Abstract
Background
Ovine babesiosis is the most important haemoparasitic tick-borne disease of small ruminants in Iran caused by Babesia ovis, B. motasi, and B. crassa. The aim of this study was to characterize the species of ovine Babesia species isolated from different geographical region of Iran.
Methods
One hundred fifty four blood samples collected from animals, which demonstrated the pale mucous membranes or hyperthermia. The specimens were transferred to the laboratory and the blood smears stained with Geimsa, the morphological and biometrical data of parasite in any infected erythrocyte have been considered. Extracted DNA from each blood samples were used in PCR and semi nested- PCR in order to confirm the presence of the species.
Results
Microscopical observation on 154 blood smears determined 38 (24.67%) and 40 (26%) samples were infected by Babesia and Theileria respectively. The mixed infections occurred in four (2.6%) samples. The results of the PCR assays showed nine (5.85%), 81 (53%) and 18 (11.7%) were distinguished as Babesia, Theileria and mixed infection, respectively. Semi nested- PCR did not confirm the presence of B. motasi.
Conclusion
The causative organism of many cases of haemoprotozoal diseases, which recorded in previous studies, could be B. ovis or Theileria lestoquardi. The result confirmed that B. ovis was only species which causes babesiosis in the study areas. It seems that the biometrical polymorphisms could exist in B. ovis in Iran. This polymorphism could be a main problem in differentiation between B. ovis and B. motasi and it could be dissolved by specific PCR analysis.
Keywords: Babesia ovis, Babesia mutasi, PCR, Semi nested – PCR
Introduction
vine babesiosis is the most important haemoparasitic tick-borne disease of small ruminants in tropical and subtropical areas of the world (1). These parasites are detected in Iran as Babesia ovis, B. motasi and B. crassa (2–4). Ovine babesiosis is an important disease in the livestock, which causes high mortality and morbidity; it causes high economical losses annually in Iran (5). Since Rhipicephalus bursa is the major and dominant vector of B. ovis in Zagros Mountainous area (6), the highest infection rate for B. ovis is reported in this area (58.81%). The infection rate in Caspian Sea, central area, desert, and semi desert areas are demonstrated as 15.93%, 13.22% and 12.04%, respectively (7).
Microscopic examination of Geimsa stained blood smears is the common method for diagnosis and identification of this piroplasms in Iran, which have some technical problems cause false morphological diagnosis and in some cases, are impossible due to carriers (8); and the low sensitivity of the method does not permit its use in epidemiological investigations (9). Seroepidemiological survey on B. ovis performed by IFAT in different geographic areas of Iran has indicated a seroprevalence of 36% in the country (7). Serological study of the parasite determined an average seroprevalence of 47.5% in Khouzestan province (10).
The lack of the specificity due to cross reactivity with other species of Babesia has been observed in serological investigations (11). Molecular techniques have been perfect methods for diagnosis of babesiosis and theileriosis, (12, 13). Shayan et al. described the using PCR technique for simultaneous differentiation between Theileria spp. and Babesia spp. on stained blood smear (8). Aktas et al. used the PCR technique for the specification of B. ovis in sheep and goats in Eastern Turkey (14). Shayan et al. recently described biometrical and genetical characterization of large B. ovis in Iran. They concluded that the morphologically large B. ovis showed a milder clinical signs compared to the small one (15). The small shape of B. motasi described in certain cases from northern Europe, was genetically distinguishable from B. ovis (12).
There are still some gaps in contributed species of Babesia, which causes babesiosis in different geographical region of Iran. Therefore, the aim of this study was to characterize the species of ovine Babesia isolated from those areas.
Materials and Methods
The study was conducted during the tick activity seasons in six different sheep rearing provinces where the ovine babesiosis was recorded by the Parasitic Diseases Control Group of Veterinary Organization as indigenous disease (Eastern Azerbaijan, Western Azerbaijan, Khouzestan, Northern Khorasan, Eillam, and Central).
Veterinary staff provided us with the latest information about infected flocks in each province. Local veterinarians have also checked the suspected animals for clinical signs of ovine babesiosis. 2.5 ml blood samples were collected from peripheral vein into the labeled vessels containing of 2.5 ml Alsever's solution1 prior to the thin blood smear preparation. Generally, 154 blood samples collected from animals, which demonstrated the pale mucous membranes or hyperthermia; the specimens were transferred to the parasitological laboratory of Veterinary Faculty in Tehran for further analysis.
