- pantetheine), 1.21 (s, 20H, -(CH2)10-), 1.41 (m, 2H, -CH2-CH2-CO-), 1.54 (m, 2H, -CO-CH2-CH2-), 2.22 (m, 2H, -NHCO-CH2-), 2.36 (m, 4H, -CH2-CO-CH2-), 2.95 (m, 2H, -CO-CH2-CH2-CH2-), 3.25 (m, 2H, -NHCO-CH2-CH2-), 3.63 (ABX, 2H, J1 = 9.7, J2 = 5.8, -CH2-O-), 3.67 (s, 1H, -CHOH-), 7.70 (t, 1H, J = 5.8, -NH-), 7.83 (t, 1H, J = 6.8, -NH-); 13C-NMR (DMSO-d6, 500 MHz): 14.38 (CH3-CH2), 19.91 (CH3-), 21.23 (CH3-), 22.54 (CH2), 23.66 (CH2), 23.76 (CH2), 29.06 (CH2), 29.15 (CH2), 29.33 (CH2), 29.36 (CH2), 29.45 (4x CH2), 31.74 (CH2), 35.29 (CH2), 35.56 (CH2), 38.29 (CH2), 39.03 (-C(CH3)2-), 39.55 (CH2), 42.32 (CH2), 71.65 (CH2), 74.32 (-CH(OH)-), 170.84 (-HN-CO-), 172.53 (-HN-CO-), 210.56 (-CO-); 31P-NMR (DMSO-d6, 500 MHz): 0.29 (1P); MS-FAB (3-NBA) (m/z (%)): 596.4 (8) [M++2Na], 595.4 (22), 589.4 (5) [M++K], 575.4 (9), 574.4 (34), 573.4 (100) [M++Na], 571.4 (9), 551.4 (13) [M++H], 550.4 (1) [M+].
Myristoyl-carba(dethia)-CoA 1 + myristoyl-carba(dethia)-isoCoA (2'-phosphate isomer) 13
To remove traces of water, 130 mg (0.105 mmol) 5 and 36 mg (0.065 mmol) 12 were dissolved in 5 mL dry pyridine and concentrated again for a total of three times. After drying in high vacuum, the reactants were dissolved in 5 mL dry pyridine and stirred for 2 days at room temperature under argon atmosphere. After evaporating the solvent, the residue was washed with diethyl ether and dried in high vacuum. The residue was purified by preparative HPLC on a C18 reversed phase column with a potassium phosphate buffer/acetonitrile gradient (A: 10 mM potassium phosphate pH 6.0, B: acetonitrile/10 mM potassium phosphate pH 6.0 = 80/20). The adenosine-2',3'-cyclophosphate eluted between 55% and 70% buffer B. After concentrating the eluate by lyophilisation, the residue was stirred in methanol with 2% TFA acid over night at room temperature to open the cyclophosphate. Preparative HPLC on a C18 reversed phase column with a water/acetonitrile gradient yielded a mixture of the two isomers 1 and 13 as white solid.
Yield: 38 mg (61%); 1H-NMR (CD3OD, 500 MHz): 0.83 (s, 3H, CH3- pantetheine), 0.89 (t, 3H, J = 7.1, CH3-CH2-), 1.06 (s, 3H, CH3- pantetheine), 1.27 (s, 20H, -(CH2)10-), 1.52 (m, 2H, -CH2-CH2-CO-), 1.70 (m, 2H, -CO-CH2-CH2-), 2.44 (m, 6H, -NHCO-CH2-, -CH2-CO-CH2-), 3.13 (m, 2H, -CO-CH2-CH2-CH2-), 3.45 (m, 2H, -NHCO-CH2-CH2-), 3.78 (ABX, 2H, J1 = 9.7, J2 = 4.8, -CH2-O-), 4.07 (s, 1H, -CHOH-), 4.21 (m, 2H, ribose 5'-position, 2′-isomer), 4.27 (m, 2H, ribose 5'-position, 3′-isomer), 4.49 (m, 1H, ribose 4'-position, 2′-isomer), 4.62 (m, 1H, ribose 4'-position, 3′-isomer), 4.72 (m, 1H, ribose 3'-position, 2′-isomer), 5.15 (m, 1H, ribose 2'-position, 2′-isomer), 6.13 (d, 1H, J = 5.6, ribose 1'-position, 3′-isomer), 6.28 (d, 1H, J = 5.6, ribose 1'-position, 2′-isomer), 8.19 (s, 1H, adenine C2-position), 8.57 (s, 1H, adenine C8-position); 31P-NMR (CD3OD, 500 MHz): −9.26 to −8.67 (m, 2P, pyrophosphate), 1.91/2.18 (s, 1P, 2′-/3′-position)
3-[4-(bis-benzyloxy-phosphoryloxy)-2(R)-hydroxy-3,3-dimethyl-butyrylamino]-propionic acid 15
1 g (4.15 mmol) D-pantothenic acid sodium salt 14 was dissolved in water and applied to a column (25×300 mm) with Dowex® 50×8 (H+-Form). The free acid was eluted with 250 mL water, and the water was removed by lyophilisation. The residue was dried in high vacuum. 900 mg (4.11 mmol) of the free acid and 1.352 mL (4.11 mmol) dibenzyl-N,N-diisopropylphosphoramidite were dissolved in 5 mL dry acetonitrile in a 100mL-round flask with septum under inert gas. 9.133 mL (4.11 mmol) of a 0.45 M tetrazole solution in acetonitrile was added, and the solution was stirred for 15 min at room temperature. Then, 1.064 g (6.16 mmol) mCPBA (1.52 g of a mixture of 70% mCPBA, 10% 3-chlorobenzoic acid and 20% water) was added, and the mixture was stirred for another 2 h at room temperature. The solvent was evaporated, and the residue was purified by silica gel flash chromatography (ethyl acetate, methanol). 15 was obtained as colourless oil.
