Abstract
Background
Modulation of the immune response is an important strategy by which establishment and growth of hydatid cyst in the internal organs of human is warranted. Induction of apoptosis in the lymphocytes might be a considerable component. This study was designed to evaluate apoptotic impact of hydatid fluid (HF) on human lymphocytes.
Methods
Human lymphocytes were treated with hydatid fluid. After 6 hours of exposure, caspase-3 activity, the central enzyme of apoptosis cascade, was measured by fluorometric assay in the HF-treated lymphocytes and control cells. In addition, the expression of Bax (a pro-apoptotic protein) and Bcl-2 (an anti-apoptotic protein) mRNA was assessed by RT-PCR after 12 hours of exposure.
Results
Both the ratio of Bax/Bcl-2 mRNA expression and Caspase-3 activity were higher in the HF-treated lymphocytes relative to the control group.
Conclusion
Apoptosis could be as a possible mechanism by which Echinococcus granulosus overwhelms host defenses.
Keywords: Hydatidosis, Fertile/Infertile Hydatid Cysts, Apoptosis
Introduction
Hydatidosis is a zoonotic helminthic disease caused by the larval stage of the platyhelminth parasite Echinococcus granulosus, which is highly prevalent in the Middle East, and especially in north of Iran (1, 2). Oncospheres hatching from eggs migrate to liver and several organs of the intermediate hosts (including man), and developed into hydatid cysts in a few weeks. In a fertile cyst, the germinal layer expresses many antigens that are important stimulators of the host immune response (3). The main antigens, Ag B and Ag 5, which are used for both diagnosis and follow-up (4), are released into the hydatid fluid (HF) (5, 6).
Apoptosis is one of the main mechanism by which host defends against this parasite. The fertile hydatid cyst containing protoscolex is unfertilized through induction of an apoptotic cascade by the host (7, 8). On the other hand, E. granulosus is capable of modulating immune responses of the host by several mechanisms (9–13). Some of these mechanisms are the interference with modulation of dendritic cells maturation (14), and induction of a non-protective Th2 cell response by AgB (9). Apoptosis is another proposed mechanism (15) that provides suitable situation for survival of the cyst by inducing apoptosis in the host immune cells. Several factors play role in apoptosis, but the caspase enzymes and Bcl-2 family are the two main families in this process. The first is a cascade of enzyme, ofwhich caspase 3 is the most important member affecting the lymphocyte apoptosis. The second is Bcl-2 family, a set of cytoplasmic proteins members that regulate apoptosis (16). In this family, the most considerable components are Bcl-2 and Bax proteins. While Bcl-2 proteins inhibit apoptosis, Bax counteracts this (17). Furthermore, Macintyre et al. studied the effect of E. granulosus hydatid fluid on the lymphocytes in 3-day cultures of the T-cell line, and suggested that the cytotoxicity of hydatid fluid could be resulted from cell-cycle arrest (18).
This study aims to assess the in vitro apoptotic features of hydatid fluid on human lymphocytes treated with fertile and infertile hydatid fluid by evaluating the expression levels of Bcl-2, Bax mRNA as well as the Caspase-3 activity, which is the central enzyme in the apoptosis cascade (19).
Materials and Methods
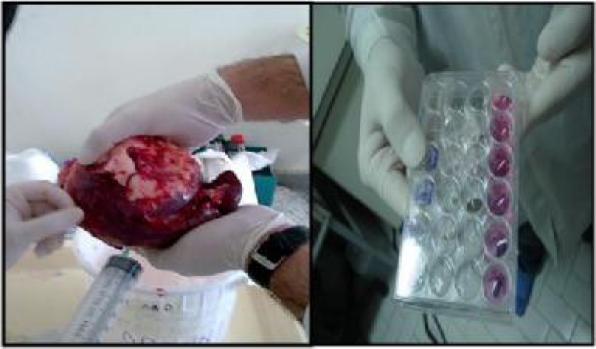
In our study in Medical Mycoparasitology laboratory and Bu Ali Research Institute of Mashhad University of Medical Science 2008, four human hydatid cysts (two fertile and two infertile cysts) excised from the lower lobe of the lungs and the spleen were used to assess apoptotic effects of hydatid fluid on human lymphocytes. Fertile cysts were determined by the presenc of free protoscolece in the hydatid fluid and the growing protoscolece attached to the germinal layer. The infertile cysts do not have these protoscolece. After diagnosis by clinical examination and imaging techniques, hydatid cysts were surgically removed un-ruptured under sterile condition and were aspirated by syringe and stored at −70 °C for further analysis (Fig. 1).