Geimsa staining
The fixed blood smears in methanol were stained with Geimsa in order to determine of the presence of haemoprotozoal parasites. Then the morphological and biometrical parameters including the long and short axis, shape and site location of parasite in any infected erythrocyte have been considered for differential diagnosis (9, 16).
DNA extraction
Babesia genomic DNA was extracted from suspected sheep blood samples as described previously (17). The DNA was air-dried, dissolved in TE buffer (10 mM Tris-HCl pH: 8, 0.1 mM EDTA), and kept at −20° C until use.
PCR analysis
In order to simultaneous differentiation between Theileria and Babesia, PCR technique was used with specific primers for Babesia and Theileria spp. derived from flanking part of hyper variable region of 18ssrRNA (Table 1). The PCR products of Theileria spp. and Babesia spp. were 426–430 bp and 389–402 bp, respectively (8, 18).The difference of approximately 30 bp in the nucleotide sequence of the PCR products is easily revealed in 1.5 % agarose gel (8).
Table 1.
The sequences for primers in PCR from the hypervariable region V4 of the 18S rRNA gene of piroplasms Babesia and Theileria and primers for Semi nested -PCR from B. ovis and B. motasi from the same corresponding gene (18)
| PCR product | Nucleotide sequences | Publication references and gene bank code | Gene | Primer |
|---|---|---|---|---|
| 389–402bp (Babesia) | 5′cacagggaggtagtgacaag3′ | Hypervariable region V4 OF 18S rRNA (Schnittger et al 2004) | 18SrRNA gene sense | P1 |
| 426–430bp (Theileria) | 5′aagaatttcacctatgacag3′ | AJ006446 | 18SrRNA gene antisense | P2 |
| 186 | 5′gtctgcgcgcggcctttgcg3′ | AY260178 | B.ovis | P3 |
| 205 | 5′ cgcgattccgttattggag3′ | AY260179 | B.motasi | P4 |
The PCR was performed on 25 µl total volume including one- time PCR buffer, 0.1U Taq polymerase (Cina gene, Iran), 0.5 µl of each primer (P1/P2, 20 mM, Cina gen), 125 µM of each deoxadenosine triphosphate, deoxythymidine triphosphate, deoxycytidine triphosphate and deoxyguanosine triphosphate (Fermenta-) and 1.5 mM MgCl2 in an automatic DNA Thermocycler (Eppendorf) with the following program : 5 min incubation at 95°C to denature double- strand DNA, 38 cycles of 45s at 94°C, 45s at 56°, 45s at 72°C and Finally, PCR was completed with the additional extension step for 10 min. The amplified products were resolved by 1.5% agarose gel electrophoresis and stained with ethidium bromide for visual detection by ultraviolet transillamination.
Semi nested-PCR
In order to differential diagnosis of B. ovis and B. motasi, semi nested- PCR technique of the PCR products were done with primer P2 as an antisense primer and P3, P4 as sense primers (Table 1) that derived from V4 region of 18ssrRNA (18). This technique was performed on 25µl total volume including one time PCR buffer, 0.1U Taq polymerase (Cina gen) dNTPs (each one, Cina gen) and 1.5 mM MgCl2 in automated Thermocycler (Eppendorf) with the following program: 5 min incubation at 95°C to denature double- strand DNA, 35 cycles of 1 min at 94°C, 1 min at 60°C, 1 min at 72°C and finally, PCR was completed with the additional extension step for 10 min. The PCR products were analyzed on 2 % agarose gel in 0.5X TBE buffer and visualized using ethidium bromide and an UV illuminator.
Statistical Analysis
The data analysis was performed by Chi-square, ANOVA, Fishers exact, and Duncan tests using SPSS 16. The differences were considered statistically significant when P≤0.05.
Results
Microscopical observation on 154 blood smears determined 38 (24.67%) and 40 (26%) samples were infected by Babesia and Theileria, respectively. The mixed infections occurred in four (2.6%) samples (Table 2).
Table 2.
