Abstract
Background
Coccidiosis of domestic fowl, caused by species of the Genus Eimeria, is responsible for important economic losses in poultry production. Because different species and/or strains can vary in pathogenicity and other biological parameters, their precise characterization is important for epizootiological studies.
Methods
Fifty samples from litter, whole intestinal tract and feces were collected from poultry houses located in different provinces of Iran. One hundred twenty male day-old broiler chicks were challenged with three selected isolates. Data on weight gain, Food Conversion Ratio (FCR), food intake, lesion scoring and shedding of oocysts per gram of feces were recorded and analyzed by the Duncan's test.
Results
In all treatments, the challenged groups had statistically significant lower weight gain than that of unchallenged control group. Isolate three caused the lowest weight gain and food intake and the worst lesion score as well as FCR. Despite originating from close geographical regions for isolates 1 and 2, the difference in biopathologic factors may be either due to different proportion of identified species or the different pathogenicity of the species present in the isolates.
Conclusion
The results highlight the importance of considering various species of Eimeria in designing the preventive, control and treatment strategies to prevent coccidiosis in different regions of Iran. Further characterization of each isolate would be the next step to provide a basis for coccidiosis research with well-characterized local isolates.
Keywords: Eimeria, Poultry coccidiosis, Iran
Introduction
Amongst various parasitic infections, coccidiosis caused by obligate intracellular protozoan parasite of the genus Eimeria is a major parasitic disease within the intensive poultry production system. Eimeria spp. are highly host-specific, with six species having an economic impact in chickens (1). Coccidiosis, as one of the most significant diseases of poultry, costs the world's commercial chicken producers at least US$ 1.5 billion every year (2). It has been shown that the disease has brought about great economic losses in the poultry industry of Iran like the other parts of the world (3). In broilers, coccidiosis control should not only address the prevention of clinical disease and mortality but also mild and subclinical infections, as even minor intestinal lesions can interfere with growth, feed conversion and therefore profitability. In addition to management measures, (litter condition, bird population in the poultry farm); the disease has largely been controlled by directly adding anticoccidial drugs to the chicken feed (4). Medication by anticoccidial drugs in chicken had been started about 30 years ago in Iran and numerous products were introduced, which are readily available and in use (3). The intensive use of anticoccidial drugs has led to the development of resistance (5). The public concern of chemical residues in meat and pollution of the environment has led to stricter regulations against the use of coccidiostats in food in Europe (6–8); besides the increasing development of drug-resistant coccidian species has stimulated searches for alternative control methods such as applying a live vaccine early in life or development of new drugs (7).
Detection of oocysts in chicken feces and examination of lesions at particular regions of the gut are common means to diagnose coccidiosis, and identification of Eimeria species has been conducted traditionally based on morphologic and pathologic aspects, such as oocyst characteristics, variations in prepatent period (time spent in coccidial generations), sporulation time, clinical signs and intestinal lesions, and histopathologic characteristics (9). In fact, characterization of the field isolates is the first step towards further investigation of the biology of the coccidiosis as a disease entity and Eimeria spp. as its causative agents. Furthermore, these well-characterized isolates can be used for further challenge studies in testing either anticoccidial drugs or vaccine efficacy.
This paper describes the isolation, identification and assessment of pathogenicity of three-mixed Eimeria field isolates among 50 field samples collected from different parts of Iran.
Materials and Methods
Sample collection
Fifty samples including twelve litter samples and thirty-eight whole chicken intestines were collected from different regions of Iran. Litter samples were collected from several representative areas of the chicken house, from the top layer of material. The samples were mixed together and 5 g of material was weighed for oocysts detection and isolation. Totally 12 litter samples from 12 poultry houses were collected for oocyst detection.
For intestine samples, the entire length of the intestine from duodenum to cloaca of at least 5 birds per poultry house, which were referred to a veterinary diagnostic laboratory, were collected in potassium dichromate. The samples were transferred to parasitology laboratory. Totally, intestinal samples were collected from 38 poultry farms. The farms were selected without regard to previous coccidiosis problems (10).