Yield: 836 mg (42%); 1H-NMR (CD3OD, 500 MHz): 0.89 (s, 3H, -C-CH3), 0.95 (s, 3H, -C-CH3), 2.51 (t, 2H, J = 6.6, HOOC-CH2-), 3.46 (m, 2H, HOOC-CH2-CH2-), 3.84 (s, 1H, -CHOH-), 3.92 (ABX, 2H, J1 = 9.4, J2 = 4.5, -CH2-O-), 5.04 (s, 2H, -CH2-C6H5), 5.06 (s, 2H, -CH2-C6H5), 7.36 (m, 10H, 2x -C6H5); 13C-NMR (CD3OD, 500 MHz): 18.64 (CH3-), 19.91(CH3-), 33.31 (HOOC-CH2-), 34.38 (HOOC-CH2-CH2-), 38.65 (-C(CH3)2-), 69.34 (-CH2-C6H5), 69.38 (-CH2-C6H5), 73.41 (-CH2-O-), 73.95 (-CHOH-), 127.75 (4x aromat. tert.), 128.27 (6x aromat. tert.), 135.81 (2x aromat. quart.), 173.80 (-NHCO-), 174.06 (HOOC-); 31P-NMR (CD3OD, 500 MHz): 0.16 (1P); MS-FAB (3-NBA) (m/z (%)): 504.2 (3), 503.2 (20) [M++H+Na], 502.2 (59) [M++Na], 482.2 (8), 481.2 (28), 480.2 (100) [M++H], 479.3 (3) [M+].
(R)-dibenzyl-3-hydroxy-2,2-dimethyl-4-oxo-4-(3-oxo-3-(3-(2-tridecyl-1,3-dioxolan-2-yl)propylamino)propylamino)butyl phosphate 11 (via peptide chemistry)
In a 50mL-round flask with septum and bubble counter, 150 mg (0.312 mmol) 15 were dissolved in 6 mL dry DMF and heated to 40°C under inert gas. 100 mg (0.624 mmol) CDI in 4 mL dry DMF were added and the mixture was stirred for 90 min, until gas formation had stopped. Then, 98 mg (0.312 mmol) amine 8, dissolved in 3 mL dry DMF, was added, and the reaction mixture was stirred over night at 40°C. The solvent was evaporated, and the residue was purified by preparative HPLC over a C18 reversed phase column with a water/acetonitrile gradient (A: H2O, B: acetonitrile). 11 eluted between 94% and 100% B. After concentrating the eluate by lyophilisation, 11 was obtained as white solid.
Yield: 208 mg (86%); 1H-NMR (CDCl3, 500 MHz): 0.80 (s, 3H, CH3- pantetheine), 0.87 (t, 3H, J = 7.0, CH3-CH2-), 1.05 (s, 3H, CH3- pantetheine), 1.24 (s, 20H, -(CH2)10-), 1.29 (m, 2H, -CH2-CH2-C(OCH2)2-), 1.55 (m, 4H, -CH2-C(OCH2)2-CH2-CH2-), 1.62 (m, 2H, -C(OCH2)2-CH2-), 2.41 (m, 2H, -NHCO-CH2-), 3.21 (m, 2H, -NHCO-CH2-CH2-), 3.55 (m, 2H, -C(OCH2)2-CH2-CH2-CH2-), 3.78 (ABX, 2H, J1 = 10.1, J2 = 7.4, -CH2-O-), 3.88 (s, 1H, -CHOH-), 3.90 (s, 4H, -C(OCH2)2-), 5.03 (m, 4H, 2x -CH2-C6H5) 6.21 (m, 1H, -NH-), 7.28 (m, 1H, -NH-), 7.34 (m, 10H, 2x -C6H5); 31PNMR (CDCl3, 500 MHz): 0.72 (1P); MS-FAB (3-NBA) (m/z (%)): 813.4 (2.5) [M++K], 799.4 (11), 798.4 (45), 797.4 (100) [M++Na], 776.4 (25), 775.4 (55) [M++H], 774.4 (3) [M+].
