Abstract
Background
Fascioliasis is considered as the most important helminthic infection of cattle and sheep. Traditional approaches using morphological and biologic characters cannot cause a certainty in the accurate and precise identification and intra-specific differences of Fasciola spp. In this study, we identified Fasciola species using ITS-1 marker and described genetic variation of each species of the parasite in isolates from Tabriz slaughterhouse in West Azerbaijan Province, north- western Iran.
Methods
Overall, 100 samples (50 from sheep and 50 from cattle) morphologically detected as Fasciola worms were studied for identification of Fasciola species by PCR-RFLP method and intra-species variation of the parasite using RAPD-PCR technique.
Results
A region of approximately 460bp in all samples was successfully amplified. There were no identifiable variations among the size of PCR products. Two and three fragments in samples correspond to F. hepatica and F. gigantica was seen, respectively, through PCR-RFLP method. No difference was seen in digestion pattern according to host (sheep or cattle). Different types of each species of the parasite was observed using RAPD-PCR technique.
Conclusion
We could have an estimate of frequency of F. hepatica and F. gigantic and different genotypes of the parasite in isolates from one locality in north- western of Iran. By extension of such studies in future to other animal hosts (buffalo and goat) and including more regions to sampling, the reliability of the results and their application for control programs in zoonotic diseases will be increased.
Keywords: Fasciola hepatica, Fasciola gigantic, PCR, Iran
Introduction
Digenean trematodes of the genus Fasciola (Platyhelminthes: Trematoda: Digenea) have a global distribution and are the common liver flukes of a range of animals (1). A few ectopic cases of human fascioliasis, in non-hepatic sites such as thyroid, eyes, or skin, have been reported (2, 3). Recent documents estimate human infection by this parasite up to 17 million cases (4). In parts of Africa,such as Kenya, Zimbabwe and Zambia infection with Fasciola represents a major animal and human health problem (5–10) and, because of reduction in milk production, adverse effects on quality and quantity of fleece, this infection causing huge economic losses in the livestock, specially sheep and cattle (11). In tropical countries, fascioliasis is considered as the most important helminthic infection of cattle in prevalence of up to 90% (12). In mainland China, F. hepatica and F. gigantica have been reported from a range of mammals including buffalo, cattle, sheep, and goat (13–16). Fascioliasis is emerging as an important chronic disease of human in Gilan Province of Iran (17). In several outbreaks between 1989 and 1999, 7000 and 10000 human cases occurred in Gilan Province, respectively (18–22). There is no major macroscopic difference between two species of Fasciola, although Fasciola gigantica is longer and narrower, its cephalic cone is shorter, and ventral sucker is larger than F. hepatica (23).
Previously, it has been demonstrated that F. hepatica is mainly found in temperate areas, F. gigantica is mainly common in tropical localities, and both F. hepatica and F. gigantica may overlap in subtropical zones (24). Traditional approaches using morphological and biologic characters cannot cause a certainty in the accurate and precise identification and intra-specific differences of Fasciola spp. (25).
Recently, using genetic approaches, a lot of information regarding the genetic characterization of Fasciola in many countries has been provided (26–28).
The low number of records on human infection with F. gigantica may be due to the lack of suitable tools to distinguish this species from F. hepatica (29). PCR methods using molecular markers such as first and/or second internal transcribed spacers (ITS-1, ITS-2) of ribosomal DNA (rDNA)can be used as a reliable tools for differentiation of Fasciola species in areas where two species overlapping (24, 30). Several recent studies using the (ITS-1 and ITS-2) as genetic markers have identified a so-called “intermediate Fasciola “between F. hepatica and F. gigantica from Korea, Japan and China (25–32).
Different species of Fasciola including intermediate forms exist in Iran (33). In spite of several studies previously documented on distribution of F. hepatica and F. gigantic in Iran, there is not any documented information on the occurrence and genetic features of the parasite in north- western of the country. Therefore, the aim of this study was to genetic describe of the Fasciola spp. using ITS-1 marker by methods of PCR-RFLP and RAPD-PCR in isolates from Tabriz slaughterhouse in West Azerbaijan province, north- western Iran.
