Abstract
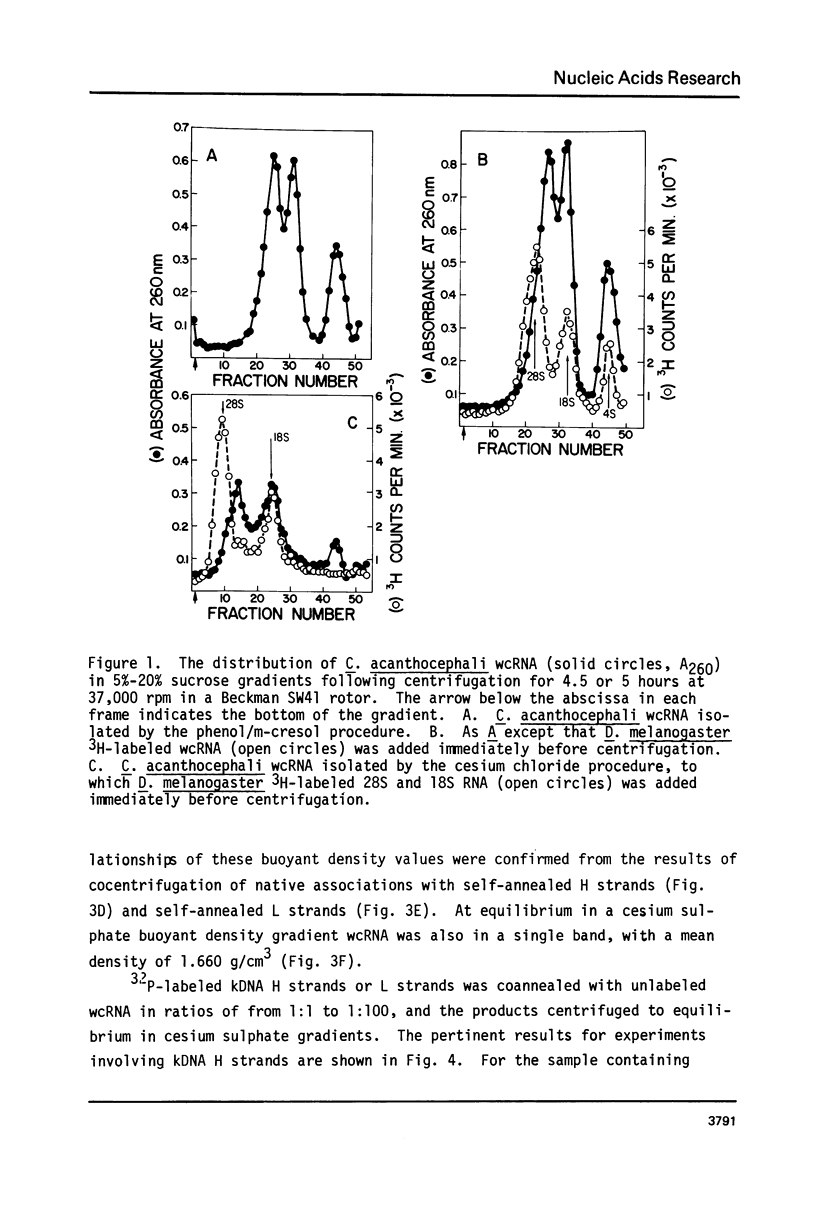
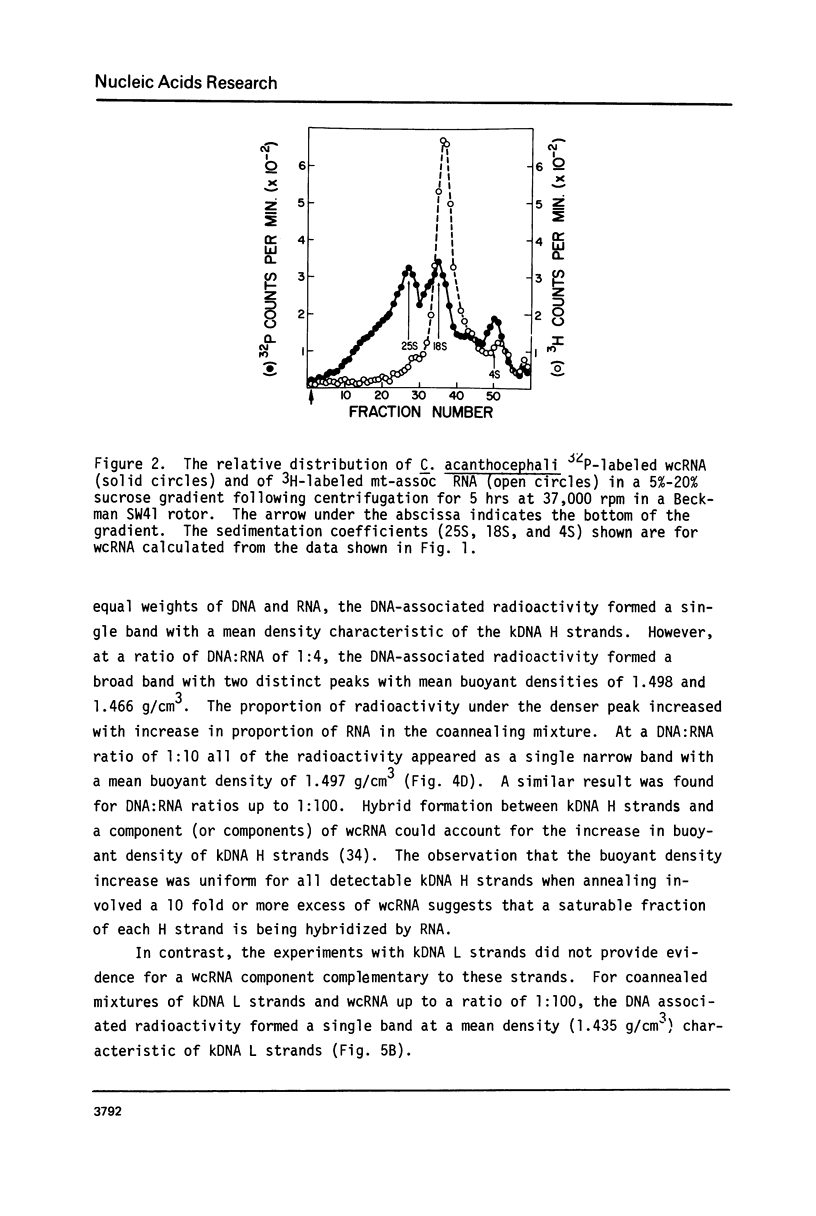
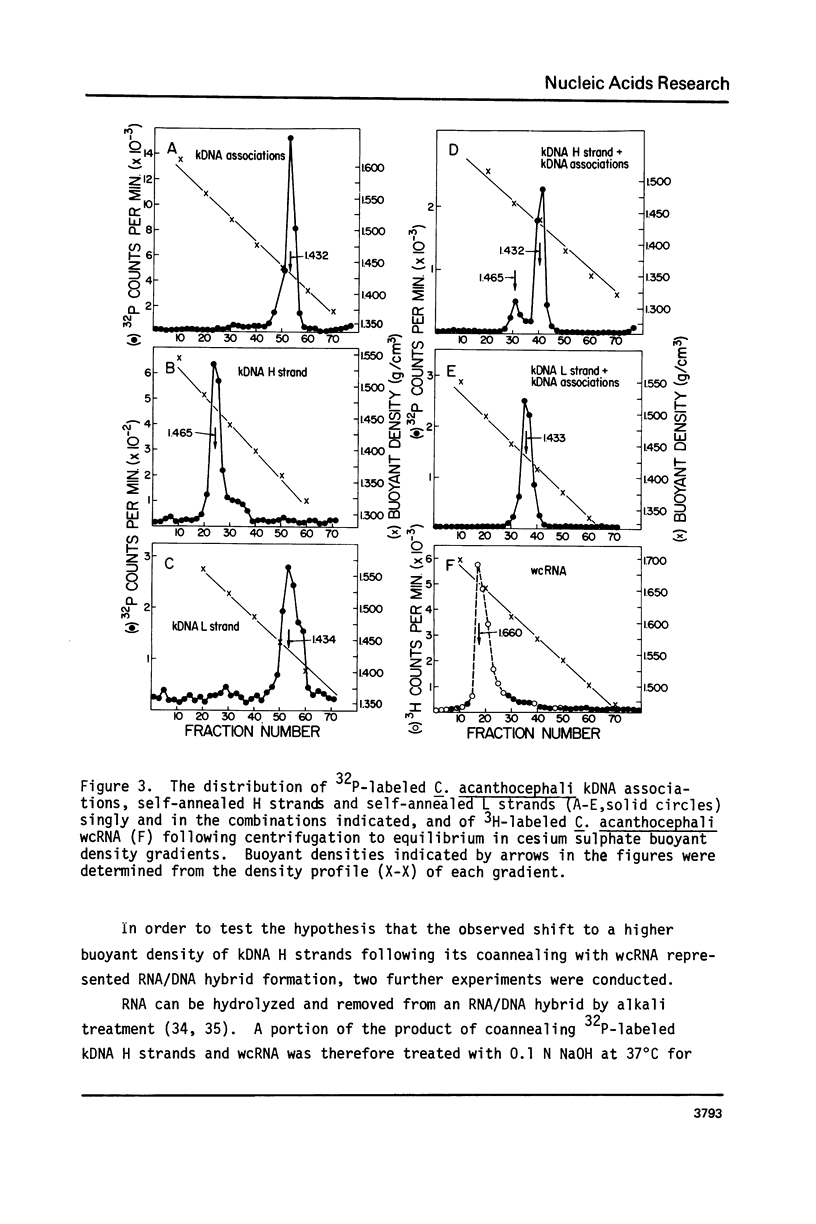
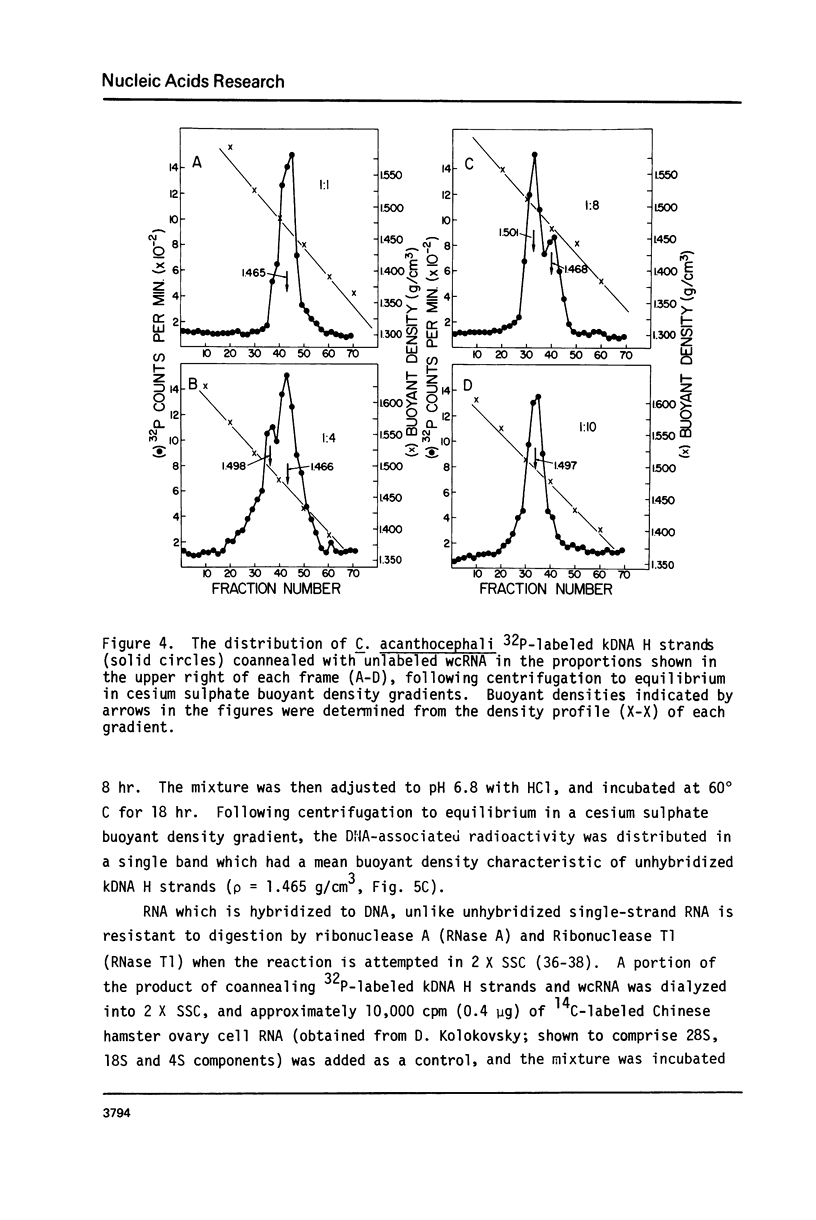
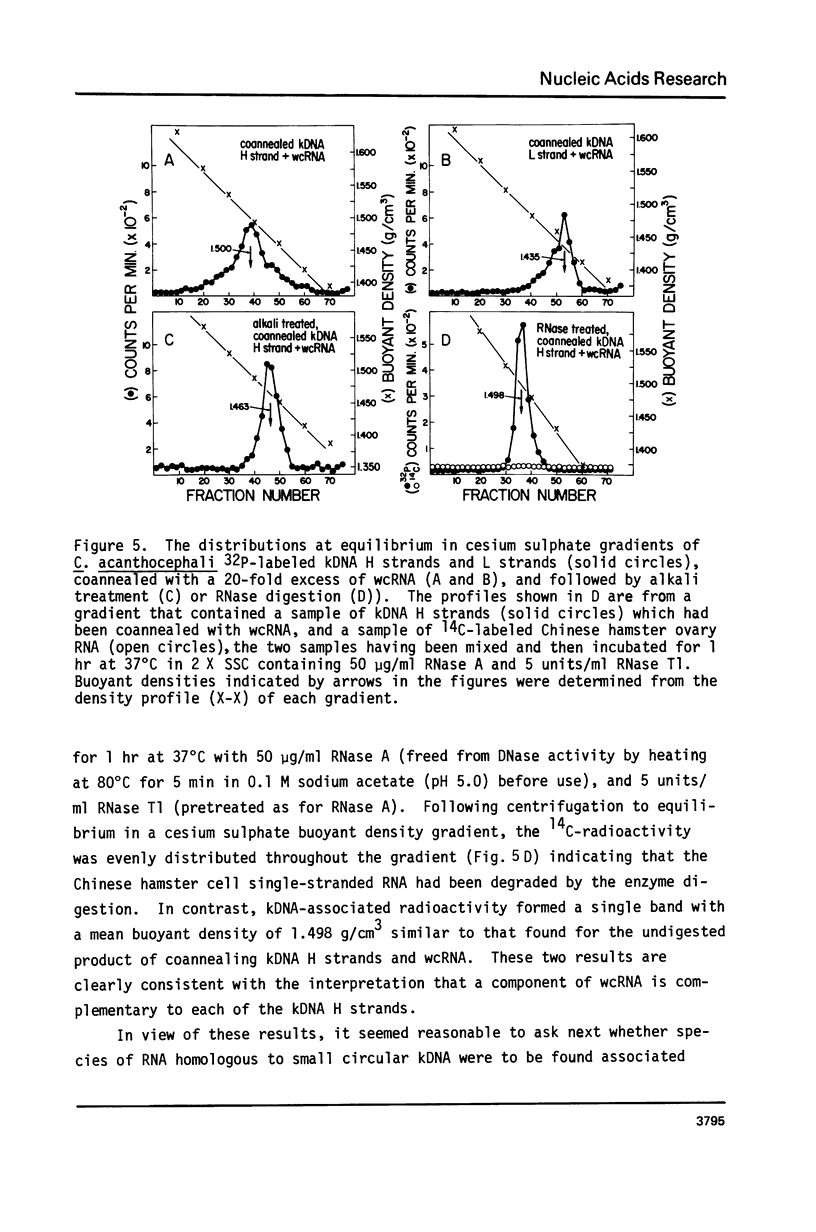
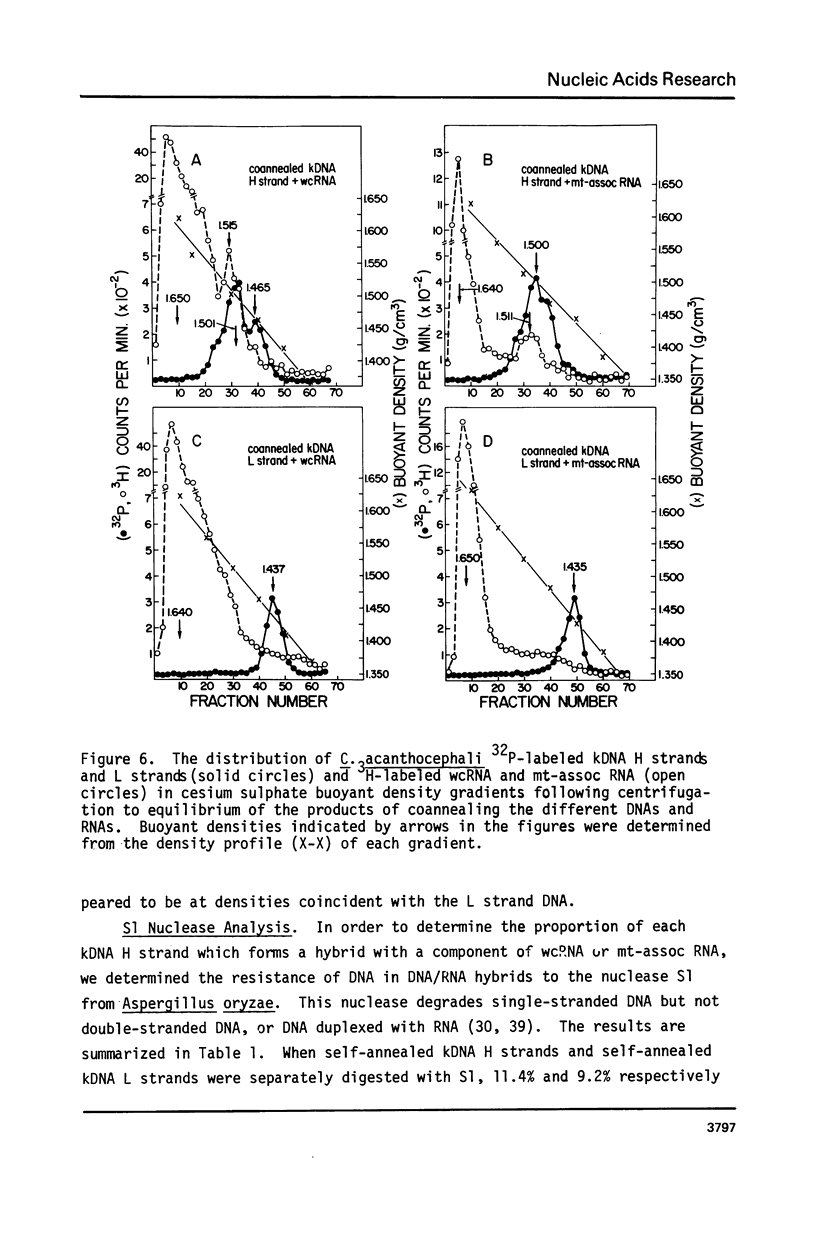
The major component of kinetoplast DNA (kDNA) in the protozoan Crithidia acanthocephali is an association of approximately 27,000, 0.8 micrometers (1.58 x 10(6) dalton) circular molecules apparently held together in a particular structural configuration by topological interlocking. We have carried out hybridization experiments between kDNA samples containing one or the other of the two complementary (H and L) strands of purified 0.8 micrometers molecules derived from mechanically disrupted associations and RNA samples prepared either from whole C. acanthocephali cells or from a mitochondrion-enriched fraction. The results of experiments involving cesium sulfate buoyant density centrifugation indicate that whole cell RNA contains a component(s) complementary to all kDNA H strands, but none complementary to kDNA L strands. Similar results were obtained using mitochondrion-associated RNA. Digestion of RNA/DNA hybrids and suitable controls with the single-strand-specific nuclease S1 indicated that 10% of the kDNA H strand