Abstract
Background
The aim of this study was to evaluate the antimalarial effects of Iranian flora Artemisia khorassanica against Plasmodium berghei in vivo and pharmacochemistry of its natural components.
Methods
The aerial parts of Iranian flora A. khorasanica were collected at flowering stage from Khorassan Province, northeastern Iran in 2008. They were air-dried at room temperature; powder was macerated in methanol and the extract defatted in refrigerator, filtered, diluted with water, then eluted with n-hexane and finally non-polar components were identified through Gas Chromatography and Mass Spectroscopy (GC-MS). Toxicity of herbal extracts was assessed on naïve NMRI mice, and its anti-malarial efficacy was investigated on infected Plasmodium berghei animals. This is the first application on A. khorssanica extract for treatment of murine malaria. The significance of differences was determined by Analysis of Variances (ANOVA) and Student's t-test using Graph Pad Prism Software.
Results
The herbal extract was successfully tested in vivo for its anti-plasmodial activity through artemisin composition, which is widely used as a standard malaria treatment.
Conclusion
Although, this study confirmed less anti-malarial effects of A. khorssanica against murine malaria in vivo, however there are some evidences on reducing pathophysiology by this medication. In complementary assay, major components were detected by GC-MS analysis in herbal extract including chrysanthenone (7.8%), palmitic acid (7.4%) and cis-thujone (5.8%). The most retention indices of the component are given as n-eicosane, palmitic acid and n-octadecane.
Keywords: Artemisia khorassanica, Iran, Malaria, Pharmacochemistry, Plasmodium berghei
Introduction
Malaria, is one of the most serious and widespread diseases encountered by human. It is an infectious disease caused by the parasite Plasmodia (P.) transmitted by the female anopheles mosquitoes. Four identified species of this parasite exist, which cause different types of human malaria (1). Although all the four species of malaria parasites can infect humans and cause illness, only P. falciparum is known to be potentially life threatening and some of infected persons die, usually because of delayed treatment (2). An annual incidence of 300-500 million clinically cases and 1-2 million death occur in the world (3–6).
As malaria vaccines remain problematic, chemotherapy still is the most important weapon in the fight against the disease (7). The antimalarial drugs including chloroquine, quinine, mefloquine, pyrimethamine and artemisinine are currently used to prevent and treat human malaria. Part of the reason for the failure to control malaria, is the spread of resistance to first-line antimalarial drugs, cross-resistance between the limited number of drug families available, and some multidrug resistance (8). Resistance has emerged to all classes of antimalarial drugs except artemisinin, an endoperoxide antimalarial drug derived as the active component of Artemisia annua, a herbal remedy used in Chinese folk medicine for 2000 years "qinghaosu" (9–12). Artemisinin is a powerful anti-malarial drug with significant activities, which is resistant to chloroquine. It is a natural product, which has the characteristics of high potency against the parasite whilst possessing low toxicity during treatment of malaria infections (13–15).
The genus Artemisia has always been of great pharmaceutical interest and is useful in traditional medicines for a treatment of the variety of diseases (11, 16, 17). A. annua is presently being cultivated on a commercial scale in China and Vietnam for its antimalarial sesquiterpene lactone. The genus is of small herbs found in Northern temperate regions and belongs to the important family Compositae (Asteraceae), one of the most bulky vegetal groupings, which comprises about1000 genera and over 20,000 species. Within this family, Artemisia is included into the family Anthemideae and comprises itself over 400 species, found in Europe and North America, but mainly are dominating the Asia (18–20). Among the Asian Artemisia flora, 150 species were recorded for China, 50 species reported to occur in Japan and 34 species of the genus are found in Iran, of which may be endemic; A. melanolepis Boiss and A. kermanensis Pold (21), A. absinthium (22), A. annua (23), A. dracunculus (24), A. aucheri (25), A. haussknechtii Boiss (26), A. scoparia, A. sieberi (27) and A. sieberi Besser (28).
Pharmacochemical analysis of artemisinin shows that the structure of this compound is rather unique among natural products as it contains the very unusual 1, 2, 4-trioxane ring system. It was sufficiently unusual that it was originally characterized as an ozonide until revised crystallographic analysis provided unambiguous structural elucidation (29–33).
For a drug to be effective against the malaria parasite, it must reach the site of action in sufficient concentration and then interact with the receptors before it is either deactivated and/or eliminated by the host or the parasite. Extensive pharmacological and biochemical evaluation revealed that this compound was a blood schizonticide preferentially imported into malaria-infected erythrocytes via the parasitophorous duct (34). It has been also shown that artemisinin and related trioxanes demonstrate useful activity against selected carcinomas (35). Due to complex chemical structure of artemisinin, the chemical synthesis of the molecule is complex, which results in very low yields and the cost becomes prohibitory to use synthetic approach for its commercial production (36).
