Abstract
Background
Trichomoniasis is a worldwide protozoan parasitic disease and metronidazole is a choice drug for its treatment. Because of disease importance in public health and its controversial ideas about the prevalence of drug resistance, this study was carried out.
Methods
Fifty-two suspected vaginal samples were collected from 2006 to 2007 in Gynecology Maryam Hospital, Tehran, Iran. All isolates were examined by microscopic, culture and PCR techniques. The PCR products were analyzed by RFLP and CSGE methods and two suspected samples were sequenced.
Results
Trichomonas vaginalis was identified from all 52 samples. Of 52 isolates, 45 samples were successfully cultured and amplified by PCR except one. Seven were positive only by PCR. Finally, ITS1 fragment was successfully amplified in 51 of 52. CSGE analysis and PCR products digestion by MspI followed by sequencing showed nucleotide mutation at position 209 (C209T) of the ITS1 fragment in two (3.9%) of them.
Conclusion
The results showed mutation in ITS1 fragment of T. vaginalis in two (3.9%) of Iranian isolates which may be related to metronidazole resistance.
Keywords: Trichomonas vaginalis, Mutation, ITS1 fragment
Introduction
Trichomoniasis is the most widespread non-viral sexually transmitted disease caused by T. vaginalis (1).
The clinical disease in women ranges from asymptomatic to symptomatic such as severe urethritis, vulvo-vaginitis, cervicitis (2), birth of preterm or low-birth-weight infant and possibility of development of cervical cancer are associated complication in affected women (3,4). The infected women are more susceptible to other transmitted diseases including HIV (5). One of the most significant clinical features of T. vaginalis is resistance to choice drug, metronidazole (6–8) which was recognized as an effective treatment with cure rate of approximately 95% (9).
Metronidazole resistance has been reported since 1962 (10) and some studies have been reported from 2.5%-5% (11, 12). More than 10% of infected patients in the United States does not respond to this drug (13), and some clinical isolates have showed decreasing susceptibility to metronidazole (14). Rasoloson et al. showed mechanism of in vitro development of resistance to metronidazole in T. vaginalis (15). Meri et al. reported the first three cases of metronidazole resistant T. vaginalis from Finland (16). Resistance testing was performed for 78 of 467 isolates of T. vaginalis, 4 ones were resistance to difference concentrations of metronidazole (17). Clinical metronidazole resistance is defined by failure infection clearance by standard treatment (8). Internal transcribed spacers (ITS1 and 2) are nonfunctional fragments in ribosomal RNA between 18s rRNA and 28s rRNA and found in all eukaryotic organisms which are transcribed, but not translated. Some investigators proposed that it has an important role in using metronidazole as choice treatment (8). Identification of the molecular methods to detect drug resistance may lead to faster results for clinical treatments (18).
This study was designed for the first time in Iran for mutation detection in ITS1 gene of T. vaginalis by molecular methods.
Materials and Methods
Sampling
As a descriptive study on fifty two vaginal samples were collected from both treated and untreated women suspected to trichomoniasis that referred to Gynecology Department of Maryam Hospital in Tehran from 2006 to 2007. Wet mounts examinations were done for all samples and all were cultured in 50 ml of TYM medium (19).
DNA extraction
Cultures were centrifuged at 430 ×g for 20 min at 4°C and pellets were washed once with PBS (pH 7.4). Total nucleic acid was extracted with the Prim Prep Genomic DNA Isolation Kit (GeNet Bio company, Chungcheongnam-Do, Korea), according to the manufacturer's manual.
PCR amplification
The ITS1 fragment was amplified by designed primers based on T. vaginalis ribosomal DNA (rDNA) gene (ACCESSION AF466750). TVITS F: 5′- ACA CCG CCC GTC GCT CCT AC -3′ and TVITSR: 5′ AAT TTG CAT TCA AAG ATT AAC- 3′. These primers were amplified 313 bp of T. vaginalis ITS1. The PCR reaction contained 500 ng DNA, 12.5 µl master mixes (Ampliqon, California, US) and 20 pmol of each of the forward and reverse primers in 25 µl of final volume. PCR reactions were carried out in 30 cycles, denaturation at 94oC for 30 sec, annealing at 52oC for 30 sec and extension at 72oC for 30 sec. The reaction was incubated for 5 min. at 94oC and 72oC as pre denaturation and post extension steps, respectively. The PCR products were subjected to electrophoresis using 2% agarose gel, stained by ethidium bromide and visualized under a UV transilluminator.