Percentage of Babesia and Theileria infection with microscopy examination on the basis of provinces
| Province | Number of sampl. | Babesia infection n (%) | Theileria infection n(%) | Mixed infection. n(%) | Negative sample. n(%) |
|---|---|---|---|---|---|
| Eastern Azerbaijan | 16 | 14(87.5) | – | 2(12.5) | – |
| Western Azerbaijan | 25 | 13(52) | – | – | 12(48) |
| Northern Khorasan | 32 | – | 2(6.25) | 2(6.25) | 28(87.5) |
| Khouzestan | 16 | 5(31.25) | 10(62.5) | – | 1(6.25) |
| Eillam | 39 | – | 20(51.28) | – | 19(48.75) |
| Central | 26 | 6(23.07) | 8(30.76) | – | 12(46.17) |
| Total number | 154 | 38(24.67) | 40(26) | 4(2,6) | 72(46.75) |
Morphological study of infected blood smears showed different occurrence of parasite site location in erythrocytes, marginal, sub marginal and central were determined as 61.71%, 26.87%, 11.41%, respectively (Table 3). Babesia piroplasma shapes were distinguished based on single round, double round, single pyriform and double pyriform with obtuse or acute angle summarized in Table 4. Morphometrical parameters recognized based on long and short axis determined seven types of measurement sizes (Table 5).
Table 3.
Percentage of Babesia piroplasma on the basis of site location
| Site location | Observed numbers in 104 RBC | Mean numbers in 104 RBC(SD) | Percentage |
|---|---|---|---|
| Marginal | 411 | 10.55(±7.49) | 61.71 |
| Sub marginal | 179 | 4.31(±3.39) | 26.87 |
| Central | 76 | 1.87(±1.8) | 11.41 |
SD(Standard Deviation)
Table 4.
Percentage of Babesia infection on the basis of different shapes of parasites
| Forms | Observed number in 104 | Mean numbers in 104RBC(SD) | Forms percentage | Minimum | Maximum |
|---|---|---|---|---|---|
| Single round | 376 | 10.8(±7.84) | 53.18 | 0 | 10 |
| Double round | 126 | 3.58(±3.2) | 17.82 | 0 | 30 |
| Double pyriform with acute angle | 125 | 1.45(±5.7) | 17.68 | 0 | 15 |
| Double pyriform with obtus angle | 55 | 2.92(±3.48) | 7.77 | 0 | 34 |
| Single pyriform | 25 | 0.63(±1.3) | 3.53 | 0 | 5 |
SD(Standard Deviation)
Table 5.
Percentage of Babesia piroplasma on the basis of measurement (µm)
| Short axis | Long axis | ||||||
|---|---|---|---|---|---|---|---|
| Measurement (µm) | 1×1 | 1.5×1 | 2×1.5 | 1.5×2 | 2×2 | 2.5×2 | 3×2 |
| Round | 130 | – | – | – | 52 | – | – |
| Pyriform | 195 | 85 | 100 | 195 | 306 | 113 | 39 |
The size of typical paired pyriforms with acute angle was with mean of 1−1.5×2 micrometer (µm) and with obtuse angle was mean of 3×2 µm. The round forms also in different sizes were detectable, for example, 1×1 and 2×2 µm. Babesia spp. in small ruminants can be recognized on the basis of morphometrical data.
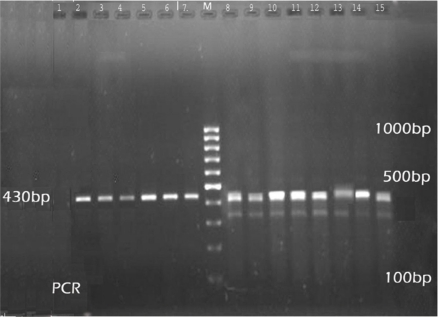
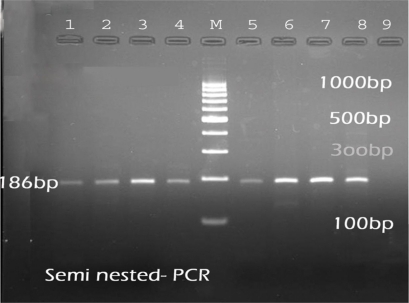
The results of the PCR assays showed nine (5.85%) were due to Babesia infection (Table 6). The PCR product of Babesia species and Theileria species is, 389- 402 bp and 426- 430 bp (Fig. 2). The results of semi nested-PCR showed that the PCR product could not be amplified with the primers specific for B. motasi, but the amplification could only be revealed by primers specific for B. ovis that detected 186bp PCR product (Fig. 3).
Table 6.