The mechanism of the action of artemisinin remains a mystery, although iron appears to be involved in activating this endoperoxide to generate cytotoxic free radicals (37). Several candidates have been hypothesized as targets of artemisinins, including haem and some parasite membrane proteins (37–39), however; none of these has been convincingly shown to be functionally relevant. Recently, some researcher proposed PfATP6, as artemisinin's target (40); however, this conclusion has also been debated (41).
For a better understanding of specific fractions with high efficacy and low side effects, chemical analysis of genus of Iranian A. khorassanica was required. Pharmacochemistry and chemical analysis of different genus of Iranian Artemisia species has been studied and the presence of variety of components including monoterpenes (42), sesquiterpenes (43, 44), sesquiterpene lactones (42, 45, 46) and essential oils (47–51) were fully reported (22–28). This genus is uniform and the chemistry is somewhat diverse. However, most species contain sesquiterpene lactones, especially 11,13- dihydro derivatives (42–51).
The aim of this study was to evaluate Iranian flora A. khorassanica for its antimalarial effects against Plasmodium berghei in vivo and pharmacochemical analysis of its natural components. This is the first report on A. khorasanica extract as flora from the Khorassan Province, northeastern Iran for treatment of murine malaria on P. berghei infected NMRI mice. The major components were also detected by Gas Chromatography and Mass Spectroscopy (GC-MS) analysis.
Materials and Methods
Plant samples
The aerial parts of Iranian flora A. khorasanica were collected at flowering stage from their natural habitats the Shahroud Mountains in Khorassan Province, northeastern Iran in 2008. Voucher specimens were deposited and identified at the Herbarium of the Research Institute of Forests and Rangelands (RIFR), Tehran, Iran.
Extraction of Herbal Extract and Non-polar Compounds
The aerial parts were air-dried at room temperature and were then powdered. The herbs powder (650 g) of A. khorassanica was macerated in methanol (1 lit) and subsequently kept for 48-72 h. It was then filtered and evaporated at reduced pressure to give a crude extract (50 ml). The extract was defatted at -15°C in refrigerator, filtered, added with water (20% w/w) and then eluted with n-hexane (50 ml). Finally, n-hexane phase was collected, evaporated by rotary evaporator (20 ml) and then non-polar components were identified through GC-MS analysis (52).
Gas Chromatography (GC): GC analysis was performed on a Shimadzu 15A gas chromatograph equipped with a spilt/spilt less (ratio 1:30), injector (250°C) and a flame ionization detector (250°C). N2 was used as carrier gas (1 mL/min) and the capillary column used was DB-5 (50 m × 0.2 mm, film thickness 0.32 pin). The column temperature was kept at 60°C for 3 min and then heated to 220°C with a 5°C/min rate and kept constant at 220°C for 5 min. Relative percentage amounts were calculated from peak area using a Shimadzu C-R4A chromatopac without the use of correction factors (53).
GC/Mass Spectrometry (MS): GC/MS analysis was performed using a Hewlett-Packard (HP-6890) with a HP-5MS column (30 m × 0.25 mm, film thickness 0.25 µm). The column temperature was kept at 60°C for 3 min, programmed to 220°C at a rate of 5°C/min, and kept constant at 220°C for 5 min. The flow rate of Helium as carrier gas was (1 mL/min). MS were taken at 70 eV, mass rang, 30 to 350 amu and scan time, 2 scan/ sec (53).
Identification of components: The components of the oil were identified by comparison of their mass spectra with those of a computer library or with authentic compounds and confirmed by comparison of their retention indices either with those of authentic compounds or with data published in the literature (54). The retention indices were calculated for all volatile constituents using a homologous series of n-alkanes (53).
Animals
Male outbred NMRI (Naval Medical Research Institute) mice (supplied by the Laboratory Animal Department, Karaj Production, and Research Complex, Pasteur Institute of Iran) were used in this study. The mice were housed at room temperature (20–23°C) on a 12 h light and 12 h dark cycle, with unlimited access to food and tap water.
Ethical considerations
Experiments with animals were done according to the ethical standards formulated in the Declaration of Helsinki, and measures taken to protect animals from pain or discomfort. It has been approved by institutional ethical review board (Ethical Committee of the Pasteur Institute of Iran), in which the antimalarial test was done.
Malaria parasite
P. berghei NY was kindly donated by Dr. M. J. Dascombe from the School of Life Sciences, University of Manchester, UK. Malaria parasite was maintained by blood passage in NMRI mice when active parasites were required; otherwise it was stored at -70°C in Alserver's solution (2.33% glucose, 0.525% NaCl and 1% sodium citrate in deionised water) and glycerol (9:1 parts by volume) (54).