PCR product analysis
All PCR products were analyzed by CSGE method (20) and RFLP using digestion by MspI restriction enzyme. The CSGE is a universal mutation detection system was developed for study into rapid and non-radioactive heteroduplex based detection for mutation screening. PCR products were mixed by control PCR product, subjected to 5 min at 98 °C and 30 min at 68 °C, mixed by five µl of loading buffer (30% glycerol, 0.25% bromophenol blue and 0.25% xylen cyanol FF), and subjected to electrophoresis on 10% CSGE gel. The PCR products were digested with MspI restriction enzyme for 3h at 37oC in the appropriate buffer and restriction patterns were seen on 3% agarose gel.
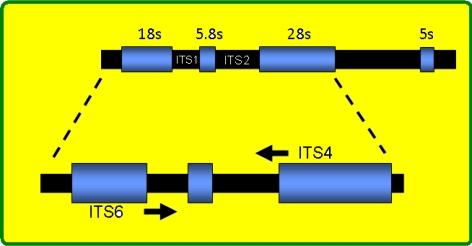
Fig. 1.
The map position of ITS1 and ITS2 in eukaryotic genome
Results
After investigating T. vaginalis isolates by three methods including direct smear, culture in TYI and PCR, 44 isolates were positive in both culture and PCR but seven were positive only by PCR. The ITS1 fragment was amplified by PCR in 51 of 52 DNA extractions. Figure 2 showed 2% agarose gel electrophoresis of amplified 313 bp as PCR product.
Fig. 2.
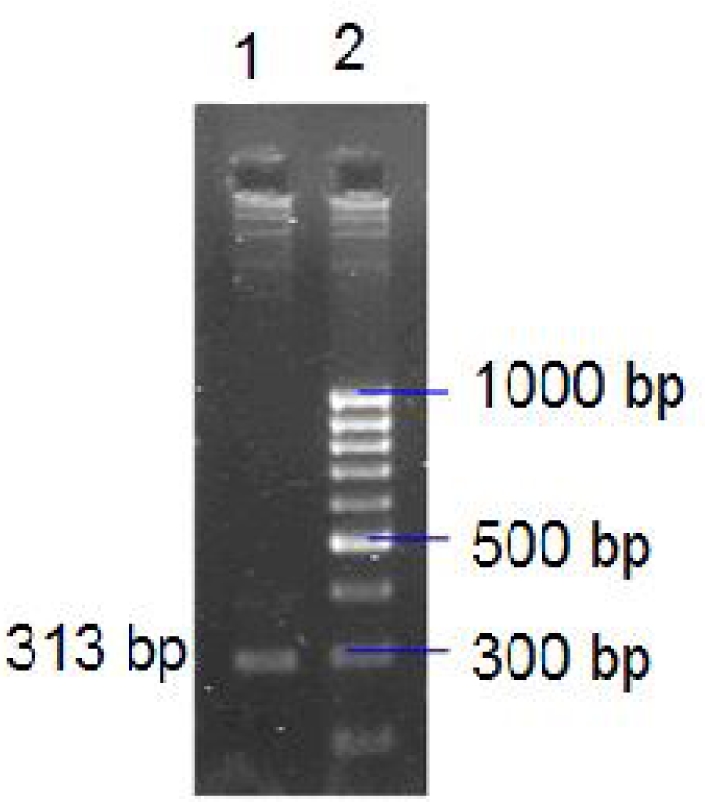
Electrophoresis of PCR product on agarose
All the 51 PCR products of T. vaginalis isolates were electrophoresed on CSGE gel and two PCR products were suspected. The suspected samples were purified and sequenced by dideoxy chain termination method. Our results demonstrated mutation in ITS1 fragment in two cases (3.9%) that were symptomatic and none of them used metronidazole before examination. Both sequences were aligned in GenBank by Blast software and demonstrated mutation related to aligned genes in GenBank.