Percentage of Babesia and Theileria infection with molecular method based on the provinces
| Province | Number of sample | Babesia infection n(%) | Theileria infection n(%) | Mixed infection n(%) | Negative sample n(%) |
|---|---|---|---|---|---|
| Eastern Azerbaijan | 16 | 4 (25) | 1(6,25) | 7(43.75) | 4 (25) |
| Western Azerbaijan | 25 | 1(4) | 1(4) | – | 23 (92) |
| Northern Khorasan | 32 | – | 26(81.25) | 3(9.37) | 3(9.37) |
| Khouzestan | 16 | – | 14(87.5) | 1(6.2) | 1(6.2) |
| Eillam | 39 | – | 33(84.61) | 2(5.12) | 4(10.24) |
| Central | 26 | 4(15.38) | 6(23.07) | 6(23.07) | 10(38.45) |
| Total number | 154 | 9(5.85) | 81(53) | 18(11.7) | 45(29.22) |
Fig. 2.
DNA isolated from the blood and analysis by PCR, PCR analysis with primers P1, P2 specific for 18S rRNA gene of Theileria and Babesia; 1- Negative control of Theileria. 2–6- Theileria (mono infection). 7- positive control of Theileria. 8–15- Mixed infection (Theileria and Babesia)
Fig. 3.
The corresponding PCR product was analyzed by Semi nested-PCR using Babesia ovis specific primers P2, P3. M- Marker 100bp. 1–4, 5–7 : B. ovis. 8- Positive control of B. ovis. 9- Negative control of B. ovis
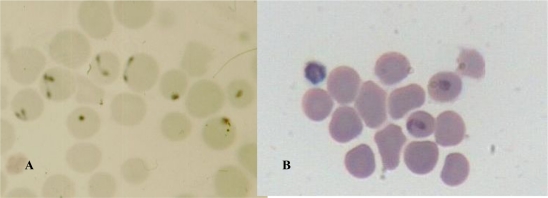
Fig. 1.
Blood smears from Babesia infected sheep was stained with Giemsa- A: Small Babesia, B : Large Babesia
The results of the microscopy analysis showed 38 (24.67%), 40 (26%) and 4 (2.6%) were Babesia, Theileria and mixed infection, respectively, while the results of the PCR assays showed 9 (5.85%), 81 (53%) and 18 (11.7%) were Babesia, Theileria and mixed infection, respectively (Table 2, 6).
In the present study with molecular diagnosis methods, the highest percentage of Babesia and Theileria infections were found in Eastern Azerbaijan 4 (25%) and Khouzestan 14 (87.5%), but no Babesia infection was reported in Northern Khorasan, Khuzestan and Ilam provinces (Table 6). Microscopical observation indicated that the highest percentage of Babesia and Theileria infections were 14 (87.5%) and 10 (62.5%) in Eastern Azerbaijan and Khuzestan provinces, respectively, but, no cases of Babesia infection was reported in Northern Khorasan and Ilam and no cases of Theileria infection in Eastern Azerbaijan and Western Azerbaijan (Table 2).
The results of statistical analysis can be emphasized that the sensitivity and specificity of molecular biology method as compared with morphometrical method; in order to determine Babesia infection were 75% and 71.69%, respectively. The sensitivity and specificity of molecular biology method, as compared with morphometrical method, in order to demonstrate Theileria infection were 46.75 % and 97.22 %, respectively.
Discussion
Babesiosis is an important disease in the livestock with high morbidity and mortality, thereby, resulting in high economical losses worldwide (13, 19, 20). B. ovis and B. motasi were described as the more common causative agents of babesiosis in Iran (4, 21) and its detection is, routinely performed by Geimsa staining of blood smears (18). Babesia spp. in small ruminants can be recognized based on biometrical and morphometrical data. Soulsby 1982 described B. ovis as small Babesia being 1–2.5 µm in length (<2.5 µm), round or comparatively rare pyriform with obtuse angle occurring at the margin of the red cells and B. motasi as a large Babesia measuring 2.5−4×2 µm (>2.5 µm) with acute angle in pyriform (22).
In this study, the size of typical paired pyriformes with acute angle was with the mean of 1−1.5×2 micrometer (µm) and with obtuse angle was with the mean of 3×2 µm. The round forms are also detectable in different sizes, for example 1×1 and 2×2 µm. In comparison to our results, Lewis et al. (23) have reported a small B. motasi in Wales as double pyriform with the mean length of one side being 2.2×3 µm, which appeared to be morphometricaly and serologically closed to the other north European B. motasi stains. Bai et al. also reported a large Babesia, which was polymorphic, including double pyriform, single pyriform, and ring form, rod like, three leafed and budding forms. The size of its typical paired pyriforms was 1.8−2.5 × 0.9−1.8 µm with mean dimensions of 2.21 ± 0.12 × 1.17 ± 0.18 µm (7).
