Abstract
Background
The study was aimed to show the effect of molecular mechanism of Aqueous Garlic Extract (AGE) on expression of IFNγ and iNOS genes in Leishmania major.
Methods
Leishmania major promastigotes (MRHO/IR/75/ER) were added to the in-vitro cultured J774 cell line, the cells were incubated for 72 hours. Various concentrations of garlic extract (9.25, 18.5, 37, 74, 148 mg/ml) were added to the infected cells. MTT assay was applied for cellular proliferation. After 72 hours of incubation, supernatants were collected and total RNA was extracted from the infected cells. The express of IFNγ and iNOS genes were studied by RT-PCR method.
Results
The colorimetric MTT assay after 3 days of incubation showed cytotoxic effect of garlic extract with an IC50 of 37 mg/ml. In addition, IFNγ and iNOS genes expression by RT-PCR indicated that garlic extract lead to over expression of these genes in J774 cell line infected with L. major.
Conclusion
Garlic extract exerts cytotoxic effect on infected J774 cell line. In addition, the hypothesis that garlic can improve cellular immunity with raising the expression of IFNγ and of iNOS genes confirmed.
Keywords: Aqueous Garlic Extract (AGE), Leishmania major, Macrophage, Gene Expression
Introduction
Leishmaniasis is an infectious disease caused by different species of Leishmania protozoa with about 12 million people currently infected and 350 million people at risk of infection (1, 2).There are two major strategies to treat leishmaniasis: chemotherapy and immunotherapy. Chemotherapy with antimonials are not very effective, instead, a number of immunotherapeutic approaches have been tested with encouraging results. Several cytokines such as IFN-γ or GM-CSF, alone or in combination with chemotherapy, have been effective in the progression of the disease treatment (3).
Garlic belongs to the Liliaceae family and is scientifically named as Allium sativum. The antibiotic properties of garlic have been known for a long time. For example, in World War II, garlic was used as an antibacterial agent for prevention of gas gangrene. The antibacterial effect of garlic on Gram positive and negative bacteria has been defined for a long time (4). Several studies have been performed on antimycotic properties of garlic including its effects on clinically important dermatophytes Aspergillus niger and Candida albicans (5). In recent years, a few studies have been performed on the effects of garlic extract for treatment of cutaneous leishmaniasis. It has been suggested that garlic modulates progression of leishmaniasis by augmentation of immune system. Identification of specific component of garlic extract, which is effective in treatment of cutaneous leishmaniasis, is important for confirmation of treatment protocol. Current drugs, such as glucantime, have several side effects and lead to direct cellular damage. If an herbal-based drug eliminates Leishmania by augmentation of immune system, it has the potential of possession a wider margin of safety (6).
This project aimed to study the effects of garlic extract on expression of IFN-γ and iNOS in macrophages infected with Leshmania major.
Materials and Methods
Preparation of aqueous garlic extract (AGE)
Two hundred g of aged garlic bulbs (Allium sativum) were divided into separate cloves. The cloves were peeled and then ground in a small pieces. The garlic aqueous extract was collected by Fromtling and Bulmer modified method (7, 8). Briefly, the minced garlic was shacked with 200 ml of sterile distilled water on a shaker for 48 hours at 37 °C. This mixture was centrifuged at 2300 rpm for 25 min. Then, the supernatant was filtered through Whatman no.1 filter paper (Whatman Corp., Bedford, Mass). The supernatant was sterilized by passing through a 0.2 µm Nalgene filter (Nalgene Labware Div., Nalge/Sybron Corp., and Rochester, N.Y.). The sterile lyophilized extract was kept frozen at −80 °C until used.
Macrophage
A BALB/c-derived macrophage-like cell line (J774) was purchased from Pasteur Institute (Tehran, Iran). Cells were grown in Dulbecco's modified minimum essential medium (DMEM) containing heat inactivated 10% FCS. All media were supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 250 ng/ml amphotericin B.
Leishmania
A clone of L. major strain (MRHO/IR/75/ER) was kindly provided by Dr. Mohebali (Tehran University of Medical Sciences,) for establishing experimental infection.Briefly, 5×105 cells/ml L. major promastigotes, were cultured in RPMI1640 medium (pH 7.2, containing 25 mM HEPES) supplemented with 10% heat inactivated fetal bovine serum (FBS) and antibiotics at 24 °C for 96 h and subcultured at cell densities of 2×107 to 2.5×107 cells/ml.