Inoculation of malaria parasites
Mice were inoculated (0.2 ml) intravenously (iv) into a tail vein with blood from a donor mouse (38% parasitaemia P. berghei) diluted with 0.85% saline to contain 2×106 parasitised red blood cells (PRBC).
Experiments and groups
A) Toxicity assay of A. khorassanica herbal extract in naïve animals
In vivo toxicity was assessed by using herbal extract on naïve NMRI male mice. Animals were divided into four groups (n=8 mice/group), including Group 1 (naïve), Group 2 (low dose), Group 3 (average dose), Group 4 (high dose). A sample of herbal extract were suspended in ethanol and normal saline (1:9), then three different concentrations (low, average and high doses) of herbal extracts including 1, 10 and 100 mg/ml were tested in vivo for its toxicity as test animals and a control group which was injected with drug vehicle. Entire animals in all groups were injected with 200 µl of related solutions subcutaneously (sc) once a day for 8 days.
B) Anti-malarial effects of herbal extract on P. berghei infected mice
Following toxicity assay, the highest dose with the lowest toxicity of herbal extract (100 mg/ml concentration) was selected to apply for its antimalarial activity on male NMRI mice infected with P. berghei. Animals were divided into two groups (n=10 mice/group), including control and test; both groups were infected with murine malaria parasite, P. berghei. Drug vehicle and herbal extract was injected sc into control and test groups respectively once a day with 200 µl of solutions for the period of three weeks.
Assessment of pathology
Parasitaemia
The clinical diagnosis was confirmed by laboratory demonstration of the malaria parasite in the stained smears. In all animals, parasitaemia was determined on different days after infection using blood smears stained with Geimsa stain (Sigma Chemical Co., USA). PRBC were counted in five different fields, each of approximately 200 cells. Results are expressed as the mean percentage (%) of erythrocytes containing Geimsa bodies. Experiments were licensed under the Animals (Scientific Procedures) Act 1986. In compliance with the conditions of this license, infected animals were humanely killed at the onset of the terminal phase of malaria (P. berghei) infection (54, 55).
Assessment of degree of hepato/ splenomegaly
Entire livers and spleens were removed post mortem at the end of the experimental period from mice after induction of terminal general anaesthesia by inhalation of diethyl ether (Sigma Co., Germany). Organ wet weights were measured and compared with controls as indices for degree of hepatomegaly and splenomegaly (56, 57).
Body weight
Body weight was measured initially and at different times of experiment (days 1, 7, 21) using a top pan balance (OHAUS Scale Corp., USA) as a major indication of pathology (56, 67).
Statistical analysis
Values are presented as the mean±SEM for groups of n mice. The significance of differences was determined by Analysis of Variances (ANOVA) and Student's t-test using Graph Pad Prism Software (GraphPad, San Diego, California, USA).
Results
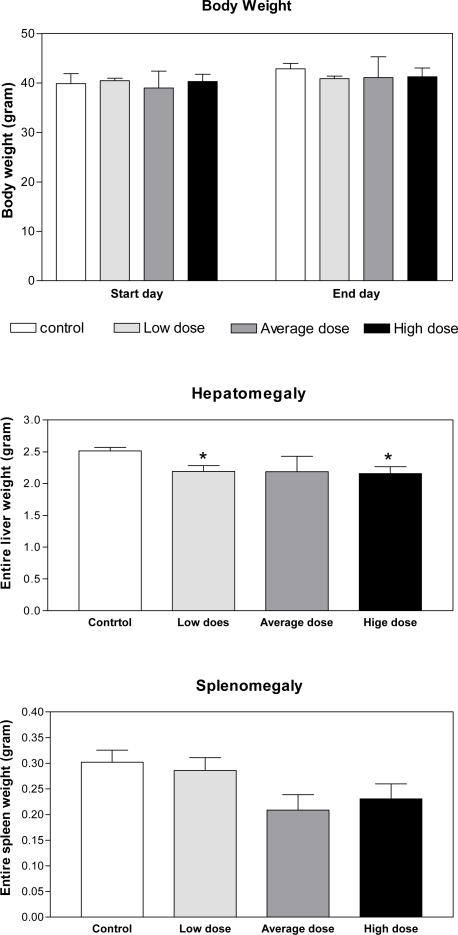
The results of toxicity assay in naïve mice indicated no significant pathophysiological changes in body weight and splenomegaly in test groups as compared with those in control after injection of low, average and high doses of A. khorassanica crude extracts. There was a small reduction (P<0.05) in hepatomegaly of test groups injected with low and high doses of herbal crude extract, which emphasises the anti- symptomatic effects of A. khorassanica (Fig. 1).
