Abstract
Background
New cases of visceral leishmaniasis (VL) have been reported recently in some parts of Mazandaran Province, north of Iran where the first human case of VL was reported in 1949. This study aimed to determine the present status of Leishmania infantum infection among humans and domestic dogs using serological and molecular methods in central parts of Mazandaran Province.
Methods
In this cross-sectional study, blood samples were randomly collected from 402 humans and forty-nine domestic dogs throughout 2009 and 2010 in the central part of Mazandaran Province including Semeskadeh and Kiakola districts where recent cases of human visceral leishmaniasis had been reported there. All the collected samples were tested by direct agglutination test (DAT) for the detection of anti-Leishmania infantum antibodies as well as convenience PCR assay on whole blood samples for detection of leishmanial infection and identification of Leishmania species.
Results
None of 402 collected human (402) and dog (49) blood samples showed anti Leishmania infantum antibodies at titers 1:3200 and 1:320 as cut-off values of DAT, respectively but only 2 of domestic dogs (4.1%) were found PCR-positive corresponding to L.infantum.
Conclusion
This study confirms the circulation of L. infantum at least among domestic dogs and highlights the sporadic pattern of VL in the studied areas. Further investigations regarding to sand flies fauna and wild canines as reservoir hosts of the disease, are recommended.
Keywords: Visceral leishmaniasis, Leishmania infantum, Seroprevalence, Direct agglutination test, convenience PCR, Iran
Introduction
Visceral leishmaniasis (VL), so-called Kala-azar, is a systemic disease caused by Leishmania donovani complex intracellular parasites, which are transmitted by different species of sand flies. The annual occurrence of human visceral leishmaniasis (HVL) cases worldwide is estimated to be 500,000 and accounts for 75,000 deaths (1, 2). Nevertheless, these diseases are still considered as neglected diseases (3). Leishmaniasis still constitutes a major public health problem and burden is increasing (4). In addition Leishmania-HIV co-infections in the adult population are being reported with increasing frequency (1). The clinical sings of VL in humans include prolonged fever, hepatosplenomegaly, substantial weight loss, progressive anemia, and death (it is fatal in left treated cased) (5). Leishmania infantum is responsible for Mediterranean visceral leishmaniasis (MVL) in children and infants in the Mediterranean basin countries including Iran and domestic dogs are considered a major reservoir hosts for MVL (6). In Iran, the main endemic foci for VL are Fars and Bushehr provinces, in the south-west, the districts of Meshkin-shahr and Kaleybar in the north-west and Qom Province in the center of Iran (7–12). “Other parts of Iran are considered as sporadic areas for VL. Visceral leishmaniasis is common (over 98%) among children under 12 years old in different endemic foci in Iran and adult cases frequently present with subclinical and asymptomatic forms in endemic regions” (9, 10).
The first case of human visceral leishmaniasis (HVL) in Iran has been reported by Pouya (13) from rural areas of Tonekabon, in western zone of Mazandaran Province, North of Iran. Moreover, at the same time, he reported the first case of canine visceral leishmaniasis in this area. Afterward, a few infantile cases of HVL were reported in different parts of this province (14–16). At the present time, it is known as an endemic disease in some parts of five provinces of Iran and other parts of country are considered as sporadic areas of VL. Over the last decade, the incidence of VL has increased in many districts of the province of Mazandaran, in northern Iran (14–16).
This study aimed to determine prevalence of human and canine visceral leishmaniasis for the first time in the province. Since there are some reports correspond to exist possibly the Leishmania visceral infection among rodents in Semeskandeh district (17) along with one report of infected dogs and jackals in around of the area by Hamidi et al. (18) as well as new report of human VL case (identified as L. infantum) in Kiakola district from the Central zone of the province (15).Therefore, we designed a preliminary molecular and seroepidemiological investigation in these suspicious districts from the Central zone of this province.