Thomford et al. (24) and Persing et al. (11) had also these inferences by their studies. They defined a Babesia like organism (WA1) and characterized it as morphologically identical to B. microti, but biologically distinct from it. Our results are similar to the findings of Bia et al. and Soulsby et al. It seems that morphometrical parameters could not be a gold standard method in the differential discrimination of Babesia spp. When the DNA samples were used for the PCR analysis, with specific primer pair (P1, P2) that facilitate simultaneously differentiating among Theileria spp. and Babesia spp., 9 out of 154 blood samples, were Babesia infection.
In order differential diagnosis between B. ovis and B. motasi, semi nested- PCR was performed with forward primers specific for B. ovis and B. motasi and a common reverse primer. The amplification could be detected by primers specific for B. ovis (P3), resulting to the expected 186 bp PCR product, but the PCR product could not be amplified with the primers specific for B. motasi (P4) (18). All examined blood smears from infected sheeps, which were previously diagnosed as B. motasi by Geimsa staining in our department, were characterized as B. ovis using PCR in this study. Shayan et al. (2008) could detect a large Babesia as B. ovis which considered wrongly as B. motasi with microscopic examination in Iran, because false positive results are commonly observed with microscopic examination of Geimsa stained blood smears. Also Shayan et al. showed that the pathogenicity of small B. ovis was higher than large one (15). Shayan et al. performed the PCR and RFLP techniques to recognize B. motasi and B. ovis in the salivary gland of Rhipicephalus spp., as vectors for babesiosis in Iran. B. ovis was only detected in salivary gland and B. motasi could not be detected in any examined ticks (18). Aktas et al. used the PCR for diagnosis of Babesia infection in sheep and goat. They showed that only B. ovis could be detected and no PCR products resulted from B. motasi (14). Our results confirm the findings of the studies by Shayan et al. 2007, 2008, Aktas et al. 2005.
Therefore, it can be concluded that the causative organism of many cases of babesiosis in previous studies could be B. ovis instead of B. motasi. We believe that under this examination, the biometrical polymorphisms could exist with in B. ovis in Iran. This polymorphism could be a main problem in differentiation between B. ovis and B. motasi by Geimsa staining, which could be dissolved by specific PCR analysis. Despite of all facts, which concluded the piroplasms of T. lestoquardi are being round to oval in the majority of cases, it should be considered as a problem in differentiation between small ovine Babesia and Theileria. It seems that the causative organism of many cases of babesiosis which identified B. ovis by Geimsa staining, could be T. lestoquardi.
Acknowledgements
The authors wish to acknowledge the valuable assistance of all veterinary practitioners from provinces where the study was conducted. This work was supported by University of Tehran. The authors declare that there is no Conflict of Interests.
Footnotes
: Alsever's solution : Citric Acid 0.55 gr+Sodium Citrate 8 gr + Dextrose 20.5 gr + Sodium Chloride 4.2 gr + D.W 1 Lit
References
- 1.Uilenberg G. International collaborative research: significance of Tick- Borne Haemoparasitic diseases of world animal health. Vet Parasitol. 2001;57(1-3):19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- 2.Delpy RLP. Agents en Iran dans le sang des animaux domestiques. Bull Path Exot. 1936;29:157–161. [Google Scholar]
- 3.Hashemi Fesharaki R, Uilenberg G. Babesia crassa n.sp (Sporozoa, Babesiidae) of domestic sheep in Iran. Vet Quarterly. 1981;3(1):1–8. doi: 10.1080/01652176.1981.9693787. [DOI] [PubMed] [Google Scholar]
- 4.Niak A, Hutner SH. New York City. 1977. Ruminant babesiosis in Iran. The Fifth International Congress of Protozoalogy. 26 June-2 July. Abstract. [Google Scholar]