Fig. 1.
Toxicity assay and pathophysiological changes induced by A. khorassanica crude extract in naive animals Pathophysiological changes including body weight, hepatomegaly and splenomegaly were evaluated in control and test groups as toxicity assay induced by injection of low, average and high doses of A. khorassanica crude extract (n=8, *P<0.05, ANOVA)
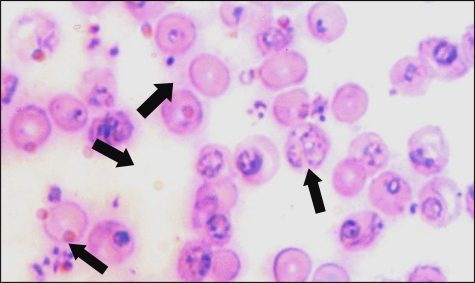
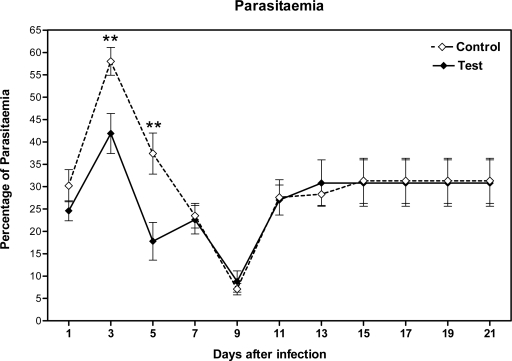
The clinical diagnosis of P. berghei infection was confirmed by laboratory demonstration of the malaria parasite in the stained smears. Parasitaemia was determined using blood smears stained with Geimsa stain from mice (Fig. 2). The observations specifically indicated the inhibitory effects of the A. khorassanica extracts on the early developmental stages of P. berghei by decreasing parasitaemia (P<0.01). This may suggest that the active constituents in the herbal extract may be toxic for P. berghei, thereby inhibiting their development to the erythrocytic stage (Fig. 3).
Fig. 2.
Plasmodium berghei blood-stage forms in Geimsa stained smears from mice. The clinical diagnosis was confirmed by laboratory demonstration of the malaria parasite in the stained smears. Parasitaemia was determined using blood smears stained with Geimsa stain. PRBC were counted in five different fields, each of approximately 200 cells. Results are expressed as the mean percentage of erythrocytes containing Geimsa positive bodies
Fig. 3.
Percentage of parasitaemia in smears from blood of malarial mice Smears were dried in air, fixed by methanol and stained with Geimsa for counting of parasites inside RBC by light microscopy, Test, A. khorassanica crude extract; Control, Drug vehicle (n=10 mice/day/group, Student's t-test, **P<0.01)
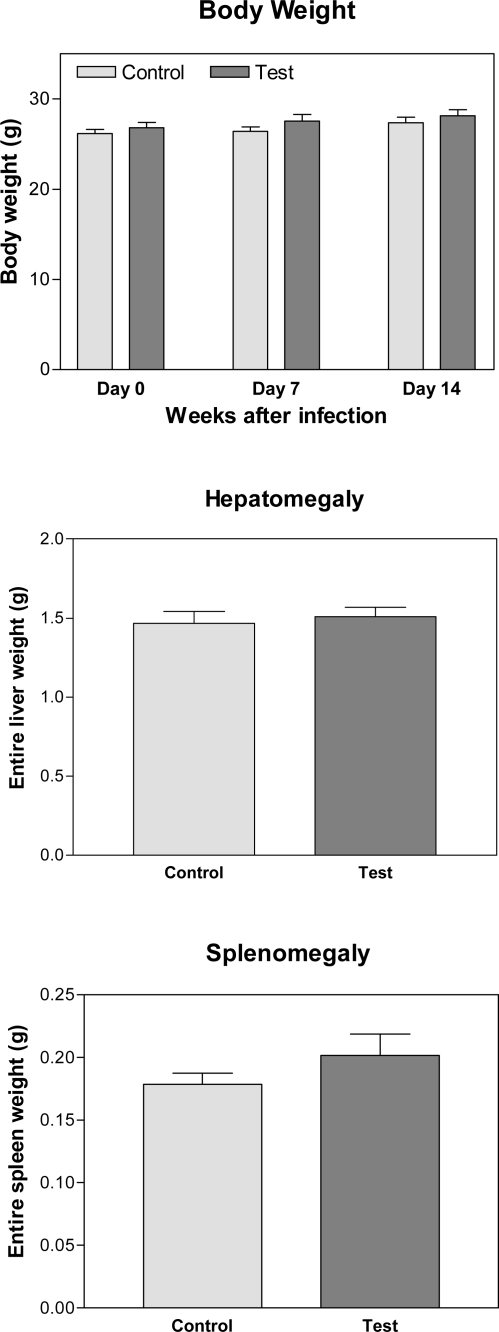
No pathophysiological changes including body weight, hepatomegaly and splenomegaly were detected in control and malarial groups as induction markers for toxicity after injection of crude extract of A. khorassanica in malarial infected animals (Fig. 4).