is involved in hybrid formation. Visualization of RNA/DNA hybrids stained with bacteriophage T4 gene 32 protein revealed that hybridation involves a single region of each kDNA H strand, equal to approximately 10% of the molecule length. These data suggest that at least 10% of the small circular component of kDNA of Crithidia acanthocephali is transcribed.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. II. Evidence for complete transcription of mitochondrial DNA. J Mol Biol. 1971 Jan 28;55(2):251–267. doi: 10.1016/0022-2836(71)90195-1. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Steinert M., Van Assel S. Maxi-circles in the kinetoplast DNA of Trypanosoma mega. Exp Cell Res. 1977 Nov;110(1):167–173. doi: 10.1016/0014-4827(77)90283-x. [DOI] [PubMed] [Google Scholar]
- Cheng D., Simpson L. Isolation and characterization of kinetoplast DNA and RNA of Phytomonas davidi. Plasmid. 1978 Jun;1(3):297–315. doi: 10.1016/0147-619x(78)90047-1. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Weislogel P. O., Hoeijmakers J. H., Borst P. Isolation and characterization of kinetoplast DNA from bloodstream form of Trypanosoma brucei. J Cell Biol. 1978 Feb;76(2):293–309. doi: 10.1083/jcb.76.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D. L., Manning J. E., Wolstenholme D. R. Physicochemical properties of kinetoplast DNA from Crithidia acanthocephali. Crithidia luciliae, and Trypanosoma lewisi. J Cell Biol. 1975 Nov;67(2PT1):378–399. doi: 10.1083/jcb.67.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D. L., Wolstenholme D. R., Boyer H. W. Heterogeneity in sensitivity to cleavage by the restriction endonucleases ECORI and HindIII of circular kinetoplast DNA molecules of Crithidia acanthocephali. J Cell Biol. 1978 Nov;79(2 Pt 1):329–341. doi: 10.1083/jcb.79.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- HONIGBERG B. M., BALAMUTH W., BOVEE E. C., CORLISS J. O., GOJDICS M., HALL R. P., KUDO R. R., LEVINE N. D., LOEBLICH A. R., Jr, WEISER J. A REVISED CLASSIFICATION OF THE PHYLUM PROTOZOA. J Protozool. 1964 Feb;11:7–20. doi: 10.1111/j.1550-7408.1964.tb01715.x. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. RNA from the insect trypanosome Crithidia luciliae contains transcripts of the maxi-circle and not of the mini-circle component of kinetoplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):407–411. doi: 10.1016/0005-2787(78)90282-4. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P. Sequence heterogeneity of the mini-circles of kinetoplast DNA of Crithidia luciliae and evidence for the presence of a component more complex than mini-circle DNA in the kinetoplast network. Biochim Biophys Acta. 1975 Nov 4;407(4):473–478. doi: 10.1016/0005-2787(75)90301-9. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Weislogel P. O., Fonck K., Borst P. The structure of kinetoplast DNA. 2. Characterization of a novel component of high complexity present in the kinetoplast DNA network of Crithidia luciliae. Eur J Biochem. 1976 Apr 15;64(1):153–160. doi: 10.1111/j.1432-1033.1976.tb10283.x. [DOI] [PubMed] [Google Scholar]
- Kleisen M. C., Borst P., Weijers P. J. The structure of kinetoplast DNA. 1. The mini-circles of Crithidia lucilae are heterogeneous in base sequence. Eur J Biochem. 1976 Apr 15;64(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10282.x. [DOI] [PubMed] [Google Scholar]
- Laurent M., Steinert M. Electron microscopy of kinetoplastic DNA from Trypanosoma mega. Proc Natl Acad Sci U S A. 1970 Jun;66(2):419–424. doi: 10.1073/pnas.66.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon W., Fouts D. L., Manning J. Sequence arrangement of the 16S and 26S rRNA genes in the pathogenic haemoflagellate Leishmania donovani. Nucleic Acids Res. 1978 Feb;5(2):491–504. doi: 10.1093/nar/5.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. I., Attardi B., Tu C., Attardi G. Evidence for complete symmetrical transcription in vivo of mitochondrial DNA in HeLa cells. J Mol Biol. 1975 Dec 25;99(4):809–814. doi: 10.1016/s0022-2836(75)80187-2. [DOI] [PubMed] [Google Scholar]
- Nichols J. M., Cross G. A. Isolation of mitochondria and mitochondrial RNA from Crithidia fasciculata. J Gen Microbiol. 1977 Apr;99(2):291–300. doi: 10.1099/00221287-99-2-291. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Renger H. C., Wolstenholme D. R. Kinetoplast and other satellite DNAs of kinetoplastic and dyskinetoplastic strains of Trypanosoma. J Cell Biol. 1971 Aug;50(2):533–540. doi: 10.1083/jcb.50.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Wolstenholme D. R. Kinetoplast deoxyribonucleic acid of the hemoflagellate Trypanosoma lewisi. J Cell Biol. 1970 Dec;47(3):689–702. doi: 10.1083/jcb.47.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Wolstenholme D. R. The form and structure of kinetoplast DNA of Crithidia. J Cell Biol. 1972 Aug;54(2):346–364. doi: 10.1083/jcb.54.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou G., Delain E. Electron microscopy of the circular kinetoplastic DNA from Trypanosoma cruzi: occurrence of catenated forms. Proc Natl Acad Sci U S A. 1969 Jan;62(1):210–217. doi: 10.1073/pnas.62.