Parasitology
Oocyst detection was performed according to the standard method (11, 12), after grounding in a blender to release the unsporulated oocysts, the tissue suspension was filtered through cheesecloth into a beaker using a spatula. Either form of the samples was washed with water through a double layer of cheesecloth or a fine mesh sieve into a beaker. The solids in the filtrate were allowed settle down by centrifugation. The supernatant was discarded and the oocysts were suspended from the pellet in a saturated salt solution. The suspension was centrifuged at a moderate speed (e.g., 1500 rpm) for 10–15 minutes to sediment the solids and allow the oocysts to remain suspended at the top of the supernatant. The oocysts were removed from the top layer of fluid by pipette. The oocyst suspension was washed three to four times to remove the salt solution. The salt-free oocyst suspension was then stored in 2.5% potassium dichromate solution at room temperature to sporulate. Three isolates containing the more numerous coccidian parasites were selected for biopathologic characterization. The collected oocysts were kept at 4 °C in 2.5% potassium dichromate solution. The sporulated oocysts were counted per 1.0 ml of solution using the hemocytometer method as described earlier (11).
Animals
For this experiment 120 male one-day-old Ross broiler chicks were randomly assigned to 4 groups at 12th day of age, containing 30 chicks in each treatment. Each group contained three replicates of 10 chicks, allocated in battery cages. During the experiment (21 days) the chickens were fed a diet based on corn, soybean (13), food, and water were provided ad-libitum. The temperature was maintained at 30-32 °C for the first week and was reduced by 1-3 °C on weekly basis. Lighting was provided 24 hours. No vaccine was used during the test period. On the start of the experiment (day 0=14th day of age) the birds were leg tagged with numbers so that individual data can be recorded.
Purification and standardization of doses of the inoculum for experimental infection
The three selected isolates were passaged through 2-3-week-old chicks by oral inoculation and the oocysts passed in the feces were collected for up to 8 days post inoculation (12). The virulence and the proper dose for challenge, so that maximum lesions but minimal mortality is caused, were estimated from the results of these initial tests.
Challenge study
The infectious dose (about 3x105) was given orally on 14th day of age. The birds were weighted on the day of inoculation and again reweighed on 21st day of age (7 days post inoculation). Data on the following parameters were recorded: weight gain, feed consumption, and feed conversion ratio, oocysts per gram of feces, lesion score, oocysts scoring and mortality. On 7th day post inoculation, three birds from each replicate were randomly selected for post mortem examination and intestinal lesion score according to Conway and McKenzie (12). Briefly, four areas of the intestinal mucosal surface were individually examined, in addition to the serosal surface. A score of 0 to +4 was recorded for each chicken for the four regions of the mucosal surface: the duodenal and upper intestine or jejunum (U), the middle intestine (M), the lower intestine or ileum (L) and the ceca (C).
An oocyst index of 0 to 5 was determined by examination of scrapings from each four segment of intestine for birds sacrificed for lesion score at 7th day post inoculation. Blind technique was applied to avoid bias. The average oocyst indexes of each segment of intestine for every necropsied bird were estimated at 120-fold magnification by counting the oocysts in five microscopic fields as follows:
0: <1 oocyst/field; 1: 1–10 oocysts/field; 2: 11–20 oocysts/field; 3: 21–50 oocysts/field; 4: 51–100 oocysts/field and 5: >100 oocysts/field (14).
Oocyst per gram of feces was assessed according to Holdsworth et al.(11) and Conway and McKenzie (12). Droppings from each group were collected for 7 days, starting on the 7th day post inoculation. The prepared samples were counted microscopically using a hemocytometer slide.
Statistical analysis
All data were subjected to ANOVA and two-way t-test to see whether the differences between groups are significant. Differences between means were considered significant at p< 0.05.
Results
Positive field samples
Fifteen out of fifty samples were positive for presence of Eimeria (Table 1). All six positive litter samples belonged to the broiler houses, the negative litter samples comprised of 4 broilers and 2 broiler breeder complexes. All the positive litter samples except one sample originated from Mazandaran Province, which is located on the southern coast of the Caspian Sea and has high relative humidity.