Materials and Methods
The samples were collected from livers of sheep and cattle from Tabriz slaughterhouse. Than, they were washed with distilled water (DW) for three times and were transferred and preserved in 70% ethanol at room temperature for further use.
We used FTA tablets for DNA extraction as described previously (23). In brief, we crushed a portion of the parasite between two microscopic slides for 1 min with 300 µl sterile DW and placed 0.4 ml of the lysate on Whatman paper (FTA Elute cards, Tokyo, Japan) and dried it at room temperature as DNA cards until further process. For DNA extraction, one part of punched DNA cards (3mm in diameter) was vortexed 3 times in DW for 1 sec. Incubation of the paper punches at 95°C for 20 min and centrifugation for 30 sec was the final processes of the DNA extraction. We stored the supernatants as DNA at −20°C.
A set of primers including forward (fascF: 5"-ACC CGT GCT GAG AAG ACG-3”) and reverse (fascR: 5”CGA CGT ACG TGC AGT CCA-3”) were used to amplify a 460bp DNA fragment in the ITS1 region of both F.hepatica and F.gigantica (23). The amplification condition was initial denaturation at 95°C for 5 min followed by 30 cycles at 95°C for 45 sec, 60°C for 45 sec, 72°C for 1 min, and final extension at 72°C for 7 min. DW was used as negative control. 1.5% agarose was used for electrophoresis and the gels were stained with ethidium bromide (2ml/ml).
For PCR-RLFP analysis, 1.5 ml of 10x supplied buffer, five micro liters of PCR product and 0.5 microliter of Tas1( fermentas) enzyme were incubated at 37°C for 2h. Separation of restricted fragments performed in 2% agarose gel in TBE buffer using ethidium bromide for staining.
Two primers including UBC90: GGG GGT TAG G and R151: GCT GTA GTG T was used for RAPD-PCR analysis. The thermal cycling condition was as follows: 95°C for 5 min, 40 cycles of 94°C for 40 sec, 32°C for 60 sec, 72°C for 60 sec, and a final extension of 72oC for 6 min. We used 2% agarose gel in TBE buffer for separation of fragments and ethidium bromide for staining the gel.
Results
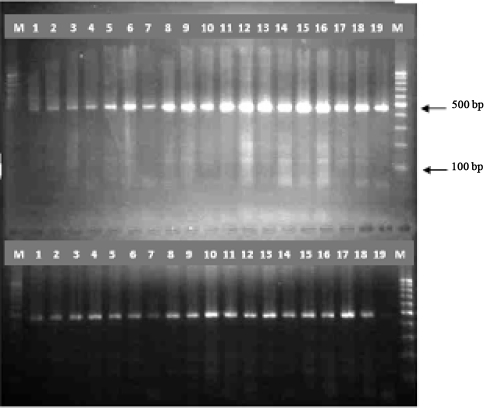
A region of approximately 460bp in all samples was successfully amplified (Fig. 1) that was predictable. There were no identifiable variations among the samples based on the size of the PCR products (Fig. 1). No band was seen in negative controls.
Fig. 1.
Agarose gel electrophoresis of ITS1-PCR products., Lane M 100-bp DNA ladder, lans 1-25 F. hepatica, lans 26-37 F.gigantica, lane C negative control
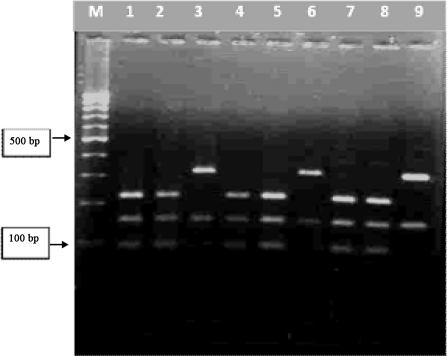
After digestion of the PCR products with TAS-1, two patterns of digestion was seen by the enzyme (Fig. 2), one digestion site producing 2 fragments (about 150 bp and 310 bp) in 75 samples related to F. hepatica and two cutting sites producing 3 fragments (about 95bp, 150bp and 220bp) in 25 samples related to F. gigantica (23) (Fig. 2). No difference was seen in digestion pattern according to host (sheep or cattle) related to each parasite (Fig. 2).
