Abstract
Background
The purpose of this comparative study was to detect superoxide dismutase (SOD) activities in Fasciola hepatica, F. gigantica parasites, infected and healthy liver tissues in order to determine of species effects and liver infection on SODs activity level.
Methods
Fasciola spp. parasites and sheep liver tissues (healthy and infected liver tissues), 10 samples for each, were collected, homogenized and investigated for protein measurement, protein detection and SOD enzyme activity assay. Protein concentration was measured by Bradford method and SODs band protein was detected on SDS-PAGE. SODs activity was determined by iodonitrotetrazolium chloride, INT, and xanthine substrates. Independent samples t-test was conducted for analysis of SODs activities difference.
Results
Protein concentration means were detected for F. hepatica 1.3 mg/ ml, F. gigantica 2.9 mg/ml, healthy liver tissue 5.5 mg/ml and infected liver tissue 1.6 mg/ml (with similar weight sample mass). Specific enzyme activities in the samples were obtained 0.58, 0.57, 0.51, 1.43 U/mg for F. hepatica, F. gigantica, healthy liver and infected liver respectively. Gel electrophoresis of Fasciola spp. and sheep liver tissue extracts revealed a band protein with MW of 60 kDa. The statistical analysis revealed significant difference between SOD activities of Fasciola species and also between SOD activity of liver tissues (P<.05).
Conclusion
Fasciola species and liver infection are effective causes on SOD enzyme activity level.
Keywords: Superoxide Dismutases, Fasciola hepatica, F. gigantica, liver
Introduction
Fascioliasis is an important animal and human disease caused by trematodes (Fasciola hepatica, F. gigantica). These flukes causing pathological lesions such as fibrosis and cirrhosis, which result from the parasites passage through the liver parenchyma. Acute and chronic fascioliasis is observed primarily in sheep, goats, and cattle, causing important economic losses due to liver injuries (1).
The production of reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, hydroxyl radical, and singlet oxygen, is dependent on oxygen utilization and can cause cellular damage by lipid and protein peroxidation (2, 3). Oxidative stress and lipid peroxidation have been related with several types of liver injuries (4). It has been demonstrated that in the cells of hosts infected with parasite, the quantity of ROS which cause lipid peroxidation are increased, thus causing cell and tissue damage (5). Products of lipid peroxidation created in different biochemical reactions are normally removed by antioxidants. Antioxidants are compounds that are involved in effective scavenging of free radicals and in suppressing the actions of reactive oxygen substances. Antioxidant barriers are extensively distributed and include both enzymatic and nonenzymatic systems. The most important enzymatic antioxidants are superoxide dismutase, glutathione peroxidase and catalase. Nonenzymatic factors that may function as antioxidants are reduced glutathione, vitamin C, vitamin E, β-carotene, ceruloplasmin and bilirubin (6). SOD enzymes catalyze the dismutation of superoxide radical into hydrogen peroxide (H2O2) and molecular oxygen (O2) and consequently present an important defense mechanism against superoxide radical toxicity7. Cell antioxidants could not protect the proteins against peroxyl radicals (3).
There is no study which shows the sod antioxidant status in the fasciola parasites and liver tissue in Iran. Therefore we designed this study to compare SOD enzyme activity in the sheep liver tissue, healthy and infected, and Fasciola spp. parasites in order to evaluate their effects on sod enzyme activity level.
Materials and Methods
Parasite and liver tissue extracts
The mature parasites of F. hepatica, F. gigantica and sheep liver tissues (healthy and infected liver tissue) were collected from sheep slaughtered at a local abattoir (Saman, Tehran, Iran).The samples (10 samples for each) were washed for three times in PBS, pH 7.4, to remove host material and stored at −20°C. After thawing samples were homogenized in homogenizing buffer (2 ml), PBS 7.2 by a glass homogenizer. Then suspension was centrifuged (10000g for 30 min at 4°C) and supernatant was stored at −20°C.
Protein concentration and electrophoresis
Protein concentration was measured by Bradford method with BSA standard solutions as duplicate. Standards were prepared as a range of 5 to 100 micrograms protein (Bovine Serum Albumin, Merk Product) in 100 µl volume. Diluted samples were obtained between 5 and 100 µg protein in assay tubes containing 100 µl samples. Dye reagent, 5 ml, was added on tubes, incubated 5 minute and measured the absorbance at 595 nm (8).