Table 1.
Geographical location, type and age of the flocks sampled in this study
| Farm | Location/province | Type | Age (days) | Anticococidials | Oocyst concentrationa |
|---|---|---|---|---|---|
| 1 | Ghasr-e-Shirin/Kermanshah | Bro | 15 | None | - |
| 2 | Hamedan | Bro | 15 | NA | - |
| 3 | Broojerd/Lorestan | Bro | 25 | Maduramycin | - |
| 4 | Varamin/Tehran | Bro | 31 | Livacox® | + |
| 5 | Broojerd/Lorestan | Bro | 25 | Salinomycin | - |
| 6 | Razan/Hamedan | Bro | 22 | NA | + |
| 7 | Hamedan | Bro | 43 | Salinomycin | +++ |
| 8 | Garmsar/Semnan | Bro | 30 | Salinimycin | - |
| 9 | NazarAbaad/Alborz | Bro | 40 | None | - |
| 10 | Qazvin/Qazvin | Bro | 45 | Salinimycin-Diclazuril | - |
| 11 | Qazvin/Qazvin | Bro | 45 | Salinimycin-Diclazuril | - |
| 12 | Qa'emshahr/Mazandaran | Bro | 32 | NA | - |
| 13 | Shirgah/Mazandaran | Bro | 21 | NA | - |
| 14b | Arth/Mazandaran | Bro | 41 | Maduramycin | ++++ |
| 15b | Duke/Mazandaran | Bro | 44 | Maduramycin | ++++ |
| 16b | Kotena/Mazandarn | Bro | 39 | NA | ++ |
| 17 | Semnan | Bro | 24 | Maduramycin | + |
| 18 | Eshtehard/Alborz | Bro | 27 | None | - |
| 19 | Garmsar/Semnan | Bro | 37 | Maduramycin | + |
| 20 | Pakdasht/Tehran | Bro | 38 | None | ++ |
| 21 | Razan/Hamedan | Bro | 32 | Maduramycin | ++++ |
| 22b | Qazvin/Qazvin | Bro | 31 | NA | + |
| 23 | Qom/Qom | Bro | 31 | NA | + |
| 24 | Shahryar/Tehran | Bro | 29 | NA | + |
| 25 | Qazvin/Qazvin | Bre | 90 | NA | - |
| 26 | Qazvin/Qazvin | Bre | 62 | NA | - |
| 27 | Hamedan/Hamedan | Bro | 40 | None | - |
| 28 | Karaj/Alborz | Bro | 38 | Salinomycin | - |
| 29 | Mahabad/West Azarbaijan | Bro | 41 | NA | - |
| 30 | Sardasht/West Azarbaijan | Bro | 37 | None | - |
| 31 | Garmsar/Semnan | Bro | 30 | Salinomycin | - |
| 32 | Nazarabad/Aalborz | Bro | 40 | None | - |
| 33 | Razan/Hamedan | Bro | 38 | None | - |
| 34 | Malayer/Hamedan | Bro | 36 | Maduramycin | - |
| 35 | Ilam/Ilam | Bro | 45 | NA | - |
| 36 | Shahryar/Tehran | Bro | 32 | NA | - |
| 37 | Mahabad/West Azarbaijan | Bro | 32 | NA | - |
| 38 | Marvdasht/Fars | Bro | 42 | NA | - |
| 39 | Mahabad/West Azarbaijan | Bro | 30 | NA | - |
| 40 | Mahabad/West Azarbaijan | Bro | 30 | NA | - |
| 41 | Kordan/Tehran | Bro | 30 | NA | - |
| 42 | Rasht/Gilan | Bro | 47 | Monensin | - |
| 43 | Eslamshahr/Tehran | Bro | 42 | Maduramycin | - |
| 44 | Qazvin/Qazvin | Bre | 70 | NA | - |
| 45b | Qa'emshahr/Mazandaran | Bro | 35 | NA | ++ |
| 46b | Qa'emshahr/Mazandaran | Bro | 40 | NA | ++ |
| 47 | Shahryar/Tehran | Bro | 25 | None | - |
| 48 | Ilam/Ilam | Bro | 32 | None | - |
| 49 | Ilam/Ilam | Bro | 31 | None | - |
| 50 | Buinzahra/Qazvin | Bro | 52 | None | - |
Bro: Broiler; Bre: Breeder; None: No anticoccidial; NA: Using and/or the kind of anticoccidial treatment in the farm were not applicable. a:determined by the intensity of oocysts present in a microscopic field: -: no oocyst, +: 10>, ++: <20>, +++: <50> and ++++: >50 oocyst in microscopic field; b:litter sample