Fig.1.
Left: Aspiration of hydatid fluid from a splenic cyst. Right: 24-well cell culture plate. From the top: Fertile hydatid fluid treated lymphocytes, Infertile hydatid fluid treated lymphocytes, Cell control (all for measurement of caspase 3 activity after 6 hours), Fertile hydatid fluid treated lymphocytes, Infertile hydatid fluid treated lymphocytes, Cell control (all for assessment of Bax , and Bcl-2 expression after 12 hours)
For later studies on Fas-L and TRAIL (as induced apoptotic molecules), the germinal and fibrous layers and adjacent normal tissue were dissected by scalpels and stored at −70 °C in sterile vials. Lymphocytes were separated from 20 ml blood sample of a healthy control by Ficoll-Hypaque gradient cen-trifug-ation method (20). Monocytes were removed by adhesion to plastic flasks in a RPMI-1640 medium in pH 7.4 at 37°C, humidified atmosphere of 5% CO2 for 2 hours. Non-adherent cells (mainly lymphocytes) were removed and counted in a hemacytometer after staining with trypan blue dye. Lymphocytes (1–2×106 cells/well) were cultured on a 24-well plate, containing 1 ml of 15% heat-inactived fetal bovine serum and RPMI-1640 medium in pH 7.4 at 37°C, in a humidified atmosphere of 5% CO2 for 24 hours.
The viability of the cells was checked again as before. After verification of the cell viability, different volumes of hydatid fluid including 25 µl, 50 µl, 100 µl, 150 µl and 200 µl were added to each well containing 1.8 ml media respectively. After six times test 200 µl of hydatid fluid (fertile and infertile) as inducer lymphocyte (optimum volume) were added to each well containing 1.8 ml media. Additional wells without hydatid fluid (HF) were used as controls to compare with HF-treated lymphocytes. Optimum volumes were determined with observation of HF-treated lymphocytes by inverted microscope after 12 hours. Inverted microscope revealed that 200 µl of hydatid fluid was optimum volume for lymphocyte shrinkage and apoptotic-body formation. The exposure time to hydatid fluid for evaluation of Caspase-3 activity and the mRNA expression levels of Bax and Bcl-2 genes was determined according to the recommendations of a study by Jafari et al. (21).
After six hours of exposure to hydatid fluid (Fig. 1), lymphocytes were separated by centrifugation at 10000g for 1 min. Caspase-3 activity was determined by the Caspase-3/CPP32 Fluorometric Assay Kit (K105) (Biovision Inc., MountainView, California, USA) as manufacturer's manual. Activity was measured by a Fluorometer (Jasco, FP-6200, Japan), as enzyme activity converts a blue emission (λ =404 nm) to a yellow-green color (λ =505 nm). Intensity was defined per milligram of total protein contents of the solubilized cells (according to total protein assessment, measured by spectrophotometer (λ =280 nm).