L. major promastigotes culture and in vitro assay of parasite growth in macrophage
Briefly, promastigotes of L. major were cultured in RPMI 1640 media containing 10% fetal calf serum (FCS) for 72 h at 24 °C. Promastigotes in the stationary growth phase were used to infect cultures of adherent macrophages at a final ratio of five parasites per macrophage. Parasites were pelleted at 1000 rpm in a rotor for 10 min at room temperature and then resuspended in RPMI 1640 containing 20% FCS at a concentration of 106 parasite/ml. Parasites were washed with RPMI 1640 and immediately prior to addition of parasites, the macrophages were washed with medium. To initiate infection, promastigotes were added to 1 ×106 macrophages. After addition of parasites, the macrophages were incubated at 33°C in 5% CO2. Infection was allowed to proceed for 24 h and then unphagocytosed parasites were removed by washing with medium, and the remaining cells were incubated for 72 h at 33°C. Macrophages were then fixed in methanol and stained with Giemsa stain for determination of intracellular parasite numbers. The mean percentages of survival in the culture medium were calculated based on the number of Leishmania in untreated culture medium as 100%.
MTT assay for cell viability and garlic treatment
Supernatants were collected after initial stimulation at 24, 48, and 72 hours. Cells were reinsulated with various concentrations of garlic extract in 9.25, 18.5, 37, 74 and 148 mg/ml. The supernatants were treated with absence of either parasite or garlic, presence of parasite and without extract and with presence of either parasite or garlic. All supernatants were then stored at −20°C until they were assessed for their of cytokines. Cell viability was examined based on the MTT assay (9). The absorbance was measured at 450 nm using ELISA reader (Awarness, Statface 3100).
All values were expressed as the mean±S.D of three independent measurements Results were expressed as the concentration that inhibited parasite growth by 50% (IC50: half-maximal inhibitory concentration).
RNA extraction and cDNA synthesis from L. major infected J774 cell
RNA was extracted base on the manufacturer protocol. Briefly, 106 infected J774cells were collected and treated with IC50 dose of AGE. By using RNA FAST kit, 1 mL of RNA extractor was added to cells. 200µL of chloroform was added to the solution and shacked gently. Finally, 100µL Isopropanol was added to the supernatant. All the solutions samples were centrifuged at 12000 RPM. Supernatant was discarded, and to the precipitates was added 20 µL of dH2O including DEPC and was frozen in −20°. cDNA library was prepared by using Accupower RT. Premix kit 1µg RNA was added to 30 pMol of Revers primer and the mixture was incubated at 70°C for 5 minutes and immediately put on ice, and then lyophilized in various vials.
RT- PCR Reform for iNOS and IFNγ Genes
The mRNA expression levels of iNOS and IFNγ genes were analyzed by semi-quantitative reverse transcriptase PCR (RT-PCR) method after 4 hours of exposure of infected cells to garlic extract.
All the primers used in this study were designed by Gene Runner & Primer Premier Software.
For iNOS and IFNγ genes the sequence of the forward and reverse primers were respectively.
For iNOS:
Forward primer 5′-TGCCGGAAGGCGGCTCATTC-3′
Reverse primer 5′CGCAGTGCGTTGCGCATACC-3′
For IFNγ:
Forward primer 5′-TGCCGGAAGGCGGCTCATTC
Reverse primer 5′-CGCAGTGCGTTGCGCATACC-3′
β-actin mRNA was used as internal control to adjust the amount of mRNA in each sample.
Briefly, Tag polymeras kit of SinaGene Company was used 30pmol of the forward primer and the reverse primer with 200µM dNTP and 2µM MgCl2 were added. After standard incubation, All PCR products were run on 1.5% agarose gel for 2 hours and stained with ethidium bromide then recorded using a transluminator. Quantification of the PCR band intensities was accomplished by Kodak 1D image analysis software (Eastman Kodak Co). The values were normalized by the value obtained from the β-actin mRNA. Data were analyzed by Wilcoxon signed rank test using GraphPad-Prism-5 software.
Statistical analysis
All the experiments were repeated at least three times, and representative results were analyzed. Statistical significance (P<0.05) was analyzed by Student's t-test using SPSS version16.
Results
Measurement of effective AGE concentration on Leishmania infected macrophage by MTT assay
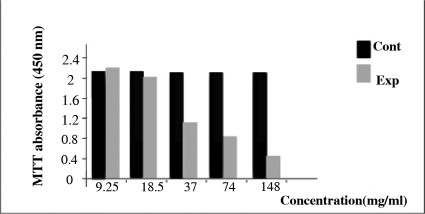
The results of MTT assay complied from three experiments in different times 18, 24, and 48 hours are shown in Fig. 1. The viability of the cells was decreased after 48 hours. The result indicated that 37 mg/ml was the best concentration for cells lyses during 48 hours (Fig. 1).