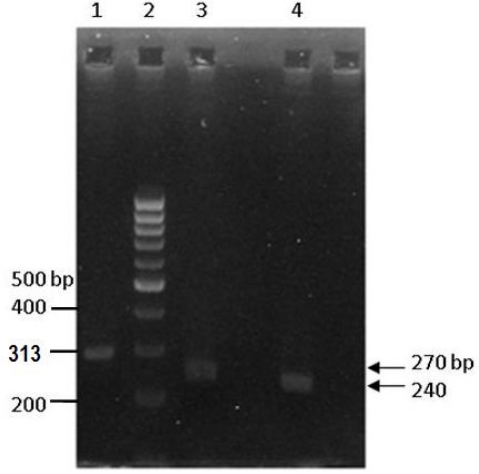
The all-51 amplified PCR products were digested by MspI restriction enzyme and electrophoresed on 3% agarose gel. Figure 3 showed RFLP pattern of mutant wiled samples on agarose gel. Sequence of mutated samples submitted to GenBank at accession numbers EU308571 and EU308572. The results showed mutation at position 209 (C209T) of the ITS1 region in which the thymidine was replaced by cytosine in accession number EU308572.
Fig. 3.
Agarose gel electrophoresis of RFLP pattern. Lane 1. PCR Product (313bp). Lane 2. 100 bp DNA Ladder marker. Lane 3. MspI digested wild type PCR Product (270 bp). Lane 4. MspI digested mutant type PCR Product (240 bp)
Discussion
The purpose of this study was detection mutations in ITS1 of T. vaginalis isolated from Iranian patients. Although correlation between metronidazole resistance and ITS1 mutations have been reported among clinical isolates of T. vaginalis (8), but others reported that correlation between the ITS1 sequence variations and drug resistance was not observed and may be related to Mycoplasma hominis (21). Previous studies reported relationship between ITS1 mutations and drug resistance, but they showed different results. The mechanism of metronidazole resistance in T. vaginalis from treatment failure is not clear (22). Additional studies are needed to adapt the differences between these studies. Internal transcribe spacer (ITS) has been considered as an appropriate candidate gene for determination of metronidazole susceptibility on T. vaginalis, the sequence of this gene have conserved among related species (21).
Despite its high prevalence, the genetic variability and drug resistance features of this parasite are poorly understood. The mutations in genomic level (like ferredoxin mutation) are the important cause of drug resistance phenomenon (23). Butler and Xioa in two separate studies showed 51% and 14% resistance to metronidazole in vitro respectively (18, 21). In other study Upcroft et al., using PCR, showed 17.4% resistance to metronidazole (24). Due to different methods, our results were different from mentioned studies.
This suggests that single mutation on ITS1 gene may be a marker for identify resistance that could lead to control and treatment strategies to avoid from prevent of their distribution. Clearly, further studies with larger samples from different endemic regions are needed to explain this contradiction.
Acknowledgement
This study was supported by Kashan University of Medical Sciences, Kashan, Iran and conducted in Cellular and Molecular Biology Research Center in Shahid Beheshti University M.C., Tehran, Iran. The authors wish to thank directors of above-mentioned universities. The authors declare that they have no conflicts of interest.