The mRNA expression levels of Bax and Bcl-2 genes were analyzed by semi-quantitative reverse transcriptase PCR (RT-PCR) after 12 hours of exposure of lymphocytes with hydatid fluid. For this purpose, lymphocytes from fertile and infertile HF-treated wells as well as controls were separated by centrifugation at 13000g for 1 min, and pellets were used for RNA extraction by TriPure Isolation Reagent (Roche, Germany) according to the manufacturer instructions. Total RNA was reverse transcribed (RT) into cDNA using MBI Revert Aid (Fermentase, LifeSciences, Lithuania). A RT-PCR was carried out to determine the mRNA expressionlevels of Bcl-2, and Bax genes. The RT-PCR was performed in a 20-µl reaction volume containing 0.5 µL of cDNA, 1.5 mM of Mgcl2, 125 µM of dNTP, 0.1 U/ µL and 0.5 pmol of specific primers. All the primers used in RT-PCR were designed by Gen Runner & Primer Premier Software (Table 1). GAPDH (Glyceraldehydes-3-phosphate deh-ydrogenase) mRNA was used as internal control to adjust the amount of mRNA in each sample. The samples were denaturized for 5 min at 95 °C, and amplified using 35 cycles of 95 °C for 30 s, 60 °C for 80 s, and72 °C for 45 s for Bcl-2 and Bcl-x genes followed by a final elongation at 72 °C for 3 min on a Corbett Research thermocycler (Sydney, Australia). The optimal numbers of cycles were selected for amplification of all three genes experimentally, to be sure that the reactions were in the exponential range of amplification. Five µL of the amplification product analyzed by electrophoreses on a 2% ethidium-stained agarose gel and documented by a gel documentation system (Syngene, U.K.). Quantifications of the PCR band intensities were accomplished by Kodak 1D image analysis software (Eastman Kodak Co). The values were normalized by the value obtained from the GAPDH mRNA. Data were analyzed by Wilcoxon signed rank test using GraphPad-Prism-5 software.
Table 1.
Primers used for RT-PCR of Bcl-2, Bax and GAPDH
| Genes | Primers | Product size |
|---|---|---|
| Bcl-2 | F: 5'CATGTGTGTGGAGAGCGTCAAC3' | 241 bp |
| R: 5'CAGATAGGCACCCAGGGTGAT3' | ||
| Bax | F: 5'TTTGCTTCAGGGTTTCATCCA3' | 151 bp |
| R: 5'CTCCATGTTACTGTCCAGTTCGT3' | ||
| GAPDH | F: 5'GGCCAAGATCATCCATGACAACT3' | 500 bp |
| R: 5'ACCAGGACATGAGCTTGACAAAGT3' | ||
Results
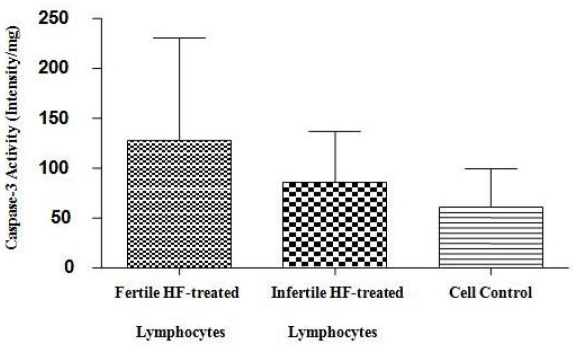
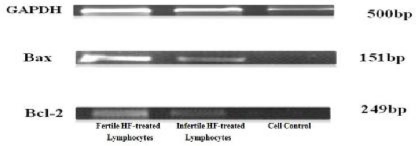
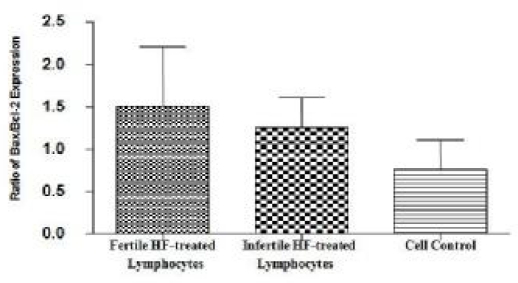
Repeatability data was measured 6 times in each well. The results of Caspase-3/CPP32 fluorometric assay (Fig. 2) show that caspase-3 activity was significantly higher in the fertile HF-treated lymphocytes compared to both infertile HF-treated lymphocytes and cell control. P-value of 0.0625 was considered as significant for assessment of Bax and Bcl-2 expression in fertile HF-treated lymphocytes compared with both infertile HF-treated and control lymphocytes. As shown in Fig. 3, expression of the Bax mRNA was significantly increased (P=0.0511) in fertile HF-treated lymphocytes compared with both infertile HF-treated and control lymphocytes while the expression of the Bcl-2 mRNA in fertile HF-treated lymphocytes was significantly decreased relative to both infertile HF-treated and control lymphocytes (P=0.0719). Furthermore, the Bax/Bcl-2 ratio was increased in fertile HF-treated lymphocytes compared to both infertile HF-treated and control lymphocytes (Fig. 4).