Based on observations during propagation, the challenge dose of the three selected farm isolates were estimated around 300000 sporulated oocyst per bird for isolate 1 (containing 12% E. acervulina, 16% E. brunetti, 44% E. maxima, 12% E. mitis, 12% E. tenella and 4% E. necatrix) and 2 (containing 24% E. acervulina, 6% E. brunetti, 34% E. maxima, 16% E. mitis, 18% E. tenella and 2% E. necatrix) and for isolate 3 it was calculated 250000 sporulated oocyst per bird (containing 40% E. acervulina, 15% E. brunetti, 25% E. maxima, 8% E.mitis, 6% E. tenella and 6% E. necatrix).
Eimeria specie
The different species of Eimeria present in the three used inoculums were identified according to shape index (15) (after measuring 100 oocysts) for each three isolates. All of the isolates contained six Eimeria species namely E. acervulina, E. brunetti, E. maxima, E. mitis, E. tenella and E. necatrix (Table 2).
Table 2.
Three field isolates and percentage of different Eimeria species in each isolate
| Islolate | E. acervulina | E. brunetti | E. maxima | E. mitis | E. tenella | E. necatrix |
|---|---|---|---|---|---|---|
| 1a | 12 | 16 | 44 | 12 | 12 | 4 |
| 2 a | 24 | 6 | 34 | 16 | 18 | 2 |
| 3 a | 40 | 15 | 25 | 8 | 6 | 6 |
1, 2, 3: Samples No.15, 14 and 21 in Table 1, respectively
Weight gain
In all treatments the challenged group had a statistically significant lower weight gain than that of unchallenged control group (P<0.05). The differences in weight gain between each two isolate were also statistically significant. The results of the weight gain are summarized in Table 3.
Table 3.
Weight gain, FCR, Productivity Index, Lesion Score and Oocyst Index in a seven-day period after challenge with three field isolate
| Isolate | Initial weight | Final weight | WG)gr) | %WG nnc | Food intake (g) | FCR (g/g) | Productivity Index | Mean LS | Mean Oocyst Index |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 414.3 | 651.36 | 237.06±2.94a | 71.79 | 4808a | 2.02 b | 5.01a | a1.80 | a1.36 |
| 2 | 406.36 | 612 | 205.64±2.63b | 63.49 | 4187b | 2.03 b | 4.33b | a1.47 | a0.75 |
| 3 | 422.83 | 568.13 | 145.3±4.48c | 43.11 | 4001b | 2.75 a | 2.26c | 2.33b | 2.61b |
| NCG | 437.2 | 785.66 | 348.46±12.35d | 100.00 | 5772c | 1.65c | 9.02d | - | - |
a, b, c, d: Different letters in a column indicates significant differences between treatments
Food consumption and FCR
According to the results, the highest and the lowest food intake during the experiment belonged to unchallenged negative control and chickens infected with isolate three, respectively (Table 3). The negative control chickens had the lowest (better) FCR at the end of experiment. The highest FCR (worst) was calculated for isolate three. FCR and food intake had a similar pattern, although the difference of FCR between isolates 1 and 2 was not significan, but numerically the value of isolate 2 was worse than isolate1. In case of food intake, values for isolates 2 and 3 were not statistically significant.
Lesion scores
The results are summarized in Table 3. Isolate three caused the most severe lesion in all segments of the intestines. The mean lesion scores of isolate one and two were not statistically significant. Lesions caused by isolate 1 was more severe in upper, middle and lower intestine than the lesions caused by isolate 2, but in the cecum, chickens inoculated with isolate 2 had higher lesion scores than those infected with isolate 1.