Materials and Methods
Study area
The study was conducted throughout 2009-2010 in two suspicious districts of the Central zone of Mazandaran Province including Semeskandeh district (as a mountainous area including 7 villages) where five kilometer far from Sari city, capital of Mazandaran, and Kiakola district (as a coastal plain including 3 villages) where it's located at the littoral of Caspian Sea, Mazandaran Province is located in the north of Iran (53°6ʹ E, 36°23ʹN). From the geographical point of view, Mazandaran Province is divided into two parts i.e. coastal plain and the mountainous area. The central zone of the province has a humid weather and also has an annual mean rainfall of 977 mm (19).
Sampling and testing
Blood samples were collected in EDTA- coated tubes from a total 20% of less than 12 years old children and 10% of their parents, as well 20% of owner dogs from 11 villages. All samples were collected by cluster sampling methods. Totally, 402 human blood samples and 49 domestic dogs' blood samples were collected. All the samples centrifuged at 1000×g for 5 min and then plasma and buffy coat were collected individual micro tubes in order to DAT and PCR examination and were stored at _20°C until examined. All plasma samples were tested by DAT and buffy coat were examined by PCR. Department of Parasitology, School of Public Health in Tehran University of Medical Sciences, supplied the DAT antigen and stored at 4°C until used. Plasma samples were tested by DAT according to the methods described by Harith et al. (20). First dilutions were prepared from 1:10 to 1:80 for dog samples and 1:10 to 1:800 for human samples. Samples with titers 1:80 for dogs, 1:800 for humans were diluted further to give final titers. Known negative and positive controls were tested in each plate. In this investigation, we considered anti-Leishmania antibodies titers at equal and above of 1:3200 and 1:320 (cut off point) as Leishmania infection for the human and dogs respectively. Total DNA was extracted from blood buffy coat as described by Motazedian et al. (21). Briefly, 200 µl of buffy coat was homogenized with 200 µl lyses buffer [50 mM Tris-HCl (pH=7.6), 1 mM EDTA and 1% Tween 20] and 10 µl of proteinase K solution (containing 19 mg of the enzyme/ml), in a 1.5 ml micro centrifuge tube. The homogenate was then incubated at 37°C overnight before 200 µl of a phenol: chloroform: isoamyl alcohol mixture was added. After being shaken vigorously, the tube holding the mix was centrifuged (10,000×g for 10 min) and then the DNA in the supernatant solution was precipitated with 400 µl cold, pure ethanol, re-suspended in 50 µl double-distilled water and then stored at 4°C until it could be tested. It was re-suspended in 100 µL sterile distillated water and stored at 4°C until it could be tested in a modified genous-specific PCR for a sequence from the kinetoplast DNA (k DNA) of Leishmania. The primers used, RV1 (5ʹ-CTT TTC TGG TCC CGC GGG TAG G-3ʹ) and RV2 (5ʹ-CCA CCT GCG CTA TTT TAC ACC A-3ʹ) amplify a 145-bp sequence from the LT1 fragment of the parasites’ kDNA minicircles, according to the methods as described by Fakhar et al. (22).
The PCR products were separated by electrophoresis in a 2% agarose gel, stained with ethidium bromide, visualized under ultra-violet trans-illumination, and sized by comparison with a 100bp ladder. Each sample found PCR-positive for Leishmanial DNA was then investigated using the PCR described by Fakhar et al. (22), which is based on the species-specific primers LINR4 and LIN17 to confirm that the DNA detected was that of L. infantum. Reference strain of L. infantum (MCAN/IR/96/Lon49) was used as standard.
Data analysis
Chi-squared (c2), Mac Nemar and Fisher exact tests were used to compare sero-prevalence values relative to gender, age groups and two studied areas. Analyses were performed with SPSS (version 13.5; SPSS Inc, Chicago,IL, USA) and Epi-Info software, with a probability (P) value of <0.05 were considered as statistically significant.