Fig. 1.
from MTT assay as indicated in macrophages on various AGE concentrations compare with control group
Expression of genes (iNOS & IFNγ) in J774cell infected with Leishmania major
The results show that garlic extract with IC50 dose influence the expression iNOS & IFNγ genes in J774cells. The amount of mRNA of these genes increase to high level. In contrast, this effect was not revealed with the uninfected cells.
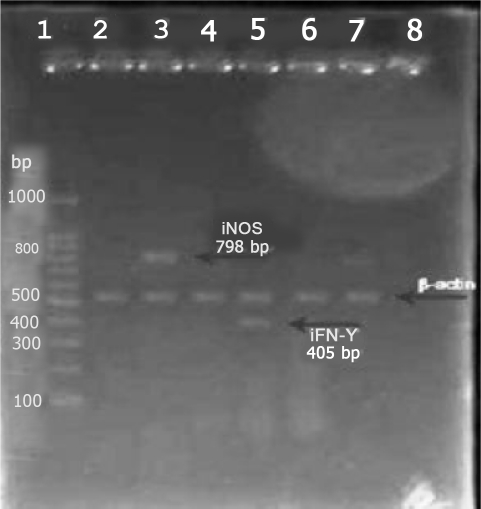
Fig. 2.
RT-PCR results from iNOS and IFNγPCR Bonds products: From left to rightLine 1: marker indicate M.W from 100 bp to 1000 bpLine 2: Lack of iNOS expression in infected macrophages without AGE.Line 3: Lack of iNOS expression in non-infected macrophage without AGE.Line 4: Lack of IFNγ expression in infected macrophages without AGE.Line 5: iNOS expression in infected macrophages with AGE.(798 bp)Line 6: IFNγ expression in infected macrophages with AGE. (405 bp)Line 7: Lack of IFNγ expression in non-infected macrophages without AGE.Line 8: Bond 538 bp as a control.
β-actin was used as the internal control for genes expression study.
Discussion
There is no vaccine against leishmaniasis, and treatment options are limited. Pentavalent antimonial still remains the first line therapy for cutaneous and visceral disease but with known side effects in most treated patients. An alternative treatment is amphotericin B, but this drug is also highly toxic and expensive. Consequently, there is need for new and less toxic drugs for treatment of the diseases (10,11). Previous studies has shown that garlic induces shift in cytokine pattern in L. major- infected Balb/c mice we were interested in investigating whether it could stimulate leishmanicidal activity in infected macrophages and to determine its mechanism of action (8). We examined the ability of garlic extract to stimulate iNOS and IFN-γ gene expression in infected J774 cell. Our results showed the effective dose of AGE for induction the L. major infected macrophage gene expression at 37 mg/ml after 48 hours treating.
Garlic extract is more effective than glucantime, and a combination of garlic and glucantime is much more effective in decreasing lesion size than either glucantime or garlic alone, besides, combined therapy leads to a Th1-type cytokine pattern similar to that induced by garlic alone (8). Our finding confirms Ghazanfari's data suggesting that garlic extract contains an immunomodulator, which modulates cytokine patterns towards a Th1-type response and the development of an effective cell-mediated response (8).
Treatment with AGE has several advantages. Preparation of AGE is very easy and even efficient at lower dose of AGE for stimulation of macrophage (12-14), AGE induces IL-12 production and subsequent NO production in macrophage, providing the best stimulation for induction of production of NO. as a lethal molecule for L. major (15). It may provide us with a highly effective approach for the treatment of advanced leishmaniasis. Gurunathan et al. reported that vaccination with DNA encoding the immunodominant single parasite Leishmania homolog of receptors for activated C kinas parasite antigen could elicit prolonged protective immunity to Leishmania major in an IL-12- and IFN-γ-dependent manner (16, 17).
Nitric oxide production is most important effector to eradicate the parasite. Probably, the increasing of iNOS that stimulated from AGE has the major role. Some studies showed IFNγ over expression in cellular immunity has an important role, too. These reports investigated the R10 fraction of AGE, and demonstrated immunomodulatory mechanism. The MW of the glycoprotein is 14 KD (18).
The study presented here has provided interesting preliminary data, which support roles of AGE effect to the outcome of Leishmania major infection via macrophage activation. Our results showed that AGE concentration of 37 mg/ml over 48 hours incubation would destroy amastigotes by over expression of iNOS & IFNγ genes in macrophages. Therefore, iNOS & IFNγ is crucial for defense against parasitic pathogens, which could affect the immune response to invading Leishmania parasites.
Acknowledgments
This study was supported by grants from Research institute for Islamic & Complementary Medicine of Tehran University of Medical Sciences (previously named as Iran University of Medical Sciences). The authors declare that there is no conflict of interests.