Fig. 2.
Digestion of PCR products with TAS-1 enzyme: lanes 1,2,4,5,7,8 F. gigantic, Lanes 3,6,and 9 F. hepatica.Lane M 100 bp DNA marker
Besides, intra-specific variation within F. hepatica and F. gigantica species were assessed by method of RAPD-PCR. The genomic DNA of all isolates was amplified by primers of UBC90 and R151. Results of two RAPD profiles are shown in (Fig. 3). Three types of F. hepatica and three types of F. gigantica were produced by primer UBC90. Using primer R151 two types of F hepatica and of two types of F. gigantica were seen.
Fig. 3.
Agarose gel electrophoresis of RAPD-PCR products of Fasciola worms isolates from Tabriz. A:RAPD-PCR patterns produced by primer UBC90, examples of three types of F. hepatica in lanes 1,4,5 and of three types of F.gigantica in lanes 6,7,8. Lane M 100 bp DNA marker. B:RAPD-PCR patterns produced by primer R151, examples of two types of F hepatica in lanes 1,4 and of two types of F. gigantica in lanes 6,7
Discussion
There are several studies have exhibited that Fasciola parasites are the causative agents of fascioliasis in animal and human in Iran (3, 17–22). Identification of the parasite species in most of the studies have been done based on the morphologic and morphometric criteria. Because of morphological similarities between Fasciola species, and, due to overlap in the values of most measurements, speciation of the parasite based on morphologic and morphometric criteria cannot be decisive, and there have been some difficulties in definite identification of Fasciola isolates, including differentiation of F. hepatica and F. gigantica where both species, and possibly intermediate forms, coexist (15, 16, 23). At present, genetic methods using molecular markers have an increasing importance in epidemiology of parasites, especially when there is not a reliable method to morphological distinguish of them (15, 16, 23).
Among different genetic markers are used to identify Fasciola species (27), the first internal transcribed spacer (ITS-1) of the rDNA has been used several times for Fasciola classification. This marker provides reliable genetic data for systematic molecular studies and intra-specific variations of the parasite (30). Using specific ITS-1, it demonstrated that, F. hepatica is only common species in some areas of Spain (34). By use of ITS-1 marker in Japan, it has been revealed that intermediate species are more similar to F. gigantica than F. hepatica. (28). In the present study, we could not find intermediate species of Fasciola parasite. Similar findings have been reported from Iran previously (23). Using 18s rDNA marker through PCR-RFLP method, the first intermediate genotype of Fasciola was reported from Fars province, Sothern Iran (35). By sequencing of ITS's-2 marker, it was revealed that isolates of the parasite in infected snails from Northern provinces of Iran were F. hepatica (36). No intermediate species of Fasciola was seen using ITS-1 and 5.8s rRNA marker in Nigeria (37).
We assessed intra-specific variation within F. hepatica and F. gigantica species by method of RAPD-PCR. Amplification of small parts of the genome of an organism can be performed by RAPD-PCR method. This technique has a great potential for describing genetic features of organisms, performing molecular epidemiology and population studies and revealing genetic relatedness of infectious agents (7, 38, 39).
In the present study, the primers produced different DNA fragments in size according to the two species of the worm. Three patterns of genomic DNA by primer UBC90 was found in both F. hepatica and F. gigantica. By using primer R151, we found two patterns of amplification in both of the parasites. The patterns of amplification of DNA of each parasite with both primers were similar in sheep and cattle, demonstrating that these patterns were not specific for cattle and sheep originated F. hepatica and F. gigantica, whereas, in some cases, different patterns by the same primers in different hosts (sheep and buffalo) were reported in isolates from other areas of the country, previously (23). This means the homogeneity of the parasite in isolates in our study is more than former report. Based on the sequencing of ITS-2 marker complete homogeneity was also reported among isolates of the parasite from Mexico, Australia, and Malaysia (27). Studies have been performed in China (27) and Spain using ITS-1 marker showed that the sequence heterogeneity was not related to host species and/or geographical origins of the isolates (28).
