Abstract
Background
Free-living amoebae (FLA) are a group of ubiquitous protozoan, which are distributed in the natural and artificial environment sources. The main aim of the current study was to identify the presence of FLA in the recreational hot springs of Sarein in Ardebil Province of Iran.
Methods
Seven recreational hot springs were selected in Sarein City and 28 water samples (four from each hot spring) were collected using 500 ml sterile plastic bottles during three month. Filtration of water samples was performed, and culture was done in non-nutrient agar medium enriched with Escherichia coli. Identification of the FLA was based on morphological criteria of cysts and trophozoites. Genotype identification of Acanthamoeba positive samples were also performed using sequencing based method.
Results
Overall, 12 out of 28 (42.9%) samples were positive for FLA which Acanthamoeba and Vahlkampfiid amoebae were found in one (3.6%) and 11 (39.3%) samples, respectively. Sequence analysis of the single isolate of Acanthamoeba revealed potentially pathogenic T4 genotype corresponding to A. castellanii.
Conclusion
Contamination of hot springs to FLA, such as Acanthamoeba T4 genotype (A. castellanii) and Vahlkampfiid amoebae, could present a sanitary risk for high risk people, and health authorities must be aware of FLA presence.
Keywords: Hot spring, Free-living amoebae, Iran, Acanthamoeba castellanii, Vahlkampfiidae
Introduction
Free- living amoebae (FLA) include many genera with high distribution in natural and artificial environment, such as air, water, dust, and soil (1). Several genera of FLA have been concerned in medical sciences, such as Acanthamoeba spp., Balamuthia, and Naegleria (1–2). On the other hand, these FLA could act as a Potential pathogen leading to fatal disease in domestic animals and humans by invasion to central nerve system (CNS) and other organs, especially in those with higher risk (3–4). Amphizoic nature of FLA lead to their presence in different water types, such as fresh, coastal, sea, mineral waters, wastewater, swimming pools, and hot springs. High temperature of hot springs (37-45 °C) can support the growth and distribution of some potentially pathogenic thermophilic FLA (5). To date, Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, and Sappinia pedata are known as causal agents for human disease (6–7).
Acanthamoeba is the most prevalent amoebae in the environmental sources, and this genus has been isolated from different water types (5, 7). To date, Acanthamoeba have been classified to 17 genotypes (T1-T17) based on the variable region of 18S rRNA gene (8–10). However, strains belong to the T4 genotype are the most important causative agent in Acanthamoeba-related disease, such as amoebic keratitis (AK), and granulomatous encephalitis (9). Interestingly, most of AK cases showed a history of wearing contact lenses and water activity before the onset of disease (9). In Iran genotypes belonged to T2, T4 and T6 have been identified in stagnant waters thus far (11–12). However, there are no reports regarding the presence of Acanthamoeba spp. in hot springs in Iran.
Among many genera of Vahlkampfiidae, Naegleria and Vahlkampfia are of medical importance (1, 7, 13). These thermophilic amoebae have global distribution in water sources and can cause FLA-related disease, such as corneal, and brain involvement (1). Naegleria fowleri can cause primary amoebic meningoencephalitis (PAM) in young and healthy people with a history of water activity (7, 14–15).
It is worthy to mention that Acanthamoeba and Vahlkampfiidae can also act as a carrier of pathogenic bacteria, such as Legionella, Pseudomonas, and Helicobacteria, and this can lead to their expanded threat for human being (16–17).
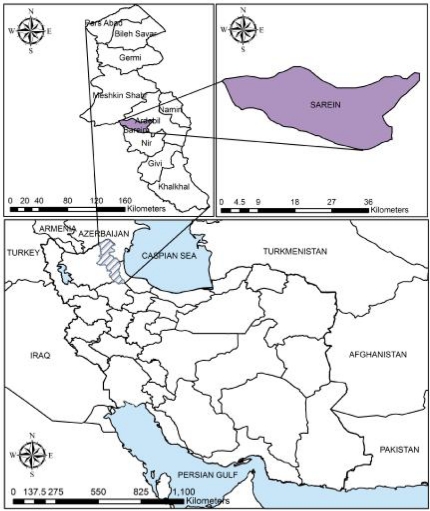
Sarein is a town of Ardebil Province, which is located in the northwest of Iran, and it is famous for various hot springs and spas as recreation areas and health purposes (Fig.1). Indeed, local people and tourists enjoy water activity in this region through all months of year. The main goal of the current study was to identify the presence of FLA in the hot springs of Sarein town using culturing method and morphological key.