Fig. 4.
Toxicity assay and pathophysiological changes induced by A. khorassanica crude extract in malarial animals. Pathophysiological changes including body weight, hepatomegaly and splenomegaly were evaluated as indices of toxicity by crude extract of A. khorassanica in control and malarial groups (n=10 mice/day/group, Student's t-test)
The chemical analysis of extract by GC-MS on a HP-6890 instrument resulted in isolation of 31 fractions and identification of effective components. The non-polar components obtained from extract A. khorassanica are listed in Table 1, in which the percentage and retention indices of the component are given. Nineteen compounds, representing 53.7% of the total constituents in the non-polar compounds of A. khorassanica were characterized by chrisanthenone (7.8%) and palmitic acid (7.4%). Monoterpenes constitute the major fraction of the oil (29.9%), while sesquiterpenes and other compounds accounted to 6.2% and 17.6% respectively.
Table 1.
The non-polar constituents obtained from extract of A. khorassanica by GC-MS. The non-polar constituents obtained from A. khorassanica were assessed by GC-MS and listed in this table, in which the percentage and retention indices of the component are given.
| Compound | Retention indices (RI) | A. khorassanica (%W/W) |
|---|---|---|
| n-nonane | 899 | 0.6 |
| dihydromyrcene | 947 | 0.5 |
| 4-methyl nonane | 958 | 0.6 |
| n-decane | 999 | 2.1 |
| 1,8-cineole* | 1033 | 4.0 |
| cis-thujone | 1102 | 5.8 |
| trans-thujone* | 1114 | 3.7 |
| isophorone | 1118 | 2.7 |
| chrysanthenone | 1123 | 7.8 |
| champhore | 1143 | 5.4 |
| n-dodecane | 1199 | 1.1 |
| n-tetradecane | 1398 | 1.5 |
| davanone | 1586 | 2.8 |
| n-hexadecane* | 1600 | 1.7 |
| beta-davanone-2-ol* | 1717 | 3.4 |
| n-octadecane* | 1800 | 1.4 |
| palmitic acid* | 1984 | 7.4 |
| n-eicosane* | 2000 | 1.2 |
Discussion
Malaria is one of the most serious and widespread diseases and as malaria vaccines remain problematic, chemotherapy still is the most important weapon against the disease. However, the increasing drug resistance continues to be the main problem, therefore, the limited clinical repertoire of effective drugs and the emergence of multi-resistant strains substantiate the need for new anti-malarials (7).
The results of this study indicated no toxicity in naïve mice with even high dose of A. khorassanica crude extracts, which confirms its lowest side effects. Although, the inhibitory effects of the A. khorassanica extract on the early decline of P. berghei parasitaemia highlights its anti-malarial activity, however this concept no longer can be observed in the late infection. This may be due to the metabolic process of A. khorassanica crude extract by mice and reduction of its concentration in body. Malaria parasite actually decreases body weight and increases hepatomegaly and splenomegaly. Crude extract of A. khorassanica represented its anti-symptomatic effects by stabilization of body, liver and spleen weights.
In this study, 31 fractions were isolated from A. khorssanica extract and effective components were identified by the chemical analysis of extract by GC-MS. The highest percentages of the components are indicated as chrysanthenone (7.8%), palmitic acid (7.4%) and cis-thujone (5.8%). The most retention indices of the component are given as n-eicosane, palmitic acid and n-octadecane. In other studies, various species of the genus Artemisia are used for their pharmacological, antimicrobial, antioxidant activity. Three species of this genus, A. scoparia, A. sieberi and A. aucheri are widely distributed in desert area of Iran (27). This is the first report on application of A. khorssanica extract on the treatment of murine malaria. The herbal extract was successfully tested in vivo for its anti-malarial activity through artemisin composition, which is widely used as a standard malaria therapy. Although, this study confirmed anti-malarial effects of A. khorssanica extracts against murine malaria in vivo during early infection, however there are more efficacies on pathophysiological symptoms by this medication. These observations provide the basis for the traditional use of this herb in treatments of malaria disease (58).
