Abstract
Background
Members of the Vannellidae family are free-living amoebae (FLA) distributed mainly in water and soil sources. The present study reports the first isolation of this genus in the biofilm source from hospital environment in Tehran, Iran.
Methods
Biofilm samples were collected from hospital environment. Cultivation was performed in non-nutrient agar covered with a heat-killed Escherichia coli. Cloning of the suspected amoebae was done. PCR amplification and Homology analysis using the Basic Local Alignment Search Tool (BLASTn) was performed to search for the most similar reference sequences.
Results
Microscopic examination showed numerous fan-shaped amoebae and peculiar cysts different to the usual shape of typical FLA. Sequence analysis of the PCR- product revealed that the suspected amoebae are highly homologous with Vannella spp. gene (99% identity and 100% query coverage) available in the gene bank database.
Conclusion
Although Vannella spp. is not proved to be pathogenic itself, but they are capable of harboring pathogenic intracellular organisms such as Microsporidian parasites. Thus, identification of such amoebae can be of clinical importance, as they could lead to transmission of other pathogens to human.
Keywords: Vannella, Biofilm, Iran
Introduction
Free-living amoebae (FLA) include various taxa with high distribution in the environmental sources such as fresh water, hot springs, sea water, mineral water, soil, dust, clay and moist habitats such as biofilms (1–3). The ubiquitous nature of these organisms leads to the common exposure of human to FLA (4). However, various genera are known as potentially pathogenic organism including Acanthamo-eba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia pedata (1, 5). To date, 17 different genotypes of Acanthamoeba has been identified according to the diagnostic fragment 3 (DF3) of the 18S rRNA gene (6) and most of these genotypes have been proved to be pathogenic in human being (2). Recently, other genera of FLA have been identified as pathogens of cornea and Central Nervous System (CNS) (7, 8). Indeed, recent reports of mixed corneal infection due to Acanthamoeba with Vahlkampfia and Hartmannella and also brain involvement due to Paravahlkampfia francinae lead to the more attention to other FLA, worldwide (7–9). Other families within FLA include Vannellidae amoebae (Vannellids) which were previously introduced as the family belonged to the Thecamoebidae. However, to date Vannellidae is a totally different family from Thecamoebidae (10). Vannellidae contains several genera such as Vannella, platyamoeba and Pessonella (10). The genus Vannella was first described by Bovee, 1965 (10, 11) and occur mainly in water sources (marine and fresh water). This genus contains approximately 40 described species identified in marine and fresh water sources (10, 12, 13). The trophozoite form is rather fan-shaped and some of them are capable to form cysts (12, 14). Few researches reported the presence of these amoebae in soil samples (12). It should be mentioned that amoebae belong to this genera could act as a trojan horse for microbial world and they could support their endosymbiont proliferation (15). This property makes non-pathogenic FLA as relevant organisms for human being. Indeed, the carrier property of Vannella for intracellular microorganism such as pathogenic Microsporidian parasites is of utmost clinical importance (15, 16).
The present study is the first report regarding the isolation of Vannellidae amoebae (Vannellids) in the biofilm sources in Iran using both morphological tests and molecular approaches.
Material and Methods
Sample Processing
Biofilm samples were collected using sterile swap from hospital environment in our previous study (17). Briefly, biofilms were dissolved in distilled sterile water, incubated for an hour and filtrated using membrane filters (Pore size 1.2 µ). Filters were then placed upside down on to non-nutrient agar covered with a heat- killed Escherichia coli. Incubation was performed at 30°C. Plates were then monitored for the presence of FLA using both light and inverted microscope.
Microscopic examination
One plate showed numerous fan-shaped amoebae and peculiar cysts different to the usual shape of typical FLA and thus fresh smear of the suspected culture-plate was examined under inverted microscope. Suspected amoebae were then submitted to cloning to obtain a single line cell and to eliminate bacterial and fungi contamination of plates for subsequent evaluation according to our previous studies (17, 18).
DNA extraction, PCR amplification and sequencing analysis
Cloned plate containing suspected amoebae were then scraped using pH 7 PBS. Pellet of amoebae were then obtained using 3000 rpm centrifuge and DNA extraction were done using Chelex kit (Instagen matrix, Biorad). PCR were performed by NA primers which amplify a partial 18s rRNA gene of some FLA such as Vannella and Hartmannella. The primers sequences were: NA1: 5′-GCT CCA ATA GCG TAT ATT AA-3′ and NA2: 5′-AGA AAG AGC TAT CAATCT GT-3′ (17). PCR reactions were performed in 30 µl Ampliqone (Taq DNA Polymerase Master Mix RED, Denmark) as a ready-made mixture. Briefly, 25 µl of Taq Master mix were used with 10 ng template DNA, 0.1 µM of each primer and distilled water. PCR cycling conditions included initial denaturing step of 94°C for 1 min, followed by 35 repetition cycles at 94°C for 35 s, annealing at 58°C for 45 s, and at 72°C for 1 min. PCR products were electrophoresed using 2% agarose gel stained with a solution of ethidium bromide and visualized under UV light.