Fig. 1.
Map of the Ardebil Province and Sarein city, Iran (Created by Arc GIS version 9.3)
Materials and Methods
Sampling
During August, September, and October of 2010, 28 water samples were collected from seven hot springs of Sarein in Ardebil Province. Fig.1 shows the map of Ardebil Province and Sarein City (created by Arc GIS version 9.3). Four samples were collected from each water sources including two samples from margin, and two from center of the hot springs. Water temperature was measured using standard thermometer. Samples were transmitted to the Department of Parasitology and Mycology, Tehran university of Medical Sciences, Tehran, Iran.
Filtration, cultivation, and cloning
Samples were filtered using sterile membrane filters (Millipore, pore size 0.45 µm). Each filters were cultured on non-nutrient agar medium enriched with Escherichia coli (NNA medium), and incubation was performed at room temperature for one month. All mentioned plates surveyed daily under light microscope (Carl Zeizz Axiolab) with the magnification of 100X and 400X. Positive samples were then cloned in order to achieve a free-bacteria and fungi plates (12).
Identification of the cloned amoebae
The criteria for identification of amoebae were based on morphology of cysts and trophozoites according to the Page, FC (1988) key (18). Acanthamoeba were detected based on double wall and wrinkled or wavy ectocyst and characteristic acantopodia of trophozoites. Molecular identification was performed for Acanthamoeba positive sample using primers JDP1-2 (JDP1 5'-GGCCCAGATCGTTTACCGTGAA-3' and JDP2 5'-TCTCACAAGCTGCTAGGGAGTCA-3') (19). DNA extraction was performed using Phenol chloroform method according to our previous study (12) and PCR was achieved in 30 µl Ampliqone (Taq DNA Polymerase Master Mix RED, Denmark) as a ready-made mixture. Twenty-five µl of Taq Master Mix were used with 5 ng template DNA, 0.1 µm of each primer and distilled water. The thermal cycling conditions were an initial denaturing step of 94 oC for 1 min and 35 repetitions at 94 oC for 35 s, annealing step