The route of inoculation is important factor to determine herbal efficacy. Although, subcutaneous injection was used in this study, other routes may be recommended for future studies. Moreover, active derivatives of Artemisia including artemether, arteether and artesunate, which are used for oral, intramuscular, rectal and intravenous administration (59). More investigations on different Plasmodia and animal hosts are needed to better clarify anti-malarial activity of Iranian flora A. khorassanica and analysis of its natural components.
Acknowledgements
This work was funded by Department of Parasitology, Pasteur Institute of Iran and collaboration with Department of Applied Chemistry, Islamic Azad University, Qom Branch, Qom, Iran and Payame Noor University of Tehran centre, Iran. We thank Department of Biochemistry, Pasteur Institute of Iran for assistance in methodology. The authors declare that they have no conflicts of interest.
References
- 1.Hardman JG, Limbird LE. Drugs used in the chemotherapy of malaria. In: Brunton L, Lazo J, Parker K, editors. The Goodman and Gilman's pharmacological bases of therapeutics. 10th ed. New York, USA: Mc Graw-Hill; 2001. pp. 1069–1100. [Google Scholar]
- 2.Peter IT, Anatoli VK. 1st ed. WDC: ASM Press; The current global malaria situation; Malaria parasite biology, pathogenesis and protection. [Google Scholar]
- 3.Martin SA, Bygbjerg IC, Joil GB. Are multilateral malaria research and control programs the most successful? Lessons from the past 100 years in Africa. Am J Trop Med Hyg. 2004;2:268–78. [PubMed] [Google Scholar]
- 4.Miller LH, Good MF, Million G. Malaria pathogenesis. Biochem Systemat Ecolog. 1994;264:1878–83. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 5.More CM. Reaching maturity-25 years of the TDR. Parasitol Today. 2002;16:522–8. doi: 10.1016/s0169-4758(00)01815-9. [DOI] [PubMed] [Google Scholar]
- 6.David AF, Philip JR, Simon LC, Reto B, Solomon N. Antimalarial drug discovery: Efficacy models for compound screening. Nat Rev. 2004;3:509–20. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 7.Turschner S, Efferth T. Drug resistance in Plasmodium: natural products in the fight against malaria. Mini Rev Med Chem. 2009;9:206–12. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]
- 8.Olliaro P, Cattani J, Wirth D. Malaria, the submerged disease. J Am Med Assoc. 1996;275:230–3. [PubMed] [Google Scholar]
- 9.White N, Olliaro P. Strategies for the prevention of anti-malarial drug resistance: rationale for combination therapy for malaria. Parasitol Today. 1996;12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- 10.He SP, Tan GY, Li G, Tan WM, Nan TG, Wang BM, Li ZH, Li QX. Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the antimalaria active ingredient artemisinin in the Chinese herb Artemisia annua L. Anal Bioanal Chem. 2009;393:1297–303. doi: 10.1007/s00216-008-2527-5. [DOI] [PubMed] [Google Scholar]
- 11.Willoughby JA, Sr, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203–13. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsenault PR, Wobbe KK, Weathers PJ. Recent advances in artemisinin production through heterologous expression. Curr Med Chem. 2008;15:2886–96. doi: 10.2174/092986708786242813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klayman DL, Lin AJ, Acton N, Scovill JP, Hock JM, Milhous WK, Theoharides AD. Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J Nat Prod. 1984;47:715–7. doi: 10.1021/np50034a027. [DOI] [PubMed] [Google Scholar]
- 14.Ro DK, Ouellet M, Paradise EM, Burd H, Eng D, Paddon CJ, Newman JD, Keasling JD. Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol. 2008;8:83. doi: 10.1186/1472-6750-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ridder S, van der Kooy F, Verpoorte R. Artemisia annua as a self-reliant treatment for malaria in developing countries. J Ethnopharmacol. 2008;120:302–14. doi: 10.1016/j.jep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop Anim Health Prod. 2006;38:497–503. doi: 10.1007/s11250-006-4390-8. [DOI] [PubMed] [Google Scholar]
- 17.Romero MR, Serrano MA, Vallejo M, Efferth T, Alvarez M, Marin JJ. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV) Planta Med. 2006;72:1169–74. doi: 10.1055/s-2006-947198. [DOI] [PubMed] [Google Scholar]