Oocyst index and oocysts per gram of feces
The mean oocyst index of the group infected with isolate three was significantly higher than the other two isolates. The mean of oocyst index of isolate one and two were not significantly different. The results are shown in Table 3.
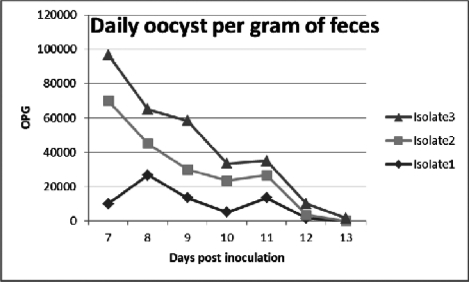
The results of OPG are shown in Fig. 1. The mean oocyst index of isolate three was statistically significant and greater than two other isolates. Numerically oocyst index of isolate one was higher (worse) than isolate two, although it was not statistically significant.
Fig. 1.
oocyst per gram of feces during a 7-day period after inoculation
Productivity index
The productivity index was calculated using the following formula (14):
The PI of the negative control group was almost 2 times higher than that of isolates 1 and 2 and about four folds higher than PI of isolate 3 (Table 3).
Discussion
In this study, 50 field samples were examined for the presence of Eimeria oocysts and the prevalence was estimated 30%. Ninety 7-day-old chickens were challenged with 3 selected field isolates and biopathological characteristics, including weight gain, food intake, FCR, intestinal lesion score, oocyst index, oocyst per gram of feces and productivity index induced by those isolates were recorded in a seven day period post inoculation. Isolate 3 was the most virulent isolate (6% E. tenella and 6% E.necatrix) according to the above-mentioned criteria. The reduction in weight gain, alteration of food intake and subsequently alteration of FCR, in comparison to negative control, highlights the economic importance of the disease.
Prevalence of positive samples was 30%. Hamidinejat et al. (16) had reported a prevalence of 31.5% by PCR in Khuzestan. In other study in Tabriz, the prevalence of Eimeria sp. was 55.96% determined by conventional parasitological method (17). In a survey of subclinical coccidiosis, 38% of the surveyed farms were positive in Khorasan (18). Sampling in winter or spring, the more intensity of birds in a farm and older flocks were the risk factors affecting the rate of infection positively (18). Season and the climatic characteristics of the sampling regions, such as temperature and humidity, positively influences the disease prevalence (19).
Regarding weight gain, chickens infected with isolate 3 had the least weight gain followed by isolate 2 and isolate 1, in sequence. In the cases of isolate 1 and 2, although they originated from a close geographical region, the difference in weight gain and food intake may be either due to different proportion of identified species or the different pathogenicity of the species present in the isolates. There is little information to allow an accurate assessment of the effect of coccidiosis on weight gains of birds in the field. It is likely that almost all broilers are suffering from a reduction in final live weight (20). Productivity index was the worst for isolate 3, respectively followed by isolate 2, 1. As expected the negative control group had the best productivity index.
The most prevalent species in the two samples collected from Mazandaran province was E. maxima (44 and 34 percent for isolates 1 and 2 respectively) whereas in isolate 3, E. acervulina was more numerous (40%). In Khuzestan the most prevalent species was E. tenella (16) , in Tabriz and Khorasan E. acervulina was more numerous (17, 18). The severity of coccidial lesion caused by isolate 3 was the worst followed by isolates 1 and 2, respectively.