PCR product was submitted to sequencing using the ABI 3130X automatic sequencer in the Research Center for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran. Homology analysis using the Basic Local Alignment Search Tool (BLASTn) was performed to search for the most similar reference sequences.
Results and Discussion
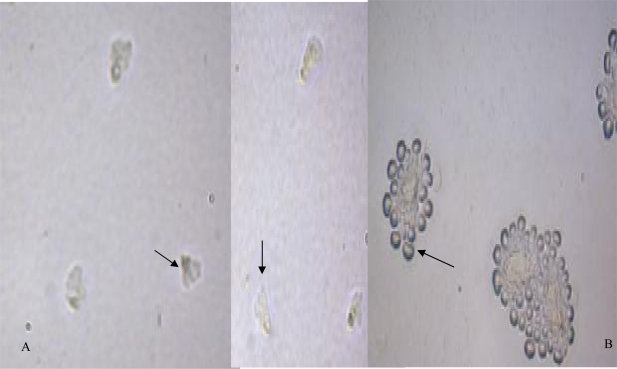
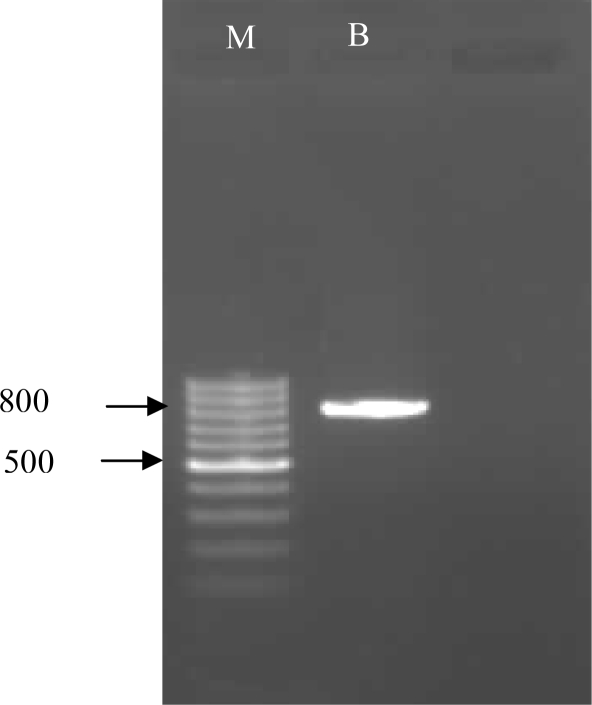
Plate culture revealed fan-shape trophozoites and unusual cysts after one week of incubation. Thecamoeba were detected according to ovoid and flat shape of their trophozoites and therefore cloning have been done to rescue our targeted amoebae from Thecamoeba and to obtain free-bacteria and fungi plate. Flabellate shaped trophozoites measured about 20 µ and cysts were detected in clump manner (Fig. 1). Microscopic examination revealed our suspected amoebae as Vannellidae. Sequence analysis of the PCR product (Fig. 2) confirmed that the suspected amoebae are highly homologous with Vannella spp. gene (99% identity and 100% query coverage) available in the gene bank database (Accession number: AY929909.1; strain 4362V/II 18S small subunit ribosomal RNA gene) and this confirms our microscopic observation. The new gene have been deposited in the gene bank database under accession number: JN009668.
Fig. 1.
A: Fan-shape trophozoit of Vannella spp X400; B Cyst of Vannella spp X100 isolated from biofilm source in Tehran, Iran
Fig. 2.
Gel electrophoresis of the 800 bp PCR-product of Vannella spp isolated from biofilm in Tehran, Iran (M=Marker, B: Biofilm sample)
This is the first report of Vannella spp. isolated from biofilm sources in Iran. Our previous study showed that biofilm could harbor various free-living amoebae such as Acanthamoeba T4 and T5 genotype and Vahlkampfia (17). The present study proved that Vannella also could proliferate in such habitats. Indeed, microorganism within biofilms could be a great source of food for FLA and therefore biofilm sources introduced as serious hazard in hospital environment (15). Improved disinfection measures are recommended in health-care settings to avoid aggregation of biofilms. Previous researches isolated Vannella from marine and fresh water environments (10, 12). Few studies also report the isolation of Vannella from soil samples (12). Although, there is no report of human infection due to Vannella, but it is worthy to mention that this genus could be a carrier of other pathogens such as Microsporidian- like protozoa (15, 16). In support of this, Hoffman et al. (1998) researches revealed that Vannella could be infected by Microsporidian organisms. The sources of Vannella containing Microsporidian organisms in Hoffman et al. study were a domestic tap- water supply (16). This is important since Vannella could protect their endosymbiont from chlorine and harsh environment and this can lead to multiplying of pathogenic organism inside the host amoebae. Indeed, infected amoebae can act as a vehicle for transmission of microorganisms to cornea. Subsequently, Scheid (2007) reported a case of keratitis in a contact lens wearer without a history of corneal trauma (19). Pseudomonas aeruginosa was identified as the etiological agent for the mentioned patient; however, Vannella was also detected in the material of the patient eye and intracellular organism detected in this amoebae resembling Microsporidian organism (19).