Fig. 2.
Caspase-3 activity in fertile HF-treated lymphocytes, infertile HF-treated lymphocytes and cell control (from left to right). Bar graph indicates the mean ± S.E.M. Increase in Caspase-3 activity was determined by comparing fluorescence of 7-amino-4-trifluoromethyl coumarin in control with HF-treated lymphocytes
Fig. 3.
Semi-quantitative RT-PCR analysis of Bax and Bcl-2 expressions in fertile HF-treated lymphocytes, infertile HF-treated lymphocytes and cell control (from left to right). GAPDH (Gluceraldehydes-3-phosphate dehydrogenase GAPDH gene) was used as an internal control. The density of the labeled bands for amplified products of Bax gene (151 bp) and Bcl-2 gene (249 bp) as well as GAPDH gene (500 bp) is shown in each group
Fig. 4.
Bax/Bcl-2 expression ratio in fertile HF-treated lymphocytes compared to infertile HF-treated lymphocytes and cell control. Bar graph indicates the mean ± S.E.M
Discussion
E. granulosus has considerable defense mechanisms against the immune response of the host, which warrants cyst survival in the host body (9). Hydatid cyst can live as long as 53 years in the human body (22). This shows that this parasite has strategies for evading protective immune responses. Sequestration and antigenic disguise (23), interference with complement activity (24), alteration of lymphocyte proliferative responses (25), and inhibition of the effector cell chemotaxis (9) are some of the proposed strategies that permit the parasite to survive for a longer period (12). Mitogenic responses induced by protoscoleces of Echinococcus species were previously showed by investigations evaluating growth kinetics of leukocyte cell lines cultured with hydatid fluid (10, 15). These responses decrease the efficacy of immune system, as mitotic cycles induced by hydatid products are not completed (15). Along with alteration in lymphocyte proliferative responses, depletion of T lymphocytes is another strategy, by which the parasite impairs the immune response of the host (26).
Although not thoroughly identified, apoptosis might be another mechanism. Cytotoxic features of E. granulosus was previously verified (18). This study shows that apoptosis was significantly higher in lymphocytes that were treated by fertile hydatid fluid regarding to control cells. Expression of the Bax gene as a pro-apoptotic molecule was increased and the expression level of Bcl-2 mRNA as an anti-apoptotic molecule was reduced in the fertile HF-treated lymphocytes relative to the infertile HF-treated lymphocytes and control. Activity of caspase-3 was also significantly higher in the first group. All these supports this notion that protoscolex of E. granulosus (which is present in fertile hydatid cysts) induces apoptosis in the lymphocytes of hosts. In consistent with our results, Macintyre et al. showed that hydatid fluid could induce T-cell mitosis with enhanced membrane expression of CD25 and CD38 on human peripheral blood lymphoblasts, and diminished that of CD28 and reduce co-stimulation with subsequent T-cell anergy or apoptosis (18). In another study, Li et al. explained apoptosis as an important mechanism of CD4+ T cell death in experimental alveolar echinococcosis. The apoptosis level of CD4+ and CD8+ T cells was significantly higher after the infection (27). Results also showed that apoptosis was more in the fertile HF-treated lymphocytes compared with the infertile HF-treated lymphocytes. This could be resulted from the presence of protoscoleces in the cysts, which may release inducer of apoptosis. However, in a study by Janssen et al., it was demonstrated that protoscoleces had no cytotoxic impact on the macrophages. Albeit in that study, it was suggested that some toxic substances are probably secreted by protoscoleces, which induce reduction in the viability of the macrophages in vitro (28).