However, E. tenella is considered to have the most pathologic effect, but in the present study, isolate 3, which was the most severe isolate based on all criteria, had the lowest proportion of this species, although the cecal lesion score for isolate 3 was notably higher than the other two isolates which may point out the difference of pathogenicity of Eimeria tenella present in this isolate. Strains of poultry coccidia that vary in pathogenicity have been recognized in various geographical areas. Fayer (22) proposed some strains for E. tenella such as Houghton, Weybridge, Wisconsin and Beltsville. It seems logic to consider seasonal conditions and prevalence of various species of coccidia in designing the preventive, control, and treatment measures to combat coccidiosis (1) in various regions of Iran. Isolate 3 had induced the highest rate of oocyst shedding, followed by isolates 2 and 1. Researchers used different criteria to evaluate coccidial infections. Some suggested that oocyst production might be a very unreliable quantitative criterion (23) as the number of oocysts produced is affected by factors such as the inherent potential of each species to reproduce in a non-immune host; immunity or resistance developed by the host; the'crowding' factor; competition with other species of coccidia or other infectious agents; nutrition of the host; and strain differences of the host. The inherent difference in reproductive potential is high for E. tenella, and E. acervulina, and low for E. maxima. Immunity, which is specific to each coccidian species, results in decreased production of oocysts after ingestion of infective oocysts (22).
The pathogenicity of coccidia depends largely on the dose and viability of oocysts and the virulence of strains (10). It is recommended to determine the distribution of different poultry Eimeria species in geographical regions of Iran and the pathogenicity of the prevalent species be distinguished, as geographically separate isolates have been found to cross-immunize poorly (24, 25) which may lead to decrease efficiency of anticoccidial vaccines which are not prepared from local strains or strains close in immunogenicity to local strains (26). Surveying the regional pattern of anticoccidial resistance will lead to better and prolonged use of available anticoccidial drugs, as there are reports of restoration of anticoccidial drug sensitivity in Eimeria spp. isolates (27).
In conclusion, further molecular identification of each isolate, identifying their anticoccidial drug sensitivity profile, assessing the efficacy of available vaccines against these isolates will be the next steps for providing a basis for coccidiosis research with well-characterized local isolates.
Acknowledgments
The authors would like to thank the faculty of Veterinary Medicine, University of Tehran for funding the project number 7506009/6/8 and Engineering Research Institute of Ministry of Agriculture for supporting the research. The authors declare that there is no conflict of interests.
References
- 1.Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15(1):58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav A, Gupta SK. Study of resistance against some ionophores in Eimeria tenella field isolates. Vet Parasitol. 2001;102:69–75. doi: 10.1016/s0304-4017(01)00512-x. [DOI] [PubMed] [Google Scholar]
- 3.Pirali-kheirabadi Kh, Zamani-Moghadam A, Abdi F, Bahonar AR. The effect of administration of anti-coccidial drugs on oocysts shedding and performance in experimental coccidiosis in broiler chicken. Int J Vet Res. 2008;2,1:67–73. [Google Scholar]
- 4.Guo FC, Suo X, Zhang GZ, Shen JZ. Efficacy of decoquinate against drug sensitive laboratory strains of Eimeria tenella and field isolates of Eimeria spp. in broiler chickens in China. Vet Parasitol. 2001;147:239–245. doi: 10.1016/j.vetpar.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Chapman HD. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 6.De Pablos LM, dos Sontos MF, Montero E, Garcia-Garnados A, Parra A, Osuna A. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol Res. 2010;107:601–604. doi: 10.1007/s00436-010-1901-3. [DOI] [PubMed] [Google Scholar]