- 18.Heywood VH, Humphries CJ. Anthemideae systematic review. In: Heywood VH, Harbord JB, Turner BL, editors. The biology and chemistry of the compositae. 2nd ed. London: Academic Press; 1977. pp. 852–88. Chapter 31. [Google Scholar]
- 19.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Zhao Y, Wang Y. Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol. 2006;72:11–20. doi: 10.1007/s00253-006-0452-0. [DOI] [PubMed] [Google Scholar]
- 21.Rechinger KH. Artemisia in Flora Iranica. In: Rechinger KH, Hedge IC, editors. Compositae, Chapter 158, Akademische Druck and Verlagsanatalt, Graz, Austria. 1986. p. 214. [Google Scholar]
- 23.Rezaeinodehi A, Khangholi S. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak J Biol Sci. 2008;11:946–9. doi: 10.3923/pjbs.2008.946.949. [DOI] [PubMed] [Google Scholar]
- 23.Khangholil S, Rezaeinodehi A. Effect of drying temperature on essential oil content and composition of sweet wormwood (Artemisia annua) growing wild in Iran. Pak J Biol Sci. 2008;11:934–7. doi: 10.3923/pjbs.2008.934.937. [DOI] [PubMed] [Google Scholar]
- 24.Maleki A, Zarasvand MA. Heavy metals in selected edible vegetables and estimation of their daily intake in Sanandaj, Iran. SE Asn J Trop Med Pub Health. 2008;39:335–40. [PubMed] [Google Scholar]
- 25.Asgary S, Dinani NJ, Madani H, Mahzouni P. Ethanolic extract of Artemisia aucheri induces regression of aorta wall fatty streaks in hypercholesterolemic rabbits. Phar-mazie. 2008;63:394–7. [PubMed] [Google Scholar]
- 26.Jalali Heravi M, Sereshti H. Determination of essential oil components of Artemisia haussknechtii Boiss. using simultaneous hydrodistillation-static headspace liquid phase microextraction-gas chromatography mass spectrometry. J Chromatog A. 2007;1160:81–9. doi: 10.1016/j.chroma.2007.05.096. [DOI] [PubMed] [Google Scholar]
- 27.Farzaneh M, Ahmadzadeh M, Hadian J, Tehrani AS. Chemical composition and antifungal activity of the essential oils of three species of Artemisia on some soil-borne phytopathogens. Commun Agric Appl Biol Sci. 2006;71(Pt B):1327–33. [PubMed] [Google Scholar]
- 28.Bagheri R, Chaichi MR, Mohseni-Saravi M, Amin GR, Zahedi G. Grazing affects essential oil compositions of Artemisia sieberi Besser. Pak J Biol Sci. 2007;10:810–13. doi: 10.3923/pjbs.2007.810.813. [DOI] [PubMed] [Google Scholar]
- 29.Gu JD, Chen KX, Jiang HL, Ji RY. A model molecule study of the O-centered and the C-centered free radical intermediates of artemisinin. Mol. Struct (Theochem) 1999;459:103. [Google Scholar]
- 30.Gu JD, Chen KX, Jiang HL, Leszczynski J. The Radical Transformation in Artemisinin: A DFT Study. J Physical Chemistry Part A. 1999;103:9364. [Google Scholar]
- 31.Castilho PC, Gouveia SC, Rodrigues AI. Quantification of artemisinin in Artemisia annua extracts by 1H-NMR. Phytochem Anal. 2008;19:329–334. doi: 10.1002/pca.1053. [DOI] [PubMed] [Google Scholar]
- 32.Ma C, Wang H, Lu X, Li H, Liu B, Xu G. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J Chromatogr A. 2007;1150:50–3. doi: 10.1016/j.chroma.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 33.Haynes RK. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem. 2006;6:509–37. doi: 10.2174/156802606776743129. [DOI] [PubMed] [Google Scholar]
- 34.Vyas N, Avery BA, Avery MA, Wyandt CM. Carrier-Mediated Partitioning of Artemisinin into Plasmodium falciparum-Infected Erythrocytes. Antimicrob Agent Chemoth. 2002;46:105–9. doi: 10.1128/AAC.46.1.105-109.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meschnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Mol Biol Rev. 1996;60:301–15. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya A, Mishra LC, Bhasin VK. In vitro activity of artemisinin in combination with clotrimazole or heat-treated amphotericin B against Plasmodium falciparum . Am Soc Trop Med Hyg. 2008;78:721–8. [PubMed] [Google Scholar]
- 37.Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–60. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 38.Asawamahasakda W, Benakis A, Meshnick SR. The interaction of artemisinin with red cell membranes. J Lab Clin Med. 1994;123:757–62. [PubMed] [Google Scholar]