were 56 oC for 45 s, and 72 oC for 1 min. PCR products were electrophoresed on a 2% agarose gel stained with a solution of ethidium bromide and detected under UV light. Sequencing analysis was performed using BLASTn in the genbank database. Vahlkampfiid amoebae were detected according to spherical and smooth wall cysts and eruptive trophozoites in plates (18).
Results
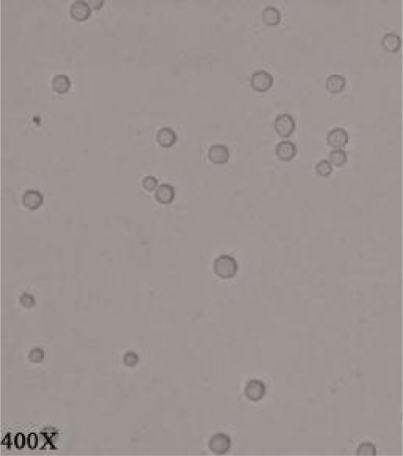
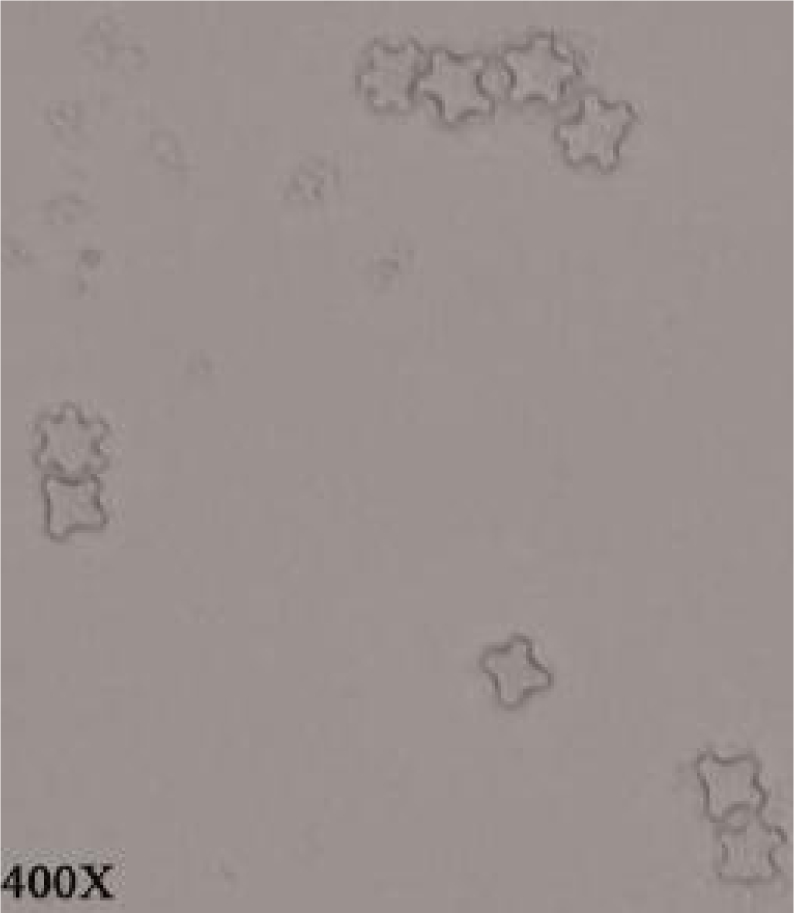
Overall, 12 out of 28 (42.9%) samples were positive for outgrowth of FLA (Table 1). Acanthamoeba castellanii and Vahlkampfiid amoebae were found in one (3.6%) and 11 (39.3%) samples, respectively (Figs.2 and 3). Only one A. castellanii strain was isolated in Pehenloo hot spring from the margin site, which was further confirmed using PCR based method. Sequencing analysis of this isolate in the GenBank database was revealed a 100% similarity with the T4 genotype corresponding to A. castellanii species. Vahlkampfiid amoebae were detected according to spherical and smooth wall cysts and eruptive trophozoites (Fig. 3). Contamination of hot springs to Vahlkampfiid amoebae where detected in Pehenloo, Ghavmishgholi, General, Abdarmanieh Sabalan, Sarisuei, Beshbajilar, and Ghahvesuei. The mean temperature of surveyed hot springs was 43.6°C (Table 1). Both collected samples of margin and center of the springs were positive.
Table 1.
Frequency of free-living amoebae in Sarein Hot Springs, and Samples Sites
| Sampling Site | Temp (°C) | No. of Samples | Total Samples | Acanthamoeba castellanii | Vahlkampfiid amoebae | ||
|---|---|---|---|---|---|---|---|
| Oct | Sep | Aug | |||||
| Pehenloo | 43 | 2 | 1 | 1 | 4 | 1 | 2 |
| General | 43 | 2 | 1 | 1 | 4 | – | 2 |
| Ghavmishgholi | 46 | 2 | 1 | 1 | 4 | – | 1 |
| Abdarmanieh Sabalan | 41 | 2 | 1 | 1 | 4 | – | 1 |
| Sarisuei | 46 | 2 | 1 | 1 | 4 | – | 2 |
| Beshbajilar | 41 | 2 | 1 | 1 | 4 | – | 2 |
| Ghahvesuei | 43 | 2 | 1 | 1 | 4 | – | 1 |
| Total | 43.6* | 14 | 7 | 7 | 28 | 1 | 11 |
Mean temperature of 7 hot springs
Fig. 2.
Acanthamoeba castellanii cysts (400 X)
Fig. 3.