- 5.Rahbari S, Nabian S, Khaki Z, et al. Clinical, Hematologic, and pathologic aspects ovine babesiosis in Iran. Vet Res. 2008;9(1):1–22. [Google Scholar]
- 6.Nabian S, Rahbari S. Occurrence of soft and hard ticks on ruminants in Zagros Mountainous Area. Iranian J Arthropod-Borne Dis. 2008;2(1):16–20. [Google Scholar]
- 7.Tavasoli M, Rahbari S. A seroepidemiological survey on Babesia ovis in different geographical region of Iran. Vet J Tehran University. 1998;53(3,4):55–59. [Google Scholar]
- 8.Shayan P, Rahbari S. Simultaneus differention between Theileria spp. and Babesia spp. on stained blood smear using PCR. Parasitol Res. 2005;97(4):281–6. doi: 10.1007/s00436-005-1434-3. [DOI] [PubMed] [Google Scholar]
- 9.Almeria S, Castella J, Ferrer D, et al. Bovine piroplasma in Minorca (Balearic Island, Spine): a comparison of PCR- based and light microscopy detection. Vet Parasitol. 2001;99(3):249–259. doi: 10.1016/s0304-4017(01)00464-2. [DOI] [PubMed] [Google Scholar]
- 10.Hashemzadeh F, Nabavi L, Seyfabad Shapouri MR, Rahbari S, Azizi F, et al. Development of an ELISA technique for the detection of B. ovis and serological survey of the parasite in Khuzestan province, southern Iran, Iranian J. University of Shiraz. 2006;7(2):15. [Google Scholar]
- 11.Persing DH, Herwaldt B L, Glaser C, et al. Infection with a Babesia like organism in northern California. N Engl J Med. 1995;2332(5):298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 12.Bai Q, Liu G, Liu D. Isolation and preliminary characterization of a large Babesia sp. From sheep and goat in the eastern part of Gansu province, China. Parasitol Res. 2002;88:16–21. doi: 10.1007/s00436-001-0563-6. [DOI] [PubMed] [Google Scholar]
- 13.Barnett SF. Economical aspects of tick borne diseases control in Britain. Bull Off Int Epiz. 1974b;81(1-2):167–182. [Google Scholar]
- 14.Aktas M, Altay K, Dumanli N, et al. Development of a PCR method for diagnosis of Babesia ovis infection in sheep and goat. Vet Parasitol. 2005;133(4,5):277–281. doi: 10.1016/j.vetpar.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Shayan P, Hooshmand E, Nabian S, Rahbari S. Biometrical and genetical characterization of large Babesia ovis in Iran. Parasitol Res. 2008;103:217–221. doi: 10.1007/s00436-008-0960-1. [DOI] [PubMed] [Google Scholar]
- 16.Habela M, Reina D, Nieto C. Navarrete I. Isolation and identification of Babesia ovis in extremadura (Spain) Vet Parasitol. 1990;35:233–238. doi: 10.1016/0304-4017(90)90058-j. [DOI] [PubMed] [Google Scholar]
- 17.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN, et al. High sensitivity of detection of human malaria parasites by the use of nested PCR. Molecular and Biochemical Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 18.Shayan P, Hooshmand E, Rahbari S, Nabian S. Determination of Rhipicephalus spp. as vectors for Babesia ovis in Iran. Parasitol Res. 2007;101:1029–1033. doi: 10.1007/s00436-007-0581-0. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed J, Yin H, Schnittger L, Jongejan F. Ticks and tick- borne diseases in Asia with special emphasis on China. Parasitol Res. 2002;88:5. doi: 10.1007/s00436-001-0574-3. [DOI] [PubMed] [Google Scholar]
- 20.Mehlborn H, Schein E. The piroplasms life cycle and sexual stages. Adv Parasitol. 1984;23:37–103. doi: 10.1016/s0065-308x(08)60285-7. [DOI] [PubMed] [Google Scholar]
- 21.Mazlum Z. Hyaloma asiaticum asiaticum (Schulze and Schlottke) 1929, Its distribution, hosts, seasonal activity, life cycle and role in transmission of bovine Theileriosis in Iran. Acarologia. 1968;10(3):437–442. [PubMed] [Google Scholar]
- 22.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. London, UK: Bailliere Tindall; 1982. pp. 706–728. [Google Scholar]
- 23.Lewis D, Holman MR, Purnell RE. Investigations on Babesia motasi isolated from Wales. Res Vet Sci. 1981;31:239–243. [PubMed] [Google Scholar]
- 24.Thomford JW, Conrad PA, Boyce WM, Holman PJ. Isolation and in vitro cultivation of Babesia parasites from free- ranging desert bighorn sheep (Ovis canadenis nelsoni) and mule deer (Odocoileus hemionus) in California. J Parasitol. 1993;79(1):77–84. [PubMed] [Google Scholar]