Results
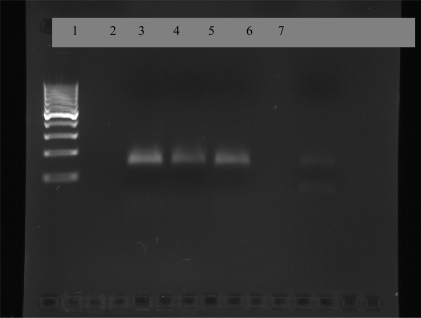
Of the 402 subjects (200 males and 202 females) investigated, 138 cases (31.9%) were showed Leishmania specific antibodies with titers 1:800, 8 cases (2%) with 1:1600 titers which those considered as suspicious cases. Also none of those were found DAT (at titers≥1:3200) and PCR positive. Out of 49 dogs ( 38 males and 11 females) examined, 17 (34.6%) were showed Leishmania specific antibodies with titers 1:80 and only two dogs (4.1%), belonged to Kiakola district, were found PCR positive in the Leishmania genus -specific PCR based on the RV1/ RV2 primer set (Fig. 1) and none of those were found PCR positive from Semskande district.
Fig. 1.
2% agarose gel electrophoresis of PCR products from buffy coat DNA of dogs Lane 1: standard marker (100 bp), Lane 2: negative samples, Lane 3: Positive control, Lane 3: Standard Leishmania infantum (145 bp), lanes 4, 5: positive dogs, Lane 6: negative samples, Lane 7: positive sample
In addition, none suspicious human cases (<1:3200 titers, as cut off) had clinical signs and only one of PCR positive dogs had clinical signs including skin lesions, alopecia and weight loss along with anti Leishmania specific antibodies at 1:80 titer.
There was no significant difference between male and female at 1:800 and 1:1600 titers of anti-Leishmania specific antibodies in human population and in dog population as well. Although, the number of human cases with 1:800 and 1:1600 titers in females were higher than males. As well as, there was no significant difference in age groups of the subjects.
Moreover, there was no significant difference between two studied areas. Although all human suspicious cases (<1:3200 titers, as cut off) have now been followed-up for at least 11 months, none has yet shown any symptoms of VL.
Discussion
For the first time, our preliminary study was carried out in Mazandaran Province. Thus we used DAT for serological test on humans, and dogs' plasma samples as a practical and sensitive test in seroepidemiological studies (20, 23). Serological results by DAT showed in spite of 34.6% human populations in the studied areas had anti-Leishmania antibodies in titers of 1:800 and 2% had anti-Leishmania antibodies in titers 1:1600 but none of them were DAT positive at titers 1:3200 and higher as cut-off values of DAT on human sera.
The direct agglutination test has been used in epidemiologic studies in several endemic areas and is used in large-scale screening of human VL as a simple, valid test with high sensitivity and specificity at cut-off titer 1:3200 (10, 23). The sensitivity and specificity of this method varies in different studies between 90–100% and 72–100% respectively (24).
As the type of sampling is noninvasive, we also used genus-specific PCR based on RV1 and RV2 specific primers for L. infantum on peripheral blood buffy coats of humans and dogs (24, 25). The results of PCR based on RV1 and RV2 primers for all human buffy coats were negative and all of them had no clinical signs.
Recently, the use of polymerase chain reaction with high sensitivity (70–100%) and specificity (100%) has become popular in different parts of the world (24, 25) and the sensitivity of 82.1% and specificity of 100% in our previous study in Fars Province, as known endemic region of VL, (22) in Iran.
The PCR technique has several advantages including the ability to work with small amounts of target material, fast detection of Leishmania in symptomatic patients and asymptomatic carriers as well as Leishmania/HIV co-infected patients and the follow up of treatment as well as the assessment of the successful cure of visceral leishmaniasis (26–28).