Vahlkampfiidae cysts (400 X)
Discussion
This study is the first survey regarding the presence of FLA in hot springs of Iran. To date, hot springs are famous for health purposes and traditional treatment option, worldwide. Sarein in northwest of Iran contain numerous hot springs and this region attract many tourists annually.
The results of the present study revealed, hot springs of Sarein were contaminated with opportunistic FLA, such as Acanthamoeba, and Vahlkampfiid amoebae, and this may lead to severe disease in high-risk people, such as contact lens wearers. This is in support with researches conducted worldwide (15, 17,20–22). In a study in Spain, 12 natural hot springs examined in which 8 springs were contaminated to different taxa including Acanthamoeba (3) and Vahlkampfiid amoebae (7, 20). In another survey in Thailand, 28 out of 69 samples were found positive for FLA, which 13.0% (9/69) were Acanthamoeba, and 34.8% (24/69) were Naegleria (5). Moreover, in another study in Thailand, 56 samples were collected from 28 natural water resources that six samples were Naegleria spp., 3 samples were Acanthamoeba, 5 were mixed, and 2 samples were unidentified (22).
Our results confirmed that Acanthamoeba is more sensitive to growth in high temperature in contrast to Vahlkampfiid amoebae which hot water may lead to the favorable condition for their proliferation. This is in support with other studies, which showed higher isolation of Vahlkampfiids compared to Acanthamoeba in hot springs (5, 22). More researches are needed for identification of different vahlkamfiids in Iran using molecular based methods.
Another interesting finding of the present study was the isolation of potentially pathogenic A. castellanii belonged to the T4 genotype. T4 strains have been proved to contain certain properties that render them to be more virulent (11). T4 genotype was also a predominant type found in AK patients in Iran (12). In another study by Rezaeian et al, 354 samples of water and soil were collected from rivers and lakes of Parishan and Dashte Arzhan in Kazerun city. They found 10 cases of FLA based on morphological criteria (2). Furthermore, the number of AK cases, which have been diagnosed up to 2004, were more than 3000 cases worldwide and more than 70 percent were contact lens wearers (23). In Iran, 45 AK cases have been diagnosis during 1995-2005 in School of Public Health at Tehran University of Medical Sciences (24).
In conclusion, contamination of hot springs to FLA, such as Acanthamoeba and Vahlkampfiid amoebae, could present a sanitary risk for high-risk people, and health authorities must be aware of FLA presence. Education to high-risk people, and improved disinfection methods must apply for preventing FLA-related disease in this region.
Acknowledgments
The authors acknowledge wholehearted cooperation of the staff of Department of Parasitology and Mycology of Tehran University of Medical Sciences. Likewise, the authors declare that they have no conflicts of interest.
References
- 1.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free living amoebae: Acanthamoeba spp, Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea . FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Rezaeian M, Bagheri F, Farnia Sh, Babai Z. Isolation of Pathogenic Amoeba (Naegleria and Acanthameoba) from Water Sources and Margin Soils of Reveirs and Lakesin Kazerun. Iranian J Publ Health. 2003;1(3):47–8. [Google Scholar]
- 3.Gianinazzi C, Schild M, Zumkehr B, Wüthrich F, Nüesch I, Ryter R, Schürch N, Gottstein B, Müller N. Screening of Swiss hot spring resorts for potentially pathogenic free-living amoebae. Exp Parasitol. 2010;126(1):45–53. doi: 10.1016/j.exppara.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Martinez AJ. Free-Living Amebas. 1st ed. CRC Press; 1985. [Google Scholar]