References
- 1.Modabber F. Leishmaniasis. In: Walgate R, Simpson K, editors. Tropical diseases research progress. Geneva: World Health Organization; 1995. pp. 77–88. [Google Scholar]
- 2.Khademvatan SH, Gharavi MJ, Rahim F, Saki J. Miltefosine induced apoptotic cell death on Leishmania major and L. tropica strains . Korean J Parasitology. 2011;49(1):1–8. doi: 10.3347/kjp.2011.49.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hepburn NC, Siddique I, Howie AF, Beckett GJ, Hayes PC. Hepatotoxicity of sodium stibogluconate therapy for American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1994;88:453–455. doi: 10.1016/0035-9203(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 4.Delaha EC, Garagusi VF. Inhibition of mycobacteria by garlic extract (Allium sativum) J Antimicrob Chemother. 1985;27(4):485–48. doi: 10.1128/aac.27.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appleton J, Tansey MR. Inhibition of growth of zoopathogenic fungi garlic extract. J Mycologia. 1975;67:882–5. [PubMed] [Google Scholar]
- 6.Kandil OM, Abdullah TH. Garlic and the immune system in humans: Its effect on killer cells. Fed Proc. 1987;46:41–6. [Google Scholar]
- 7.Panosian CB, Sypek JP, Wyler DJ. Cell contact-mediated macrophage activation for antileishmania defence. I. Lymphocyte effector mechanism that is contact dependent and noncytotoxic. J Immunol. 1984;133:3358–65. [PubMed] [Google Scholar]
- 8.Ghazanfari T, Hassan ZM, Ebtekar M, Ahmadiani A, Naderi G, Azar A. Garlic induces a shift in cytokine pattern in Leishmania major-infected BALB/c mice. Scand J Immunol. 2000;52(5):491–5. doi: 10.1046/j.1365-3083.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- 9.Khademvatan SH, Gharavi MJ, Akhlaghi L. Induction of apoptosis by miltefosine in Iranian strain of Leishmania infantum promastigotes. Iran J Parasitol. 2009;4(2):23–30. [Google Scholar]
- 10.Olliaro PL, Bryceson AD. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today. 1993;9:323–32. doi: 10.1016/0169-4758(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 11.Herwaldt BL, Berman JD. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi-Renani K, Mahmoodzadeh A, Cheraghali AM, Esfahani A. Effect of Garlic Extract on Cutaneous Lei-shmaniasis and the Role of Nitric Oxide. IJMS. 2002;27(3):97–100. [Google Scholar]
- 13.Khalid NM, Mohomed HE, Toum AM, Mubark MA, Magzoub MA. Treatment of Cutaneous leishmaniasis with some Local Sudanese Plants (Neem, Garad & Garlic) Türkiye Parazitoloji Dergisi. 2004;28(3):129–132. [Google Scholar]
- 14.Mattner J, Wandersee-Steinhäuser A, Pahl A, Röllinghoff M, Majeau GR, Hochman PS, Bogdan C. Protection against Progressive Leishmaniasis by IFN-(1) J Immunol. 2004;172:7574–82. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 15.Bogdan C, Gessner A, Rollinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. J Immunobiology. 1993;189:356–361. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- 16.Stenger S, Donhaauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–6. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhland A, Kima PE. Activation of PI3K/Akt signaling has a dominant negative effect on IL-12 production by macrophages infected with Leishmania amazonensis promastigotes. Exp Parasitol. 2009;122(1):28–36. doi: 10.1016/j.exppara.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 19.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic and failing heart. J Mol Cell Biochem. 2010;333(2):191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 20.Dutta A, Mandal G, Mandal C, Chatterjee M. In vitro antileishmanial activity of Aloe vera leaf exudate: a potential herbal therapy in leishmaniasis. Glycoconj J. 2007;24(1):81–6. doi: 10.1007/s10719-006-9014-z. [DOI] [PubMed] [Google Scholar]
- 21.Trun W, Kiderlen AF, Kolodziej H. Nitric oxide synthase and cytokines gene expression analyses in Leishmania-infected RAW 264.7 cells treated with an extract of Pelargonium sidoides. Phytomedicine. 2006;13(8):570–5. doi: 10.1016/j.phymed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Isaac-Marquez AP, Lezama-Davila CM. Detection of pathogenic bacteria in skin lesions of patients with chiclero's ulcer. Reluctant response to antimonial treatment. Mem Inst Oswaldo Cruz. 2003;98:1093–5. doi: 10.1590/s0074-02762003000800021. [DOI] [PubMed] [Google Scholar]
- 23.Tsao SM, Hsu C, Yin MC. Garlic extract and two diallyl sulphides inhibit methicillin resistant Staphylococ-cus aureus infection in BALB/cA mice. J Antimicrob Chemother. 2003;52:974–980. doi: 10.1093/jac/dkg476. [DOI] [PubMed] [Google Scholar]