It was previously verified that hydatid fluid toxins have lytic effects on mouse peritoneal macrophages (29). Thus, whether by secreted toxins from the protoscoleces or directly by the antigens of protoscoleces, apoptosis pathways are activated after exposure to hydatid fluid containing protoscoleces. This shows that fertility of hydatid cyst might be an important factor in the survival of cyst in the host's body. This apoptotic feature offers a significant advantage to this pathogenic parasite, as in the fertile stage, the organism defend by induction of apoptosis in the leukocytes invading to the newly growing cyst. Induction of apoptosis and a possible anergy in the T-cells might result in a significant decrease in the effectiveness of leukocytes, and make them incapable of eliminating the parasite. This process is more prominent in the earlier stages of infection, in which the concentration of leukocytes are not high enough to resist the parasite (18).
In conclusion, the results of this study represent apoptosis as an important mechanism by which E. granulosus overwhelms host defenses. Further investigations with larger sample size are needed to confirm this finding.
Acknowledgements
This study was conducted and financially supported by Mashhad University of Medical Sciences (MUMS). This article is derived from the master's thesis of the last author (Thesis No. A-247). We should appreciate scientific aids from dear Dr. Ali Akbar Shamsian and Mr. Gholamreza Farnoosh, as well Dr. Esmaeeli for statistical consults. In addition, we should thank all staff of Ghaem Hospital Mycoparasitology lab and Bu-Ali Research center Immunobiochemistry Lab for their co-operation. The authors declare that there is no conflict of interests.
References
- 1.Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006;(55 Suppl):S197–202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008;102(4):283–95. doi: 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 3.Siracusano A, Riganò R, Ortona E, Profumo E, Margutti P, Buttari B, Delunardo F, Teggi A. Immunomodulatory mechanisms during Echinococcus granulosus infection. Exp Parasitol. 2008;119(4):483–9. doi: 10.1016/j.exppara.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo C, Last JA, González-Sapienza GG. The immunogenicity of Echinococcus granulosus antigen 5 is determined by its post-translational modifications. Parasitology. 2005;131(5):669–77. doi: 10.1017/S0031182005008309. [DOI] [PubMed] [Google Scholar]
- 5.Mamuti W, Sako Y, Nakao M, Xiao N, Nakaya K, Ishikawa Y, Yamasaki H, Lightowlers MW, Ito A. Recent advances in characterization of Echinococcus antigen B. Parasitol Int. 2006;(55 Suppl):S57–62. doi: 10.1016/j.parint.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Rickard MD, Lightowlers MW. A strategy for production of monoclonal antibodies to Echinococcus granulosus antigen 5 and antigen B. Int J Parasitol. 1992;22(7):1013–6. doi: 10.1016/0020-7519(92)90062-p. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera G, Cabregos ME, Morassutti AL, Cabezon C, Orellana J, Hellman U, Zaha A, Galanti N. DNA Damage, RAD9 and Fertility/Infertility of Echinococcus granulosus Hydatid Cysts. Journal of Cellular Physiology. 2008;216:498–506. doi: 10.1002/jcp.21418. [DOI] [PubMed] [Google Scholar]
- 8.Paredes R, Jimenez V, Cabrera G, Iraguen D, Galanti N. Apoptosis as a possible mechanism of infertility in Echinococcus granulosus hydatid cysts. J Cell Biochem. 2007;100:1200–9. doi: 10.1002/jcb.21108. [DOI] [PubMed] [Google Scholar]
- 9.Riganò R, Profumo E, Bruschi F, Carulli G, Azzarà A, Ioppolo S, Buttari B, Ortona E, Margutti P, Teggi A, Siracusano A. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect Immun. 2001;69(1):288–96. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macintyre AR, Dixon JB. Echinococcus granulosus: regulation of leukocyte growth by living protoscoleces from horses, sheep, and cattle. Exp Parasitol. 2001;99(4):198–205. doi: 10.1006/expr.2001.4662. [DOI] [PubMed] [Google Scholar]
