Abstract
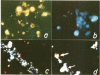
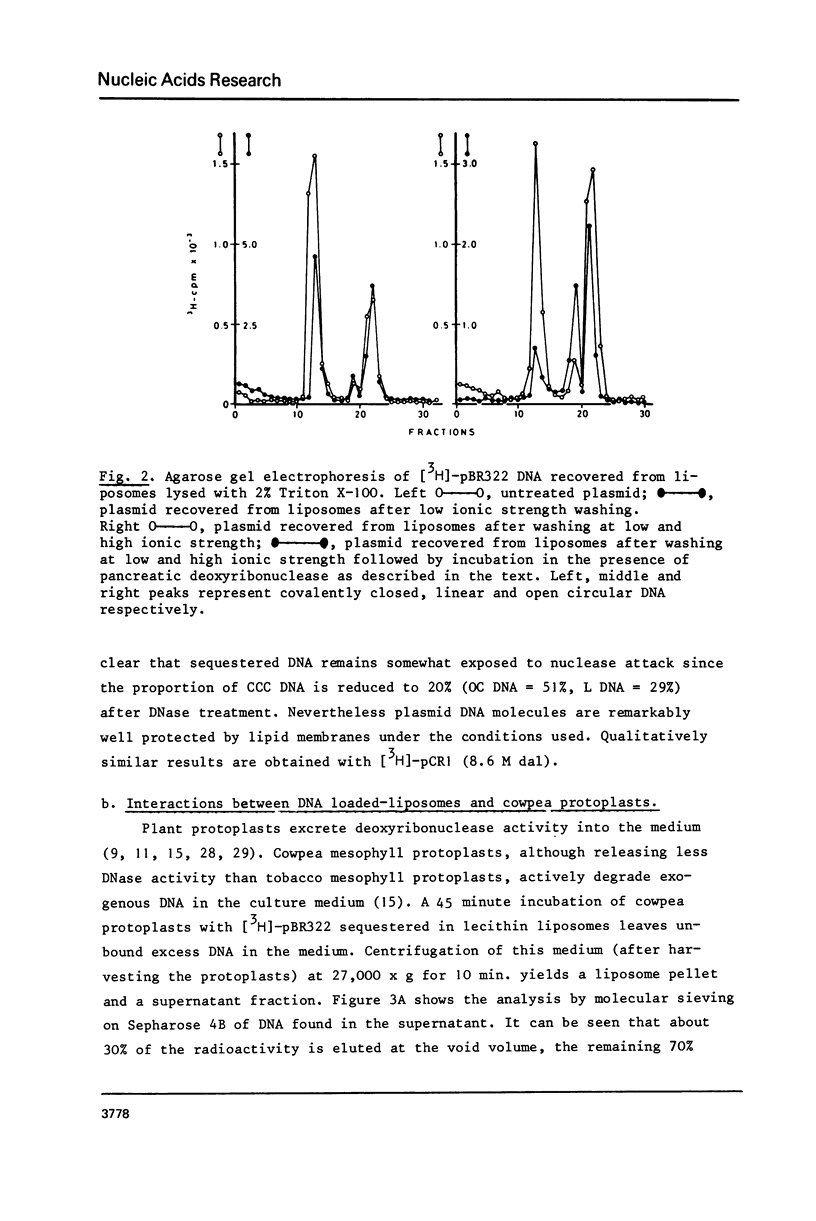
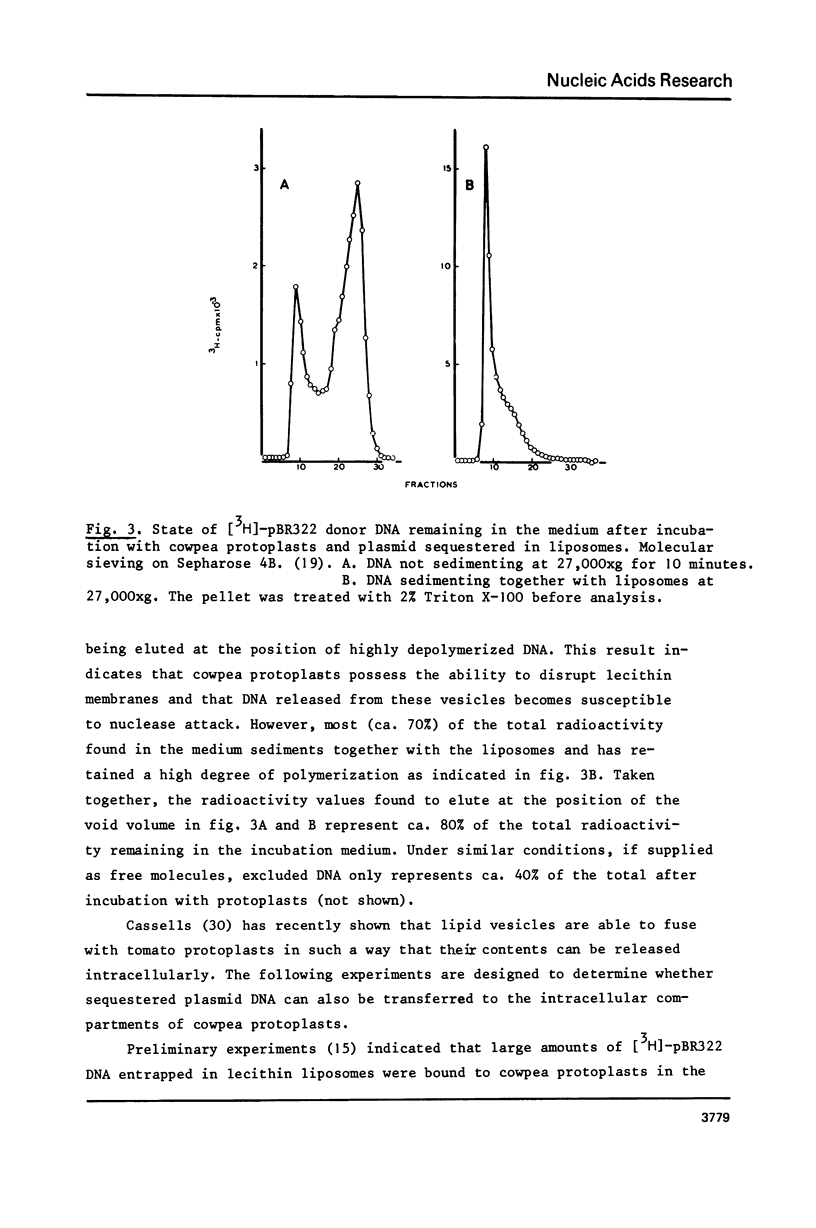
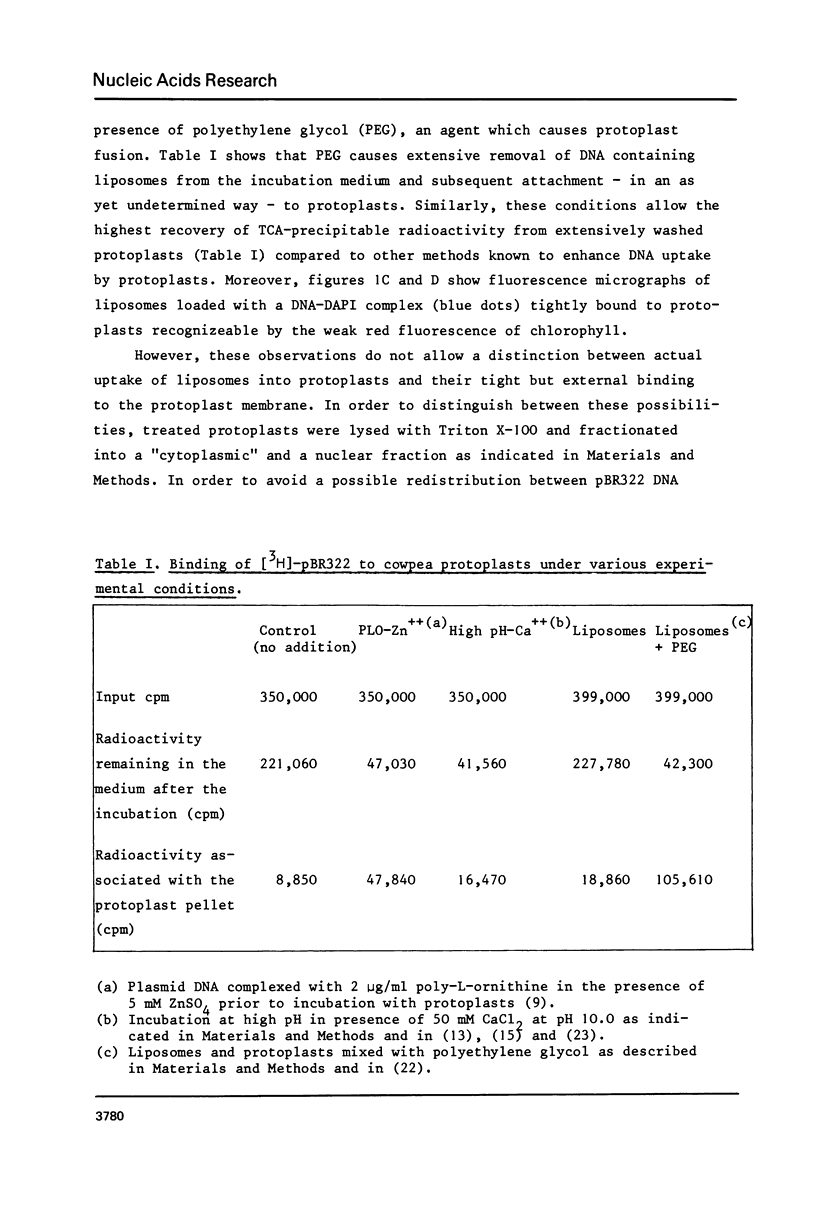
Lecithin and lecithin/cholesterol liposomes formed in aqueous solutions of DNA entrap covalently closed circular, open circular and linear DNA molecules of size up to at least 13 kilobases. The sequestered DNA molecules are efficiently protected against exogenous deoxyribonuclease action although nicking and linearization of circular DNA can be observed. The size of these liposomes ranges from approximately 0.5 to 7.5 mu with an average of 2.5--4 mu. DNA filled liposomes strongly interact with plant protoplasts under conditions inducing protoplast fusion. Results suggest that sequestered plasmid DNA can be transferred to protoplast nuclei.
Full text
PDF




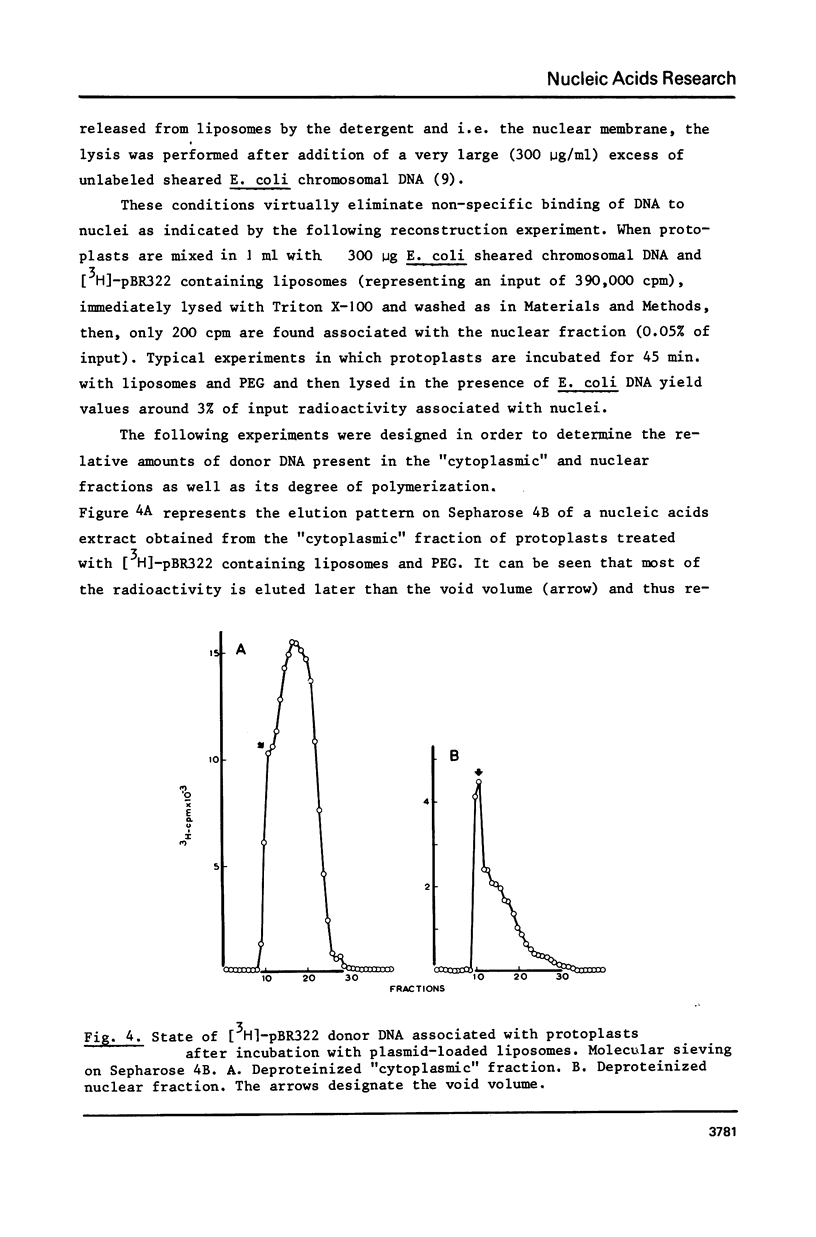
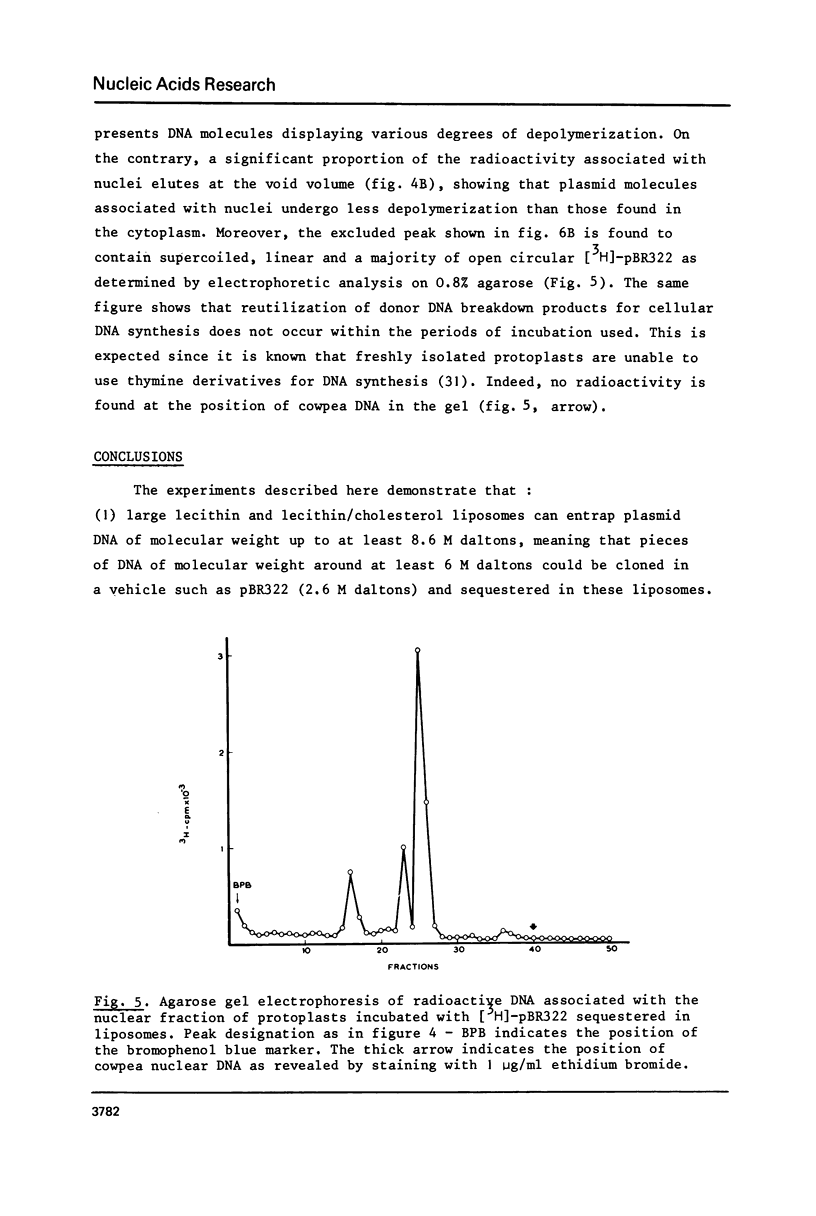