As has been showed previously (23), we observed that DNA extraction method using FTA Elute card is easy, without special equipment need and is economical and the DNA prepared from FTA Elute card can be preserved for a long time.
According to the results of this study we emphasize again that the PCR- RFLP method can be useful tool for epidemiological surveys on both human and livestock fascioliasis and should be considered for identification of Fasciola species in geographical areas such as Iran, where both F. hepatica and F. gigantica appear to be coexist and clinical, immunological / pathological methods and morphological features do not permit a discrimination between them at veterinary fields with overlapping distribution areas.
In conclusion, in the present study, using ITS-1 marker through PCR- RFLP and RAPD-PCR methods, we could have an estimate of F. hepatica and F. gigantica variability and different genotypes in isolates from one locality in north- western of Iran. Although we could show a relative intra-species heterogeneity in the isolated parasites in one locality of the country, but by extension of such studies in future to other animal hosts (buffalo and goat) and including more regions to sampling, the reliability of the results and their application for control programs in zoonotic diseases will be increased.
Acknowledgements
This study was financially supported by Tabriz Research Center of Infectious and Tropical Diseases, Tabriz University of Medical Sciences, Iran. The authors declare that there is no conflict of interests.
References
- 1.Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FM. Veterinary parasitology. 2nd ed. Oxford, UK: Blackwell Science; 1996. [Google Scholar]
- 2.Mohsenin H, Ebrahimi MA. Human fascioliasis in Iran, report of a case with Fasciola hepatica in biliary ducts. Bulletin De La Societe De Patholigie Exotique. 1969;62:871–874. [PubMed] [Google Scholar]
- 3.Dalimi A, Jabarvand M. Fasciola hepatica in the human eye. Trans R Soc Trop Med Hyg. 2005;99:798–800. doi: 10.1016/j.trstmh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins DR. Homing in helminthes. Am J Trop Med Hyg. 1992;46:626–634. doi: 10.4269/ajtmh.1992.46.626. [DOI] [PubMed] [Google Scholar]
- 5.Haseeb A N, el-Shazly AM, Arafa MA, Morsy AT. A review on fascioliasis in Egypt. J Egypt Soc Parasitol. 2002;32:317–354. [PubMed] [Google Scholar]
- 6.Keyyu JD, Kassuku AA, Msalilwa LP, Monrad J, Kyvsgaard NC. Cross-sectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in Iringa district, Tanzania. Vet Res Commun. 2006;30:45–55. doi: 10.1007/s11259-005-3176-1. [DOI] [PubMed] [Google Scholar]
- 7.Mekroud A, Titi A, Benakhla A, Rondelaud D. The proportion of liver excised in Algerian abattoirs is not a good indicator of Fasciola hepatica infections in local cattle breeds. J Helminthol. 2006;80:319–321. [PubMed] [Google Scholar]
- 8.Mungube EO, Bauni SM, Tenhagen BA, Wamae LW, Nginyi JM, Mugambi JM. The prevalence and economic significance of Fasciola gigantica and Stilesia hepatica in slaughtered animals in the semi-arid coastal Kenya. Trop Anim Health Prod. 2006;38:475–483. doi: 10.1007/s11250-006-4394-4. [DOI] [PubMed] [Google Scholar]
- 9.Pfukenyi DM, Mukaratirwa S, Willingham AL, Monrad J. Epidemiological studies of Fasciola gigantica infections in cattle in the Highveld and lowveld communal grazing areas of Zimbabwe. Onderstepoort J Vet Res. 2006;73:37–51. [PubMed] [Google Scholar]