In order to separate of SOD band proteins and to confirm their existence, samples were added to each well at 15 mA per SDS-PAGE gel, 15%, for 6 hours and finally the gel stained by comassie blue staining (8).
SOD enzymes Activity assay
SOD enzyme activity was determined using RANSOD kit (Randox Labs, Crumlin, UK), which was based on the method of McCord and Fridovich (9). Xanthine and xanthine oxidase were used to generate superoxide anion radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride quantitatively to form a red formazan dye. SOD inhibits the reaction by converting the superoxide radical to oxygen. Homogenized liver tissues, Fasciola spp. parasites and standard solutions were used for the assay of SOD. Absorbance was measured at 505 nm on a Cecil 1021 UV/visible spectrophotometer (Cecil Instruments Ltd Milton Technical Centre Cambridge ENGLAND) for 30s after the addition of xanthine oxidase as start reagent and 3 minute after reaction as duplicate samples (Table 1).
Table 1.
Superoxide Dismutase Activity Assay of Samples (Fasciola spp & liver tissues) and Standard SOD Solutions using RANSOD kit
| Solutions | Uninhibited tube* | Inhibited tubes | Standards (St1-5) tubes |
|---|---|---|---|
| Substrates | 850 (µl) | 850 (µl) | 850 (µl) |
| Phosphate buffer,7.0 | 25 (µl) | 0 | 0 |
| Xanthine oxidase | 125 (ul) | 125 (ul) | 125 (ul) |
| Samples (F. hepatica, F.gigantica, healthy and infected liver) | 0 | 25 (µl) | 0 |
| Standard solutions (Superoxide Dismutase) | 0 | 0 | 25 (µl) 0.2, 0.5, 1.0, 2, 4 (U/mg) |
Uninhibited tube or negative control includes all components except SOD or samples
Inhibited percents of standards and samples were calculated by following formula. 100-(▵AStd/min×100)/(▵AS1/min) =%inhibition (Where S1 is ▵A2-A1/3 uninhibited tube and ▵Std equal A2-A1/3 of inhibited tubes). A standard curve was prepared by using the standard provided in the kit and the SOD activity value for each sample was read from this curve. The SOD activity was expressed as U/ml reagent. One unit is the amount of SOD that inhibits the rate of formazan dye formation by 50%.
To detect the statistical difference between specific SOD activities of samples, two-sample independent t-test was conducted.
Results
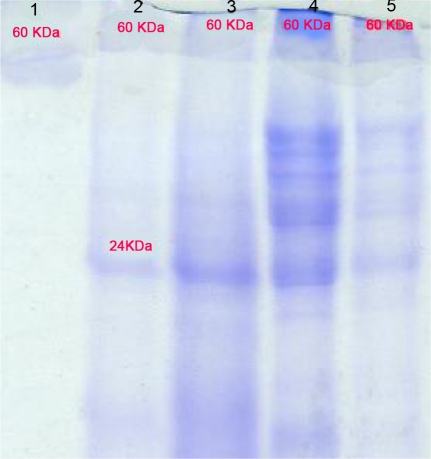
Protein concentration for F. hepatica 1.3 mg/ml, F. gigantica 2.9 mg/ml, healthy liver tissues 5.5 mg/ml and infected liver tissue 1.6 mg/ml were detected respectively (with same weight sample mass). Absorbances measures, calculated inhibition percent, total activity and specific activities of SOD enzyme in sample solutions are presented at the Table 2 (mean of 10 samples for each). Sample solutions showed one bands protein MW of 60 kDa in SDS-PAGE gel (Fig. 1).
Table 2.