In conclusion, although Vannella is not proved to be pathogenic yet, but they are capable of harboring pathogenic organisms such as Microsporidian parasites and thus identification of this genus could lead to further studies regarding bacteria and amoebae relations. The present study gave information regarding the presence of Vannella in biofilm sources in hospital environment in Iran. More Hygienic measures and improved disinfection methods is recommended to health authorities to prevent FLA- related disease.
Acknowledgments
Dr. M Niyyati was supported by a Research Grant from the National Elites Foundation for distinguished Young Assistant Professors. This study was supported by Shahid Beheshti University of Medical Sciences through grant No 7075-91-01-89. The authors declare that they have no conflicts of interest.
References
- 1.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free living amoebae: Acanthamoeba spp, Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea . FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan NA. Acanthamoeba, biology and pathogenesis. 1st ed. Great Britine: Caister Academic Press; 2009. [Google Scholar]
- 3.Marciano-Cabral F, Jamerson M, Kaneshiro ES. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010;8(1):71–82. doi: 10.2166/wh.2009.129. [DOI] [PubMed] [Google Scholar]
- 4.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 5.Qvarnstrom Y, da Silva AJ, Schuster FL, Gelman BB, Visvesvara GS. Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J Infect Dis. 2009;199:1139–1142. doi: 10.1086/597473. [DOI] [PubMed] [Google Scholar]
- 6.Nuprasert W, Putaporntip C, Pariyakanok L, Jongwutiwes S. Identification of a novel T17 genotype of Acanthamoeba from environmental isolates and T10 genotype causing keratitis in Thailand. J Clin Microbiol. 2010;48:4636–4640. doi: 10.1128/JCM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Rezaeian M, Niyyati M. Pathogenic Free Living Amebas In Human. 1st ed. Tehran: TUMS Publication; 2010. [Google Scholar]
- 9.Visvesvara GS, Sriram R, Qvarnstorm Y, Bandyopadhyay K, Silva A, Pieniazek NJ, Cabral GA. Paravahlkampfia francinae n. sp. masquerading as an agent of primary amoebic meningoencephalitis. J Eukaryot Microbiol. 2009;56:357–366. doi: 10.1111/j.1550-7408.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Page FC. A new key to freshwater and soil Gymnamoebae. Amble-side, Cumbria: Freshwater Biological Association/The Ferry House; 1988. [Google Scholar]
- 11.Bovee EC. An emendation of the ameba genus Flabellula and a description of Vannella gen. nov. Trans Am Micros Soc. 1965;84:217–227. [Google Scholar]
- 12.Smirnov AV, Brown S. First isolation of a cyst-forming Vannella species, from soil—Vannella persistens n. sp. (Gymnamoebia, Vannellidae) Protistology. 2000;1:120–123. [Google Scholar]
- 13.Smirnov AV, Nassonova ES, Chao E, Cavalier-Smith T. Phylogeny, Evolution, and Taxonomy of Vannellid Amoebae. Protist. 2007;158:295—324. doi: 10.1016/j.protis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Amaral-Zettler LA, Cole J, Laatsch AD, Nerad TA, Anderson OR, Reysenbach A. Vannella epipetala n. sp. Isolated from the Leaf Surface of Spondias mombin (Anacardiaceae) Growing in the Dry Forest of Costa Rica J Eukaryot Microbiol. 2006;53(6):522–530. doi: 10.1111/j.1550-7408.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 15.Michel R, Schmid EN, Böker T, Hager DG, Müller K-D, Hoffmann R, Seitz HM. Vannella sp. harboring Microsporidia-like organisms isolated from the contact lens and inflamed eye of a female keratitis patient. Parasitol Res. 2000;86:514–520. doi: 10.1007/s004360050704. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann R, Michel R, Schmid EN, Muller K-D. Natural infection with microsporidian organisms (KW19) in Vannella sp. (Gymnamoebia) isolated from a domestic tap-water supply. Parasitol Res. 1998;84:164–166. doi: 10.1007/s004360050377. [DOI] [PubMed] [Google Scholar]
- 17.Lasjerdi Z, Niyyati M, Haghighi A, Shahabi S, Tahvildar Biderouni F, Taghipour N, Eftekhar M, Nazemalhosseini Mojarad E. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran. Iran Parasitol Res. 2011 doi: 10.1007/s00436-011-2288-5. [DOI] [PubMed] [Google Scholar]
- 18.Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Mohebali M, Maghsood AH, Motevalli-Haghi A, Martín-Navarro CM, Farnia Sh, Valladares B, Rezaeian M. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121:242–5. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Scheid P. Mechanism of intrusion of a microsporidian- like organism into the nucleus of host amoebae (Vannella sp.) isolated from a keratitis patient. Parasitol Res. 2007;101:1097–1102. doi: 10.1007/s00436-007-0590-z. [DOI] [PubMed] [Google Scholar]