- 7.Yim D, Kang SS, Kim DW, Kim SH, Lillehoj HS, Min W. Protective effects of Aloe vera-based diets in Eimeria maxima-infected broiler chickens. Exp Parasitol. 2010 doi: 10.1016/j.exppara.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Barriga OO. A review on vaccination against protozoa and arthropoda of veterinary importance. Vet Parasitol. 1994;55:29–55. doi: 10.1016/0304-4017(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara F, Taira K, Nagai S, Onaga H, Onuma M, Nunoya T. Detection of five avian Eimeria species by species-specific real-time polymerase chain reaction assay. Avian Dis. 2008;52:652–656. doi: 10.1637/8351-050908-Reg.1. [DOI] [PubMed] [Google Scholar]
- 10.McDougald LR, Da Silva JM, Solis J, Braga M. A survey of sensitivity to anticoccidial drugs in 60 isolates of coccidian from broiler chickens in Brazil and Argentina. Avian Dis. 1986;31(2):287–292. [PubMed] [Google Scholar]
- 11.Holdsworth PA, Conway DP, McKenzie ME, Dayton AD, Chapman HD, Mathis GF, Skinner JT, Mundt HC, Williams RB. World association for advancement of veterinary parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 2004;121:189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Conway DP, McKenzie ME. 3rd ed. Iowa: Blackwell publishing; 2007. Poultry coccidiosis diagnosis and testing procedures. [Google Scholar]
- 13.Ninth Revised ed. Washington DC. National Academy Press; 1994. Nutrient Requirements of Poultry. [Google Scholar]
- 14.Daugschies A, Gässlein U, Rommel M. Comparative efficacy of anticoccidials under the conditions of commercial broiler production and in battery trials. Vet Parasitol. 1998;76:163–171. doi: 10.1016/s0304-4017(97)00203-3. [DOI] [PubMed] [Google Scholar]
- 15.McDougald LR. Coccidiosis. In: Saif M, editor. Diseases of Poultry. Ames: Iowa State Press; 2003. pp. 974–991. [Google Scholar]
- 16.Hamidinejat H, Seifiabad Shapouri MR, Mayahi M, Pourmehdi Borujeni M. Characterization of Eimeria species in commercial broilers by PCR based on ITS1 region of rDNA. Iranian J Parasitol. 2010;5(4):48–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Nematollahi A, Moghaddam Gh, Niyazpour F. Prevalence of Eimeria sp. among broiler chicks in Tabriz. Res J Poult Sci. 2008;2(3):72–74. [Google Scholar]
- 18.Razmi GR, Kalideri GA. Prevalence of subclinical coccidiosis in broiler-chicken farms in the municipality of Mashhad, Khorasan, Iran. Prev Vet Med. 2000;44:247–253. doi: 10.1016/s0167-5877(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 19.Etuk EB, Okoli IC, Uko MU. Prevalence and management issues associated with poultry coccidiosis in Abak agricultural zone of Akwa Ibom state, Nigeria. Int J Poult Sci. 2004;3(2):135–139. [Google Scholar]
- 20.Williams RB. A compartmentalized model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int J Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 21.ITPnews. Price chart. official website of ITPNews accessed on January 9. 2011: http://itpnews.com/fa/local-prices/view/42/18-10-1389.
- 22.Fayer R. Epidemiology of protozoan infections: the coccidian. Vet Parasitol. 1980;6:75–103. [Google Scholar]
- 23.Oikawa H, Kawaguchi K. Survey on drug resistance of chicken coccidian collected from Japanese broiler farms in 1973. Japanese Vet Res. 1975;37:357–362. doi: 10.1292/jvms1939.37.357. [DOI] [PubMed] [Google Scholar]
- 24.Anwar MI, Akhtar M, Hussain I, Muhammad F, Haq AU. Effects of local gametocyte and Livacox vaccines on live body weight gain and lymphoid organs in chickens. Pakistan Vet J. 2008a;28(3):136–138. [Google Scholar]
- 25.Allen PC, Jenkins MC. Cross protection studies with Eimeria maxima strains. Parasitol Res. 2005;97:179–185. doi: 10.1007/s00436-005-1423-6. [DOI] [PubMed] [Google Scholar]
- 26.Anwar IM, Akhtar M, Hussain I, Haq AU, Muhammad M, Hafeez MA, Mahmood MS, Bashir S. Field evaluation of Eimeria tenella (local isolates) gametocytes vaccine and its comparative efficacy with imported live vaccine, Livacox. Parasitol Res. 2008b;104:135–143. doi: 10.1007/s00436-008-1171-5. [DOI] [PubMed] [Google Scholar]
- 27.Peek HW, Landman WJM. Higher incidence of Eimeria spp. field isolates sensitive for diclazuril and monensin associated with the use of live coccidiosis vaccination with ParacoxTM-5 in broiler farms. Avian Dis. 2006;50:434–439. doi: 10.1637/7486-121205R.1. [DOI] [PubMed] [Google Scholar]