- 10.Phiri AM, Phiri IK, Chota A, Monrad J. Trematode infections in freshwater snails and cattle from the Kafue wetlands of Zambia during a period of highest cattle–water contact. J Helminthol. 2007;81:85–92. doi: 10.1017/S0022149X07387786. [DOI] [PubMed] [Google Scholar]
- 11.Dargie JD. The impact of production and mechanisms of pathogenesis of termatode infections in cattle and sheep. In: Vadit MJ, editor. parasitology – quo.proceedings of the 6th international C parasitology. Canberra: Australian Academy of Science; 1986. pp. 453–463. [DOI] [PubMed] [Google Scholar]
- 12.Spithill TW, Dalton JP. Progress in development of liver fluke vaccines. Parasitol Today. 1998;14:224–22. doi: 10.1016/s0169-4758(98)01245-9. [DOI] [PubMed] [Google Scholar]
- 13.Yin HZ, Ye BY, Wang PQ. Studies on the choromosomes and isozymes of Fasciola hepatica and Fasciola gigantica . J Fujian Normal Univ. 1990;6(1):85–90. in Chinese with English abstract. [Google Scholar]
- 14.Du L Y, zhang YQ, liang ZY, Li WJ. Investigation of Fasciola spp. infection in cattle and sheep in the neighboring area between Human and Hubei provinces. Chin J Zoonosis. 1994;10(55) in Chinese. [Google Scholar]
- 15.Xu QB, Shen YL, Daugschies A. Detection of difference among Fasciola hepatica and Fasciola gigantica with RAPD. Chin J Vet Sci. 2001;21:443–446. in Chinese. [Google Scholar]
- 16.Shen YL, Xu LX, Gu YF, Xu QB, Daugschise A. Comparative studies on species differentiation in Fasciola by morphology and RAPD. Adv Anim Med. 2002;23:44–46. Chinese. [Google Scholar]
- 17.Ashrafi K, Valero MA, Panova M, Periago MV, Massoud J, Mas-Coma S. Phenotypic analysis of adults of Fasciola hepatica, Fasciola gigantica and intermediate forms from the endemic region of Gilan, Iran. Parasitol Int. 2006;55:249–260. doi: 10.1016/j.parint.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Massoud J. Fascioliasis outbreak in man and drug test (triclabendazole) in Caspian littoral, northern part of Iran. Bulletin Société Française de Parasitologie. 1989;8:438. [Google Scholar]
- 19.Asmar M, Milani A, Yadgari D. Seroepidemiological investigation of fascioliasis in northern Iran. Med J IR Iran. 1991;5:23–27. [Google Scholar]
- 20.Massoud J. Present status of fasciolosis in Iran. Document SCH/SG/93/WP. Geneva: World Health Organization; 1993. [Google Scholar]
- 21.World Health Organization. Control of food-borne trematode infectious; 1995. Technical Report series No. 849. Geneva. [Google Scholar]
- 22.Rokni MB, Massoud JO, Neill SM, Parkinson M, Dalton JP. Diagnosis of human fasciolosis in the Gilan Province of northern Iran: application of cathepsin L-ELISA. Diagn Microbiol Infect Dis. 2002;44:175–179. doi: 10.1016/s0732-8893(02)00431-5. [DOI] [PubMed] [Google Scholar]
- 23.Rokni M B, Mirhendi H, Mizani A, Mohebali M, Sharbatkhori M, Beigom Kia E, Abdoli H, Izadi SH. Identification and differentiation of Fasciola hepatica and gigantica using a simple PCR-restriction enzyme method. Exp Parasitol. 2010;124:209–213. doi: 10.1016/j.exppara.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Mac-coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Itagaki T, Tsutsumi K. Triploid from of Fasciola in Japan: genetic relationships between Fasciola hepatica and Fasciola gigantica determined by ITS-2 sequence of nuclear rDNA. Int J Parasitol. 1998;28:777–781. doi: 10.1016/s0020-7519(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 26.Itagaki T, Kikawa M, Sakaguchi K, Shimo J, Terasaki K, Shibahara T, Fukuda K. Genetic characterization of parthenogenic Fasciola sp in Japan on the basis of the sequences of ribosomal and mitochondrial DNA. Parasitology. 2005 (a);131:679–685. doi: 10.1017/S0031182005008292. [DOI] [PubMed] [Google Scholar]