References
- 1.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis . Clin Microbiol Rev. 1998;11(2):300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiviat NB, Paavonen JA, Brockway J, Critchlow CW, Brunham RC, Stevens CE, et al. Cytologic manifestations of cervical and vaginal infections. I. Epithelial and inflammatory cellular changes. JAMA. 1985;253(7):989–996. [PubMed] [Google Scholar]
- 3.Cotch MF, Pastorek JG, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24(6):353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994;23(4):682–690. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]
- 5.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7(1):95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP, Garber GE. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis . Clin Microbiol Rev. 2004;17(4):783–793. doi: 10.1128/CMR.17.4.783-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durel P, Roiron V, Siboulet A, Borel LJ. Systemic treatment of Trichomoniasis with a derivative of nitroimidazole, 8823 RP. Br J Vener Dis. 1960;36:21–26. doi: 10.1136/sti.36.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, Secor WE. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol. 2000;38(8):3004–3009. doi: 10.1128/jcm.38.8.3004-3009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for disease control and prevention. Sexually transmitted diseases treatment guidelines. Morb Mortal Wkly Rep. 1993;42(RR-14):70–72. [PubMed] [Google Scholar]
- 10.Robinson SC. Trichomonal vaginitis resistant to metronidazole. Can Med Assoc J. 1962;86(14):665. [PMC free article] [PubMed] [Google Scholar]
- 11.Perez S, Fernandez-Verdugo A, Perez F, Vazquez F. Prevalence of 5-nitroimidazole resistant Trichomonas vaginalis in Oviedo, Spain. Sex Transm Dis. 2001;28(2):115–116. doi: 10.1097/00007435-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Schimd G, Narcisi E, Mosure D, Secor WE, Higgins J, Moreno H. Prevalence of metronidazole-resistant Trichomonas vag-inalis in a gynecology clinic. J Reprod Med. 2001;46(6):545–549. [PubMed] [Google Scholar]
- 13.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14(1):150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Land KM, Clemens DL, Johnson PJ. Loss of multiple hydrogenosomal proteins associated with organelle metabolism and high-level drug resistance in Trichomonads. Exp Parasitol. 2001;97(2):102–110. doi: 10.1006/expr.2001.4587. [DOI] [PubMed] [Google Scholar]
- 15.Rasoloson D, Vanacova S, Tomkova E, Razga J, Hrdy I, Tachezy J, et al. Mechanism of in vitro development of resistance to metronidazole in Trichomonas vaginalis . Microbiology. 2002;148(pt8):2467–2477. doi: 10.1099/00221287-148-8-2467. [DOI] [PubMed] [Google Scholar]
- 16.Meri T, Jokiranta TS, Suhonen L, Meri S. Resistance of Trichomonas vaginalis to metronidazole: Report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J Clin Microbiol. 2000;38(2):763–7. doi: 10.1128/jcm.38.2.763-767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krashin JW, Koumans EH, Bradshaw-Sydnor AC, Braxton JR, Evan Secor W, Sawyer MK, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37(7):440–444. doi: 10.1097/OLQ.0b013e3181cfcd8c. [DOI] [PubMed] [Google Scholar]
- 18.Butler SE, Augostini P, Secor WE. Mycoplasma homonis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol Res. 2010;107(4):1023–1027. doi: 10.1007/s00436-010-1975-y. [DOI] [PubMed] [Google Scholar]
- 19.Limoncu ME, Kilimcioglu AA, Kurt O, Ostan I, Ozkutuk N, Ozbilgin A. Two novel serum-free media for the culture of Trichomonas vaginalis . Parasitol Res. 2007;100(3):599–602. doi: 10.1007/s00436-006-0292-y. [DOI] [PubMed] [Google Scholar]
- 20.Ganguly A. An update on conformation sensitive gel electrophoresis. Hum Mutat. 2002;19(4):334–342. doi: 10.1002/humu.10059. [DOI] [PubMed] [Google Scholar]
- 21.Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, Song LY, et al. Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol Res. 2006;100(1):123–130. doi: 10.1007/s00436-006-0215-y. [DOI] [PubMed] [Google Scholar]
- 22.Dunne RL, Dunn LA, Upcroft P, O'Donoghue PJ, Upcroft JA. Drug resistance in sexually transmitted protozoan Trichomonas vaginalis . Cell Res. 2003;13(4):239–249. doi: 10.1038/sj.cr.7290169. [DOI] [PubMed] [Google Scholar]
- 23.Wiwanitkit V. Identification of weak points prone for mutation in ferredoxin of Trichomonas vaginalis . Indian J Med Microbiol. 2008;26(2):158–159. doi: 10.4103/0255-0857.40532. [DOI] [PubMed] [Google Scholar]
- 24.Upcroft JA, Dunn LA, Wal T, Tabrizi S, Delgadillo-Correa MG, Johnson PJ, et al. Metronidazole resistance in Trichomonas vaginalis from highland women in Papua New Guinea. Sexual Health. 2009;6(4):334–338. doi: 10.1071/SH09011. [DOI] [PubMed] [Google Scholar]