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Bruening G. The use of an abrasive in the isolation of cowpea leaf protoplasts which support the multiplication of cowpea mosaic virus. Virology. 1975 Mar;64(1):272–276. doi: 10.1016/0042-6822(75)90099-9. [DOI] [PubMed] [Google Scholar]
- Cocking E. C. Uptake of foreign genetic material by plant protoplasts. Int Rev Cytol. 1977;48:323–343. doi: 10.1016/s0074-7696(08)61748-9. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature. 1978 Aug 31;274(5674):923–924. doi: 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Erikson R. L. The resolution of DNA on beaded agarose. Biochim Biophys Acta. 1969 Jun 17;182(2):583–584. doi: 10.1016/0005-2787(69)90216-0. [DOI] [PubMed] [Google Scholar]
- Fernandez S. M., Lurquin P. F., Kado C. I. Incorporation and maintenance of recombinant-DNA plasmid vehicles pBR313 and pCR1 in plant protoplasts. FEBS Lett. 1978 Mar 15;87(2):277–282. doi: 10.1016/0014-5793(78)80351-2. [DOI] [PubMed] [Google Scholar]
- Hoffman R. M., Margolis L. B., Bergelson L. D. Binding and entrapment of high molecular weight DNA by lecithin liposomes. FEBS Lett. 1978 Sep 15;93(2):365–368. doi: 10.1016/0014-5793(78)81141-7. [DOI] [PubMed] [Google Scholar]
- Hughes B. G., White F. G., Smith M. A. Fate of bacterial plasmid DNA during uptake by barley protoplasts. FEBS Lett. 1977 Jul 1;79(1):80–84. doi: 10.1016/0014-5793(77)80355-4. [DOI] [PubMed] [Google Scholar]
- Keller W. A., Melchers G. The effect of high pH and calcium on tobacco leaf protoplast fusion. Z Naturforsch C. 1973 Nov-Dec;28(11):737–741. doi: 10.1515/znc-1973-11-1215. [DOI] [PubMed] [Google Scholar]