Six primer pairs were compared for detecting L. infantum DNA by Gao et al. (29). The primer pairs RV1-RV2 (0.1 parasite/ml blood) were most sensitive and suitable in detecting the asymptomatic infection of L. infantum and the prevalence of the asymptomatic infection is high in human population in the endemic area. In our study, we applied RV1-RV2 primers set as described above. The PCR on peripheral blood sample can be used for treatment response evaluation and it is also very efficient for early detection of the disease especially in patients with cryptic infection, small children and immunocompromised patients (30) and also subclinical and asymptomatic infections in endemic areas (22).
Serological results by DAT showed 34.7% dog population had anti-Leishmania antibodies at titers 1:80 but none of them showed anti-Leishmania infantum antibodies as cut-off titer 1:320. The results of PCR based with RV1, RV2 primer set revealed two dogs were found positive. Both of them were from the Kiakola district, one of them exactly was from the village where new human VL case had previously reported. Only one of PCR positive dogs had clinical signs. None of those was seropositive and had anti-Leishmania antibodies in titers 1:80. It is noticeable that the symptomatic dog lives in the same place where new human case of VL has been reported by Rahmati et al. (15).
After one year, in following up the first dog (asymptomatic dog) had not developed in clinical signs but another one was died. So it seems the evidences of circulation L. infantum in the sporadic area.
Recent epidemiological reports in endemic regions of CVL, such as Iran, indicate that asymptomatic dogs' infections with L. infantum occur in more than 50%-70% of the seropositive dogs in the field investigations (9, 12, 31). The canidae families are main reservoir hosts because the parasites are multiplied in skin macrophages and readily transferred by feeding sand flies (32). In addition, the wild carnivores such as jackals and foxes are considered reservoir hosts in sylvatic cycle of MVL, principally in sporadic areas of Iran (9).
The diagnosis of VL is complex because commonly occurring diseases such as malaria, typhoid, and tuberculosis have clinical features similar to VL. Some VL cases have been misdiagnosed as autoimmune hepatitis, acute lymphoblastic leukemia, and malignant lymphoma (33, 34). Most of these misdiagnosed patients are reported from non endemic regions where physicians do not expect the occurrence of the disease. Moreover, atypical cells and different blast may be observed in bone marrow aspiration of VL patients (34).Consequently, it seems that in the Mazandaran Province, some of VL cases (particularly, atypical and subclincal or asymptomatic) due to lack of specific and sensitive diagnostic tests such as DAT and PCR as well as lack of physician's awareness of existing the VL in the region (as a non endemic region) are most likely misdiagnosed. On the other hand some patients had referred to Tehran (as capital of Iran) hospitals in order to more management, early and accurate diagnosis.
As a whole, according to aforementioned and our investigations look like VL occur as cryptic forms in some parts of the province and it may be a potential risk for emerging or reemerging a new focus in future. Though, recently one autochthonous case of VL with no history of traveling to endemic areas has been reported from the region. However, it is difficult to explain that how she acquired infection. We can not rule out the possibility that this L. infantum strain is less virulent and might be circulating in some animal reservoirs. It is likely that the disease has been re-introduced in the region and is spread by some local species of sandfly.
This preliminary study confirms the circulation of L. infantum at least among dogs population and highlights the sporadic pattern of VL in the studied areas. Moreover, several evidences such as neglecting MVL in the province, lack of physician's awareness, discontinue or non-effective spray insecticides against malaria vectors, environmental changes and climate conditions, nomadic movements and increasing incidence of VL (35) recommend the reemergence of MVL in this non-endemic area, where the first human case of visceral leishmaniasis had been reported in Iran.
Since all human and the majority of dog population were asymptomatic as well, it showed role of asymptomatic dogs as a possible reservoir host for VL. As a whole, further investigations regarding sandflies fauna and animal reservoirs and human populations are required in this province.
Acknowledgements
The authors would like to thank the Office of Vice- Chancellor Research Affairs of Mazandaran University of Medical Sciences for their financial support (project number: 88-62) and all Health workers for their humanity assistant. DAT antigen was made by leishmaniasis laboratory from the School of Public Health, Tehran University of Medical Sciences (Project No: 130/6/10447). The authors declare that there is no conflict of interests.