Myristoyl-carba(dethia)-CoA 1 (via enzymatic cleavage of cyclophosphate)
To remove traces of water, 130 mg (0.105 mmol) 5 and 36 mg (0.065 mmol) 12 were dissolved in 5 mL dry pyridine and concentrated again for a total of three times. After drying in high vacuum, the reactants were dissolved in 5 mL dry pyridine and stirred for 2 days at room temperature under argon atmosphere. After evaporating the solvent, the residue was washed with diethyl ether and dried in high vacuum. The residue was purified by preparative HPLC on a C18 reversed phase column with a potassium phosphate buffer/acetonitrile gradient (A: 10 mM potassium phosphate pH 6.0, B: acetonitrile/10 mM potassium phosphate pH 6.0 = 80/20). The adenosine-2',3'-cyclophosphate eluted between 55% and 70% buffer B. After concentrating the eluate by lyophilisation, the residue was dissolved in 1 mL 5 mM EDTA/water, the pH was adjusted to 6.3, and 150 u ribonuclease T2 were added. The solution was incubated at 30°C for 24 h, and reaction was followed by analytical HPLC. The enzyme was then denatured by a short incubation at 80°C and could be removed by centrifugation. After sterile filtration, the mixture was separated by preparative HPLC on a C18 reversed phase column with a potassium phosphate/acetonitrile gradient (A: 10 mM potassium phosphate pH 6.0, B: acetonitrile/potassium phosphate=80/20). Product 1 eluted between 44% and 49% B within 24 min. The combined product fractions were concentrated by lyophilisation, and buffer salts were removed by preparative HPLC on a C18 reversed phase column with a water/acetonitrile gradient (A: H2O, B: acetonitrile). After lyophilisation and drying in high vacuum, the potassium salt of 1 was obtained as a white solid.
Yield: 33 mg (51%); 1H-NMR (CD3OD, 500 MHz): 0.86 (s, 3H, CH3- pantetheine), 0.89 (t, 3H, J = 7.0, CH3-CH2-), 1.06 (s, 3H, CH3- pantetheine), 1.27 (s, 20H, -(CH2)10-), 1.52 (m, 2H, -CH2-CH2-CO-), 1.70 (m, 2H, -CO-CH2-CH2-), 2.44 (m, 6H, -NHCO-CH2-, -CH2-CO-CH2-), 3.13 (m, 2H, -
CO-CH2-CH2-CH2-), 3.45 (m, 2H, -NHCO-CH2-CH2-), 3.81 (ABX, 2H, J1 = 9.4, J2 = 4.5, -CH2-O-), 4.06 (s, 1H, -CHOH-), 4.35 (m, 2H, ribose 5'-position), 4.50 (m, 1H, ribose 4'-position), 4.75 (m, 1H, ribose 2'-position), 6.09 (d, 1H, J = 5.2, ribose 1'-position), 8.26 (s, 1H, adenine C2-position), 8.68 (s, 1H, adenine C8-position); 13C-NMR (CD3OD, 500 MHz): 13.45 (CH3-CH2), 21.15 (CH3-), 22.74 (CH2), 23.20 (CH3-), 23.50 (CH2), 23.85 (CH2), 23.86 (CH2), 29.33 (CH2), 29.49 (CH2), 29.60 (CH2), 29.65 (CH2), 29.67 (CH), 29.75 (CH2), 29.76 (CH2), 29.78 (CH2), 29.80 (CH2), 32.08 (CH2), 35.48 (CH2), 35.70 (CH2), 38.73 (CH2), 39.12 (-C(CH3)2-), 39.54 (CH2), 42.53 (CH2), 72.36 (CH2), 74.09 (-CH(OH)-), 74.96 (CH), 83.28 (CH), 88.41 (CH), 88.43 (C), 110.00 (C), 172.82 (-HN-CO-), 174.61 (-HN-CO-), 212.31 (-CO-); 31P-NMR (CD3OD, 500 MHz): −9.57 (m, 1P, pyrophosphate), −9.25 (m, 1P, pyrophosphate), 1.41 (s, 1P, 3'-position ribose); MS-FAB (glycerol) (m/z (%)): 1035.9 (100) [M+-2H+2K], 998.3 (88) [M++K], 959.3 (8) [M+]; HRMS-FAB (glycerol), calcd (C36H64N7O17P3K): 998.3209, found: 998.3249.
Supplementary Material
Acknowledgments
This work was supported by grant CA132121 (to LT) from the U.S. National Institutes of Health.