Absorbances measures, percentage inhibition calculated, total activity and specific activity of SOD enzyme in sample solutions (mean of 10 samples for each)
| Sample tubes | Absorbance 1 | Absorbance2 | A2-A1 | A2-A1/3 | Calculated inhibition percent* | Total activity of SOD(U/ml)** | Specific activity of SOD(U/mgl)*** |
|---|---|---|---|---|---|---|---|
| solutionUn inhibited | 0.118 | 0.204 | 0.086 | 0.028 | 0 | 0 | 0 |
| F. hepatica extract | 0.135 | 0.202 | 0.67 | 0.022 | 21.42 | 0.76 | 0.58 |
| F. gigantica extract | 0.144 | 0.183 | 0.039 | 0.013 | 53.5 | 1.58 | 0.55 |
| extractHealthy liver | 0.162 | 0.174 | 0.012 | 0.004 | 85.7 | 2.84 | 0.51 |
| Infected liver extract | 0.155 | 0.178 | 0.23 | 0.007 | 75 | 2.28 | 1.43 |
The percent inhibition of the test samples correlates with SOD activity using a SOD standard curve.
One unit of SOD inhibits the rate of increase in absorbance at 550 nm by 50% under the conditions of the assay.
Specific activity is total activity of SOD per milligram of total protein.
Fig. 1.
SDS-PAGE analysis of Fasciola spp. parasites and liver tissue extracts. The proteins were analyzed on 15% gel. Lane 1, superoxide dismutase marker, Lane 2, somatic extract of Fasciola hepatica; lane 3, somatic extract of Fasciola gigantica, lane 4, crude extract of healthy liver tissue, lane 5, crude extract of infected liver
Discussion
SODs has been demonstrated from various helminthes of different species such as Schistosoma mansoni, Onchocerca volvulus, Dirofilaria immitis, Brugia pahangi, and Fasciola hepatica (10–14).
Although the adult worms have a generally anaerobic metabolism and live in the bile duct, where the oxygen pressure is relatively low, oxygen is necessary for other functions such as egg generation, which generate reactive oxygen species (ROS). In addition to this normal endogenous oxidative tension, the parasite is exposed to reactive oxygen species produced by host responding cells such as macrophages, eosinophils, neutrophils, and platelets. To protect themselves against oxidative stress mechanisms of hosts, parasites have developed antioxidant enzyme systems (14). It has been suggested that antioxidant inhibition of host oxidative stress may play a protective role in the parasite life cycle (15). At the present work, detected specific enzyme activity in F. hepatica was greater than F. gigantica. In this regard, researchers have suggested a possible role for this enzyme in the resistance of F. hepatica to superoxide-mediated killing (16) . Recently, the investigators have showed, over-expression of SOD can reduce oxidative damage and extend life span (17) . The higher antioxidant level in F. gigantica may help to increase of parasite life span, however further studies are needed for this conclusion.
In this research, the specific enzyme activity of infected liver was more than in the healthy liver. Antioxidant enzyme has a cellular protective role against oxidative stress resulting in liver tissue damage as a result of parasitic invasion (18). The somatic extract of F. spp. and liver tissues exhibited 1 band of 60 kDa on SDS-PAGE gel. This protein band has reported from excretory- secretory products of F. hepatica (19).
The statistical analysis revealed significant difference between SOD activity of F. hepatica and F. gigantica (P<.05). Statistical results also showed significant difference between healthy and infected liver tissues SOD enzyme activity (P<0.05). Therefore, briefly, parasite species and liver infection could be considered as effective causes on SOD enzyme activity level (20).
Acknowledgments
This work was supported by the Research grant 86-03-27-7755 (2008 to 2010) from the Tehran University of Medical Sciences. We are deeply grateful to M.B. Molaei Rad for his technical assistance on SDS-page and to Dr. M. Madah and H. A. Rahmi for collecting infected liver in the abattoirs of Tehran and Ilam cities. The authors declare that there is no conflict of interests.