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. M., Atwood K. C., Spiegelman S. On the redundancy of DNA complementary to amino acid tranfer RNA and its absence from the nucleolar organizer region of Drosophila melanogaster. Genetics. 1966 Aug;54(2):663–676. doi: 10.1093/genetics/54.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Da Silva A. Isolation and characterization of kinetoplast DNA from Leishmania tarentolae. J Mol Biol. 1971 Mar 28;56(3):443–473. doi: 10.1016/0022-2836(71)90394-9. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Spencer R., Cross G. A. Lability of RNA from the large cytoplasmic ribosomal subunit of the protozoon Crithidia oncopelti. J Gen Microbiol. 1976 Mar;93(1):82–88. doi: 10.1099/00221287-93-1-82. [DOI] [PubMed] [Google Scholar]
- Steinert M., Assel S. Large circular mitochondrial DNA in Crithidia luciliae. Exp Cell Res. 1975 Dec;96(2):406–409. doi: 10.1016/0014-4827(75)90274-8. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Tewari K. K. Ribosomal-RNA genes in the chloroplast DNA of pea leaves. Biochim Biophys Acta. 1974 Aug 15;361(1):73–83. doi: 10.1016/0005-2787(74)90210-x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Watts R. L., Mathias A. P. The use of bentonite in the isolation of plant polyribosomes. Biochim Biophys Acta. 1967;145(3):828–831. doi: 10.1016/0005-2787(67)90142-6. [DOI] [PubMed] [Google Scholar]
- Weislogel P. O., Hoeijmakers J. H., Fairlamb A. H., Kleisen C. M., Borst P. Characterization of kinetoplast DNA networks from the insect trypanosome Crithidia luciliae. Biochim Biophys Acta. 1977 Sep 20;478(2):167–179. doi: 10.1016/0005-2787(77)90180-0. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. 3. Kinetic complexity of kinetoplast and nuclear DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):267–276. doi: 10.1016/0005-2787(73)90165-2. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. II. Biophysical properties of minicircular DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):254–266. doi: 10.1016/0005-2787(73)90164-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Renger H. C., Manning J. E., Fouts D. L. Kinetoplast DNA of Crithidia. J Protozool. 1974 Nov;21(5):622–631. doi: 10.1111/j.1550-7408.1974.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N. Use of gene 32 protein staining of single-strand polynucleotides for gene mapping by electron microscopy: application to the phi80d3ilvsu+7 system. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4506–4510. doi: 10.1073/pnas.72.11.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]