Footnotes
Supporting Information The Supporting Information include Figures S1–4 of 1H-NMR and 31P-NMR spectra of the isomeric mixture of myristoyl-carba(dethia)-CoA 1 and myristoyl-carba(dethia)-isoCoA 13 and of the pure isomer myristoyl-carba(dethia)-CoA 1. Furthermore, details for the synthesis of 3-(2-tridecyl-1,3-dioxolan-2-yl)propan-1-amine 8 are given.
References
- i.Resh MD. Nat. Chem. Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- ii.Towler D, Glaser L. Proc. Natl. Acad. Sci. USA. 1986;83:2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iii.Boutin JA. Cell. Signal. 1997;9:15–35. doi: 10.1016/s0898-6568(96)00100-3. [DOI] [PubMed] [Google Scholar]
- iv.Felsted RL, Glover CJ, Hartman K. J. Natl. Cancer Inst. 1995;87:1571–1573. doi: 10.1093/jnci/87.21.1571. [DOI] [PubMed] [Google Scholar]
- v.Lakshmikuttyamma A, Selvakumar P, Tuchek J, Sharma RK. Prog. Neurobiol. 2008;84:77–84. doi: 10.1016/j.pneurobio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- vi.Lodge JK, Jackson-Machelski E, Toffaletti DL, Perfect JR, Gordon JI. Proc. Natl. Acad. Sci. USA. 1994;91:12008–12012. doi: 10.1073/pnas.91.25.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vii.Gottlinger HG, Sodroski JG, Haseltine WA. Proc. Natl. Acad. Sci. USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- viii.Bryant M, Ratner L. Proc. Natl. Acad. Sci. USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ix.Chow M, Newman JF, Filman D, Hogle JM, Rowlands DJ, Brown F. Nature. 1987;327:482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- x.Persing DH, Varmus HE, Ganem D. J. Virol. 1987;61:1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xi.Sikorski JA, Devadas B, Zupec ME, Freeman SK, Brown DL, Lu HF, Nagarajan S, Mehta PP, Wade AC, Kishore NS, Bryant ML, Getman DP, McWherter CA, Gordon JI. Biopolymers. 1997;43:43–71. doi: 10.1002/(SICI)1097-0282(1997)43:1<43::AID-BIP5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- xii.Georgopapadakou NH. Expert Opin. Investig. Drugs. 2002;11:1117–1125. doi: 10.1517/13543784.11.8.1117. [DOI] [PubMed] [Google Scholar]
- xiii.Prasad KK, Toraskar MP, Kadam VJ. Mini Rev. Med. Chem. 2008;8:142–149. doi: 10.2174/138955708783498159. [DOI] [PubMed] [Google Scholar]
- xiv.Ebiike H, Masubuchi M, Liu P, Kawasaki K, Morikami K, Sogabe S, Hayase M, Fujii T, Sakata K, Shindoh H, Shiratori Y, Aoki Y, Ohtsuka T, Shimma N. Bioorg. Med. Chem. Lett. 2002;12:607–610. doi: 10.1016/s0960-894x(01)00808-3. [DOI] [PubMed] [Google Scholar]
- xv.Kawasaki K, Masubuchi M, Morikami K, Sogabe S, Aoyama T, Ebiike H, Niizuma S, Hayase M, Fujii T, Sakata K, Shindoh H, Shiratori Y, Aoki Y, Ohtsuka T, Shimma N. Bioorg. Med. Chem. Lett. 2003;13:87–91. doi: 10.1016/s0960-894x(02)00844-2. [DOI] [PubMed] [Google Scholar]
- xvi.Paige LA, Zheng GQ, DeFrees SA, Cassady JM, Geahlen RL. J. Med. Chem. 1989;32:1665–1667. doi: 10.1021/jm00128a001. [DOI] [PubMed] [Google Scholar]
- xvii.Wagner AP, Retey J. Eur. J. Biochem. 1991;195:699–705. doi: 10.1111/j.1432-1033.1991.tb15756.x. [DOI] [PubMed] [Google Scholar]
- xviii.Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1959;81:1265–1265. [Google Scholar]
- xix.Brown GM. J. Biol. Chem. 1959;234:370–378. [PubMed] [Google Scholar]
- xx.Martin DP, Bibart RT, Drueckhammer DG. J. Am. Chem. Soc. 1994;116:4660–4668. [Google Scholar]
- xxi.Bannwarth W, Trzeciak A. Helv. Chim. Acta. 1987;70:175–186. [Google Scholar]
- xxii.Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:663–675. [Google Scholar]
- xxiii.Kaufmann HP, Stamm W. Chem. Ber. 1958;91:2121–2126. [Google Scholar]
- xxiv.Keep NH, Smith GA, Evans MC, Diakun GP, Leadlay PF. Biochem. J. 1993;295:387–392. doi: 10.1042/bj2950387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.