- 5.Lekkla A, Sutthikornchai C, Bovornkitti S, Sukthana Y. Free-living ameba contamination in natural hot springs in Thailand. Southeast Asian J Trop Med Publ Health. 2005;36(4):5–9. [PubMed] [Google Scholar]
- 6.Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Carmen M, Sutherland K, Valladares B. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res. 2009;106:279–81. doi: 10.1007/s00436-009-1592-9. [DOI] [PubMed] [Google Scholar]
- 7.Rezaeian M, Niyyati M. Pathogenic Free Living Amebas In Human. 1st ed. Tehran: TUMS Publication; 2010. [Google Scholar]
- 8.Corsaro D, Venditti D. Phylogenetic evidence for a new genotype of Acanthamoeba (Amoebozoa, Acanthamoebida) Parasitol Res. 2010;107(1):233–8. doi: 10.1007/s00436-010-1870-6. [DOI] [PubMed] [Google Scholar]
- 9.Khan NA. Acanthamoeba, biology and pathogenesis. 1st ed. Great Britine: Caister Academic Press; 2009. [Google Scholar]
- 10.Nuprasert W, Putaporntip C, Pariy-akanok L, Jongwutiwes S. Iden-tification of a novel T17 genotype of Acanthamoeba from environmental isolates and T10 genotype causing keratitis in Thailand. J Clin Microbiol. 2010;48(12):4636–40. doi: 10.1128/JCM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maghsood AH, Sissons J, Rezaeian M, Nolder D, Warhurst D, Khan NA. Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J Med Microbiol. 2005;54(8):755. doi: 10.1099/jmm.0.45970-0. [DOI] [PubMed] [Google Scholar]
- 12.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Mohebali M, Maghsood AH, Motevalli-Haghi A, Martín-Navarro CM, Farnia S, Valladares B, Rezaeian M. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121(3):242–5. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohe-bali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Roberts L, Janovy J. Foundations of parasitology. 8th ed. New Yourk: McGraw-Hill; 2010. [Google Scholar]
- 15.Sheehan K, Fagg J, Ferris M, Henson J. Geothermal Biology and Geochemistry in Yellowstone National Park. 2003. Thermophilic amoebae and Legionella in hot springs in Yellowstone and Grand Teton National Parks; pp. 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winiecka-Krusnell J, Linder E. Bacterial infections of free-living amoebae. Res Microbiol. 2001;152(7):613–9. doi: 10.1016/s0923-2508(01)01240-2. [DOI] [PubMed] [Google Scholar]
- 17.Kuroki T, Yagita K, Yabuuchi E, Agata K, Katsube Y, Endo T. Isolation of Legionella and Free-Living Amoebae at Hot Spring Spas in Kanagawa, Japan. Kansenshogaku Zassi. 1998;72(10):1050–5. doi: 10.11150/kansenshogakuzasshi1970.72.1050. [DOI] [PubMed] [Google Scholar]
- 18.Page F. A New Key to Freshwater and Soil Gymnamoebae. 1st ed. UK: Amb-leside; 1988. [Google Scholar]
- 19.Schroeder J, Booton G, Hay J, Niszl I, Seal D, Markus M, Fuerst PA, Byers TJ. Use of subgenic18s ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoeba from humans with keratitis and from sewage sluge. J of clinical Microbiol. 2001;39(5):1903–11. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penas-Ares M, Paniagua-Crespo E, Madriñan-Choren R, Marti-Mallen M, Arias-Fernandez MC. Isolation of free-living pathogenic amoebae from thermal spas in N.W. Spain. Water, Air, & Soil Pollution. 1993;78(1-2):83–90. [Google Scholar]
- 21.Rivera F, Lares F, Gallegos E, Ramirez E, Bonilla P, Calder A, Martinez J. Pathogenic amoebae in thermal waters of three reostors of Hidalgo, Mexico. Environmental Res. 1989;50:289–95. doi: 10.1016/s0013-9351(89)80010-6. [DOI] [PubMed] [Google Scholar]
- 22.Anchalee W, Manasanant B. Occurence of thermotolerant Naegleria and Acanthamoeba in some natural water sources in Chiang Mai. Chiang Mai Med J. 2009;48(3):117–24. [Google Scholar]
- 23.Maghsood A, Rezaian M, Rahimi F, Ghiasian S, Farnia S. Contact Lens-Associated Acanthamoeba Keratitis in Iran. Iranian J Pub Health. 2005;2(2):40–7. [Google Scholar]
- 24.Rezeaian M, Farnia S, Niyyati M, Rahimi F. Amoebic keratitis in Iran (1997–2007) Iranian J Parasitol. 2007;2(3):1–6. [Google Scholar]