References
- 1.Soulsby EJL. 7th. Edition. Tindall, London, UK: Bailliere; 1986. Helminths, Arthropods and Protozoa of Domesticated Animals; pp. 24–54. [Google Scholar]
- 2.Sanchez-Campos S, Tunon MJ, Gonzalez P, Gonzalez-Gallego J. Oxidative stress and changes in liver antioxidant enzymes induced by experimental dicroceliosis in hamsters. Parasitol Res. 1991;85:468–474. doi: 10.1007/s004360050579. [DOI] [PubMed] [Google Scholar]
- 3.Gieseg S, Duggan S, Gebicki JM. Peroxidation of proteins before lipids in U937 cells exposed to peroxyl radicals. Biochem J. 2000;350:215–218. [PMC free article] [PubMed] [Google Scholar]
- 4.4 Panozzo PM, Basso D, Balint L, Biasin MR, Bonvicini P, Metus P. Altered lipid peroxidation/glutathione ratio in experimental extrahepatic cholestasis. Clin Exp Pharmacol Physiol. 1995;22:266–271. doi: 10.1111/j.1440-1681.1995.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarin K, Kumar A, Prakash A, Sharma A. Oxidative stress and antioxidant defense mechanism in Plasmodium vivax malaria before and after chloroquin treatment. J Malariol. 1993;30:127–133. [PubMed] [Google Scholar]
- 6.Murray RK, Mayes AA, Granner DK, Rodwell VW. 1993. Harper's Biochemistry (in Turkish) p. 183. Translated by Menteş G and B. Ersöz. BarIş Printing House, Istanbul, Turkey. [Google Scholar]
- 7.Cell Technology, Inc [online] 2006. [cited 2010 Apr 27]; Available from URL: http://www.celltechnology.com/index.htm.
- 8.Maizels RM, Blaxter ML, Robertson BD, Selkirk ME. Parasite antigen and parasite genes: A laboratory manual for molecular parasitology. 1st ed. UK: Cambridge University Press; 1991. pp. 93–5. [Google Scholar]
- 9.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 10.James ER, McLean Jr DC, Perler. F. Molecular cloning of an Onchocerca volvulus extracellular Cu-Zn superoxide dismutase. Infect Immun. 1994;62:713–716. doi: 10.1128/iai.62.2.713-716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan Hl, Crouch RK, James ER. Dirofilaria immitis superoxide dismutase: purification and characterization. Mol. Biochem. Parasitol. 1991;49:245–252. doi: 10.1016/0166-6851(91)90068-h. 1991. [DOI] [PubMed] [Google Scholar]
- 12.Tang LX, Ou X, Henkle-Duhrsen K, Selkirk ME. Extracellular and cytoplasmic CuZn superoxide dismutases from Brugia lymphatic filarial nematode parasites. Infect Immun. 1994;62:961–967. doi: 10.1128/iai.62.3.961-967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong ZD, Kosman J, Thakur A, Rekosh D, Loverde PT. Identification and purification of a second form of Cu/Zn superoxide dismutase from Schistosoma mansoni . Infect Immun. 1992;60:3641–3651. doi: 10.1128/iai.60.9.3641-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TS, Jung Y, Na BK, Kim KS, Chung PR. Molecular Cloning and Expression of Cu/Zn-Containing Superoxide Dismutase from Fasciola hepatica . Infection and Immunity. 2000;68(7):3941–3948. doi: 10.1128/iai.68.7.3941-3948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan HL, Crouc RK, James ER. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988;4:218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- 16.Piedrafita D, Estuningsih E, Pleasance J, Prowse R, Raadsma HW, Meeusen EN, Spithill TW. Peritoneal Lavage Cells of Indonesian Thin-Tail Sheep Mediate Antibody-Dependent Superoxide Radical Cytotoxicity in Vitro against Newly Excysted Juvenile Fasciola gigantica but Not Juvenile Fasciola hepatica . Infection and Immunity. 2007;75(4):1954–1963. doi: 10.1128/IAI.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126(3):365–79. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.De(er1 Y, Ertekin1 A, De(er S, Mert H. Lipid Peroxidation and Antioxidant Potential of Sheep Liver Infected Naturally with Distomatosis. Türkiye Parazitol Derg. 2008;32(1):23–26. [PubMed] [Google Scholar]
- 19.Piacenza L, Radi R, Goñi F, Carmona C. CuZn superoxide dismutase activities from Fasciola hepatica. Parasitology. 1998;117(6):555–62. doi: 10.1017/s0031182098003394. [DOI] [PubMed] [Google Scholar]
- 20.Kim YG, Jeon DY, Yang MK. Superoxide Dismutase Activity and Lipid Peroxidation in the Liver of Guinea Pig Infected with Leptospira interrogans . Free Radical Research. 1997;26(1):1–6. doi: 10.3109/10715769709097779. [DOI] [PubMed] [Google Scholar]