- 27.Lin RQ, Dong SJ, Nie K, Wang CR, Li AX, Song HQ, Huang WY, Zhu XQ. Sequence analysis of the first internal transcribed spacer of rDNA supports the existence of an intermediate Fasciola between F. hepatica and F. gigantica in mainland China. Parasitol Res. 2007 Aug;101(3):813–7. doi: 10.1007/s00436-007-0512-0. Epub 2007 Mar 15. [DOI] [PubMed] [Google Scholar]
- 28.Alasaad S, Hung CQ, Li QY, Granados JE, Garcia-Romero C, Perez JM, Zhu XQ. Characterization of Fasciola samples from different host species and geographical localities in Spain by sequences of internal transcribed spacers of rDNA. Parasitol Res. 2007;101:1245–1250. doi: 10.1007/s00436-007-0628-2. [DOI] [PubMed] [Google Scholar]
- 29.Hammond JA. Human infection with the liver fluke Fasciola gigantica . Trans R Soc Trop Med Hyg. 1974;68:253–254. doi: 10.1016/0035-9203(74)90123-0. [DOI] [PubMed] [Google Scholar]
- 30.Huang WY, He B, Wang CR, Zhu XQ. Characterization of Fasciola species from Mainland China by ITS-2 ribosomal DNA sequence. Vet Parasitol. 2004;120:75–83. doi: 10.1016/j.vetpar.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Agatsuma T, Arakawa Y, Iwagami M, Honzako Y, Cahyaningsih U, Kang SY, Hong SJ. Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica . Parasitol Int. 2000;49:231–238. doi: 10.1016/s1383-5769(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 32.Itagaki T, Kikawa M, Terasaki K, Shibahara T, Fukuda K. Molecular characterization of parthenogenic Fasciola sp In Korea on the basis of DNA sequences of ribosomal ITS1 and mitochondrial NDI gene. J Vet Med Sci. 2005 (b);67:1115–1118. doi: 10.1292/jvms.67.1115. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam AS, Massoud J, Mahmoodi M, Mahvi AH, Periago MV, Artigas P, Fuentes MV, Bargues MD, Mas-Coma S. Human and animal fascioliasis in Mazandaran province, northern Iran. Parasitol Res. 2004;94:61–69. doi: 10.1007/s00436-004-1169-6. [DOI] [PubMed] [Google Scholar]
- 34.Marcilla A, Bargues MD, Mas-Coma S. A PCR-RFLP assay for the distinction between Fasciola hepatica and Fasciola gigantica . Mol Cell Probes. 2002;16:327–333. doi: 10.1006/mcpr.2002.0429. [DOI] [PubMed] [Google Scholar]
- 35.Karimi A. Genetic diagnosis of Fasciola species based on 18S ribosomal DNA sequences. J Biol Sci. 2008;8:1166–1173. [Google Scholar]
- 36.Ashrafi K, Massoud J, Holakouie Naieni K, Jo-Afshani MA, Mahmoodi M, Ebadati N, Rezvani SM, Artigas P, Mas-Coma S, Bargues MD. Nuclear ribosomal DNA ITS-2 sequence characterization of Fasciola hepatica and Galba truncatula . Iranian J Publ Health. 2007;36:42–49. [Google Scholar]
- 37.Blair D. Molecular variation in fasciolids and Paragonimus . Acta Tropica. 1993;53:227–89. doi: 10.1016/0001-706x(93)90034-9. [DOI] [PubMed] [Google Scholar]
- 38.Aldemir OS. Differentiation of cattle and sheep originated Fasciola hepatica by RAPD-PCR. Revue Méd Vét. 2006;157(2):65–67. [Google Scholar]
- 39.Rokni MB, Mirhendi H, Behnia M, Fasihi Harandi M, Jalalizand N. Molecular Characterization of Fasciola hepatica Isolates by RAPD-PCR and Ribosomal ITS1 Sequencing. Iran Red Crescent Med J. 2010;12(1):27–32. [Google Scholar]