- 39.Bhisutthibhan J, Pan XQ, Hossler PA, Walker DJ, Yowell CA, Carlton J, Dame JB, Meshnick SR. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem. 1998;273:16192–8. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 40.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. J Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 41.Mercereau-Puijalon O, Fandeur T. Antimalarial activity of artemisinins: identification of a novel target? Lancet. 2003;362:2035–6. doi: 10.1016/S0140-6736(03)15146-X. [DOI] [PubMed] [Google Scholar]
- 42.Rustaiyan A, Bamoniri A, Raffatrad M, Jakupovic J, Bohlman F. Eudesmane derivatives and highly oxygenated monoterpenes from Iranian Artemisia species. Phytochem. 1987;26:2307–10. [Google Scholar]
- 43.Weyerstahl P, Schneider S, Marschall H, Rustaiyan A. The essential oil of Artemisia sieberi . J Flav Frag. 1993;8:139–45. [Google Scholar]
- 44.Weyerstahl P, Schneider S, Marschall H, Rustaiyan A. Terpenes and Terpene Derivatives, XXXI. New Bisabolene derivatives and a Salsolene Ketone from Artemisia sieberi Bess. Liebigs Annalen der Chemie. 1993;2:111–6. [Google Scholar]
- 45.Rustaiyan A, Sigari H, Jakupovic J, Grenz M. A sesquiterpene lactone from Artemisia diffusa. Phytochem. 1989;28:2723–5. [Google Scholar]
- 46.Rustaiyan A, Zare K, Ganji MT, Sadri HA. A melampolide and two dihydro artemorin derivatives from Artemisia gypsacea . Phytochem. 1989;28:1535–6. [Google Scholar]
- 47.Rustaiyan A, Balalaei S, Mohammadi F, Masoudi S, Yari M. Comparison of the volatile oils of Artemisia santolina Schrenk and Artemisia gypsacea Krasch., M. Pop. et Lincz. ex Poljak. from Iran. J Ess Oil Res. 2000;12:330–2. [Google Scholar]
- 48.Rustaiyan A, Komeilizadeh H, Masoudi S, Monfared A, Yari M, Kardar M, Shahgholi M. Composition of the volatile oil of Artemisia deserti Krash. and Artemisia oliveriana J. Gayex DC. from Iran. J Sci IR Irn. 2000;11:213–5. [Google Scholar]
- 49.Sefidkon F, Jalili A, Mirhaji T. Essential oil composition of three Artemisia spp. from Iran. J Flav Frag. 2000;17:150–2. [Google Scholar]
- 50.Morteza-Semnani K, Akbarzadeh M, Moshiri K. Essential oil composition of Artemisia fragrans Willd. from Iran. J Flav Frag. 2005;20:330–1. [Google Scholar]
- 51.Nematollahi F, Rustaiyan A, Larijani K, Nadimi M, Masoudi S. Essential oil composition of Artemisia biennis Wild. and Pulicaria undulate (L.) C.A. Mey., two compositae herbs growing wild in Iran. J Ess Oil Res. 2006;18:339–41. [Google Scholar]
- 52.Rustaiyan A, Nahrevanian H, Kazemi M. A new antimalarial agent; Effect of extracts of Artemisia diffusa against Plasmodium berghei . Pharmacog Mag. 2009;4:1–7. [Google Scholar]
- 53.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream, Illinois: Allured Publishing Corporation; 2001. pp. 61–424. [Google Scholar]
- 54.Nahrevanian H, Dascombe MJ. Nitric oxide and reactive nitrogen intermediates during lethal and non-lethal strains of murine malaria. Parasit Immunol. 2001;23:491–501. doi: 10.1046/j.1365-3024.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 55.Nahrevanian H, Dascombe MJ. Expression of inducible nitric oxide synthase (iNOS) mRNA in target organs of lethal and non-lethal strains of murine malaria. Parasit Immunol. 2002;24:471–8. doi: 10.1046/j.1365-3024.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 56.Nahrevanian H, Farahmand M, Aghighi Z, Assmar M, Amirkhani A. Pharmacological evaluation of anti-leishmanial activity by in vivo nitric oxide modulation in Balb/c mice infected with Leishmania major MRHO/IR/75/ER; An Iranian strain of cutaneous leishmaniasis. Exp Parasitol. 2007;116:233–40. doi: 10.1016/j.exppara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Dascombe MJ, Nahrevanian H. Pharmacological assessment of the role of nitric oxide in mice infected with lethal and nonlethal species of malaria. Parasit Immunol. 2003;25:149–59. doi: 10.1046/j.1365-3024.2003.00618.x. [DOI] [PubMed] [Google Scholar]
- 58.Yazdanparast R, Shahriyary L. Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vascul Pharmacol. 2008;48:32–7. doi: 10.1016/j.vph.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 59.van Agtmael MA, Eggelte TA, van Boxtel CJ. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999;20:199–205. doi: 10.1016/s0165-6147(99)01302-4. [DOI] [PubMed] [Google Scholar]