- 11.Macintyre AR, Dixon JB, Bleakley JS, Green JR. Echinococcus granulosus: assays for hydatid immunoregulatory factors using established lymphoid cell lines. Parasite Immunol. 2000;22(10):475–85. doi: 10.1046/j.1365-3024.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanan JH, Chain BM. Modulation of dendritic cell differentiation and cytokine secretion by the hydatid cyst fluid of Echinococcus granulosus . Immunology. 2006;118(2):271–8. doi: 10.1111/j.1365-2567.2006.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persat F, Vincent C, Schmitt D, Mojon M. Inhibition of human peripheral blood mononuclear cell proliferative response by glycosphingolipids from metacestodes of Echinococcus multilocularis . Infect Immun. 1996;64(9):3682–7. doi: 10.1128/iai.64.9.3682-3687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riganò R, Buttari B, Profumo E, Ortona E, Delunardo F, Margutti P, Mattei V, Teggi A, Sorice M, Siracusano A. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect Immun. 2007;75(4):1667–78. doi: 10.1128/IAI.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macintyre AR, Dixon JB, Green JR. Growth kinetics of leukocyte cell lines cultured with hydatid fluid of Echinococcus granulosus equinus. Parasite Immunol. 2000;22(12):651–7. doi: 10.1046/j.1365-3024.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 997;3(1):614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 18.Macintyre AR, Dixon JB, Green JR. Mitosis and differentiation in T-cells under cytotoxic action of Echinococcus granulosus hydatid fluid. Vet Parasitol. 2001;96(4):277–89. doi: 10.1016/s0304-4017(01)00384-3. [DOI] [PubMed] [Google Scholar]
- 19.Gregory CD, Devitt A. CD14 and apoptosis. Apoptosis. 1999;4:11–20. doi: 10.1023/a:1009673914340. [DOI] [PubMed] [Google Scholar]
- 20.Kalmar JR, Arnold RR, Warbington ML, Gardner MK. Superior leukocyte separation with a discontinuous one-step Ficoll-Hypaque gradient for the isolation of human neutrophils. J Immunol Methods. 1988;110(2):275–81. doi: 10.1016/0022-1759(88)90115-9. [DOI] [PubMed] [Google Scholar]
- 21.Jafari AI, Sankian M, Ahmadpour Sh, Varasteh A, Haghir H. Evaluation of Bcl-2 Family Gene Expression and Caspase-3 Activity in Hippocampus STZ-Induced Diabetic Rats. Cell Mol Neurobiol. 2009;29:133–140. doi: 10.1007/s10571-008-9305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spruance SL. Latent period of 53 years in a case of hydatid cyst disease. Arch Intern Med. 1974;134:741–742. [PubMed] [Google Scholar]
- 23.Dixon JB. Echinococcosis. Comp Immunol Microbiol Infect Dis. 1997;20:87–94. doi: 10.1016/s0147-9571(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira AM, Wurzner R, Hobart MJ, Lachmann PJ. Study of the in-vitro activation of the complement alternative pathway by Echinococcus granulosus hydatid cyst fluid. Parasite Immunol. 1995;17:245–251. doi: 10.1111/j.1365-3024.1995.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 25.Emery I, Liance M, Deriaud E, Vuitton DA, Houin R, Leclerc C. Characterization of T-cell immune responses of Echinococcus multilocularis infected C57BL/6J mice. Parasite Immunol. 1996;18:463–472. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 26.Baz A, Hernandez A, Dematteis S, Carol H, Nieto A. Idiotypic modulation of the antibody response of mice to Echinococcus granulosus antigens. Immunology. 1995;84:350–354. [PMC free article] [PubMed] [Google Scholar]
- 27.Li FR, Shi YE, Shi DZ, Vuitton DA, Craig PS. Study on CD4+ cells deletion mechanism in experimental alveolar echinococcosis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2003;21(4):197–202. [PubMed] [Google Scholar]
- 28.Janssen D, Osuna A, Lazuen J, de Rycke PH. Comparative cytotoxicity of secondary hydatid cysts, protoscoleces, and in vitro developed microcysts of Echinococcus granulosus . J Helminthol. 1992;66(2):124–31. doi: 10.1017/s0022149x00012700. [DOI] [PubMed] [Google Scholar]
- 29.Janssen D, De Rycke PH, Osuna A. Dose-dependent effects of hydatid fluid toxins from Echinococcus granulosus on mouse peritoneal macrophages. Folia Parasitol. 1993;40(2):109–13. [PubMed] [Google Scholar]