- Kleinhofs A. Prospects for plant genome modification by nonconventional methods. Annu Rev Genet. 1977;11:79–101. doi: 10.1146/annurev.ge.11.120177.000455. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R., Jacobs M. DNA-mediated genetic correction of thiamineless Arabidopsis thaliana. Nature. 1974 May 3;249(452):17–21. doi: 10.1038/249017a0. [DOI] [PubMed] [Google Scholar]
- Lurquin P. F., Behki R. M. Uptake of bacterial DNA by Chlamydomonas reinhardi. Mutat Res. 1975 Jul;29(1):35–51. doi: 10.1016/0027-5107(75)90019-6. [DOI] [PubMed] [Google Scholar]
- Lurquin P. F. Integration versus degradation of exogenous DNA in plants: an open question. Prog Nucleic Acid Res Mol Biol. 1977;20:161–207. doi: 10.1016/s0079-6603(08)60473-0. [DOI] [PubMed] [Google Scholar]
- Lurquin P. F., Kado C. I. Escherichia coli plasmid pBR313 insertion into plant protoplasts and into their nuclei. Mol Gen Genet. 1977 Jul 20;154(2):113–121. doi: 10.1007/BF00330826. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Orloff S., Butler J. D., Triche T., Lalley P., Schulman J. D. Entrapment of metaphase chromosomes into phospholipid vesicles (lipochromosomes): carrier potential in gene transfer. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1361–1365. doi: 10.1073/pnas.75.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik N. S., Widholm J. M. Inhibition of deoxyribonuclease activity in the medium surrounding plant protoplasts. Plant Physiol. 1978 Aug;62(2):272–275. doi: 10.1104/pp.62.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Quantitative analysis of the fate of exogenous DNA in Nicotiana protoplasts. Plant Physiol. 1977 Feb;59(2):301–308. doi: 10.1104/pp.59.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimm B. H., Crothers D. M. SIMPLIFIED ROTATING CYLINDER VISCOMETER FOR DNA. Proc Natl Acad Sci U S A. 1962 Jun;48(6):905–911. doi: 10.1073/pnas.48.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]