References
- 1.World Health Organization. Geneva: WHO; 2000. Leishmaniasis and Leishmania/HIV Co-infection.Document WHO/CDS/CSR/ISR/2000.1. [Google Scholar]
- 2.Banuls AL, Hide M, Prugnolle F. Elsevier Ltd; 2007. Advances in Parasitology. Vol 64. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2010. First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases WHO/HTM/NTD/2010.1,PP:91-96. [Google Scholar]
- 4.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans Royal Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 5.Caldas AJMBB, Costa J, Aquino D, Silva AAM, Barral-Netto M, Barral A. Are there differences in clinical and laboratory parameters between children and adults with American leishmaniasis? Acta Trop. 2006;97:252–258. doi: 10.1016/j.actatropica.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Mazloumi Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania. infantum . Am J Trop Med Hyg. 2002;67:511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- 7.Asgari Q, Fakhar M, Motazedian MH. Nomadic kala-azar in South of Iran. Iranian J Publ Health. 2006;35:85–6. [Google Scholar]
- 8.Mohebali M, Hamzavi Y, Edrissian GH, Forouzani A. Seroepidemiological study of visceral leishmaniasis among humans and animal reservoirs in Bushehr Province. Islamic Republic of Iran. East Mediterr Health J. 2001;7:912–917. [PubMed] [Google Scholar]
- 9.Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi Sh, Zarei Z, Akhoundi B, Naeini K, Manouchehri AR, Fakhar M. Epidemiological aspects of canine visceral leishmaniasis in the Islamic Republic of Iran. Vet Parasitol. 2005;129:243–259. doi: 10.1016/j.vetpar.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Edrissian GH, Nadim A, Alborzi AV, Ardehali S. Visceral leishmaniasis: the Iranian experiences. Arch Iranian Med. 1999;1:22–26. [Google Scholar]
- 11.Fakhar M, Mohebali M, Barani M. Identification of endemic focus of Kala-azar and Seroepidemiological study of visceral Leishmania infection in human and canine in Qom Province. Armaghan-Danesh (In Persian) 2004;9:43–52. [Google Scholar]
- 12.Fakhar M, Motazedian MH, Asgari Q, Mohebali M, Mehrabani D. A new endemic focus of visceral leishmaniosis in southern Iran. Armaghane-Danesh (In Persian) 2006;11:104–110. [Google Scholar]
- 13.Pouya Y. Study of visceral leishmaniasis in Gilan and Mazadndran provinces of Iran. J Med Fac Tehran. 1949;7:359–361. [Google Scholar]
- 14.Sawad koohi R. Proceeding of 7th Congress of Infectious and Tropical Diseases; Iran: Tehran; 1998. Study of visceral leishmaniasis cases in Mazandaran Province during 1992-1997. [Google Scholar]
- 15.Rahmati B, Fakhar M, Sawad koohi R, Mahdavi S, Parsaei M.R, Bahrami S, Mohebali M, et al. Reemerging of visceral leishmaniasis in Mazandaran Province, North of Iran; Iran: Guilan; 2010. pp. 10–13. May. Proceeding of 11th Iranian Microbiology Congress and Eastern Mediterranean Microbiology Congress. [Google Scholar]
- 16.Shirzadi MR, Sharifian J, Zeinali M, editors. 1st ed. Ministry of Health and Medical Education Publications: Tehran; 2009. Successful in zoonosis control programmes; pp. 76–104. [Google Scholar]
- 17.Gholami Sh, Shahabi S, Mobedi I. Prevalence of leishmaniasis in rodents’ population in Jangle area of Semeskandeh district from Sari township, Mazandaran Province; Iran: Tehran; 1999. Proceeding of 8th Congress of Infectious and Tropical Diseases. [Google Scholar]
- 18.Hamidi AN, Nadim A, Edrissian GH, Tahvildar Bidruni G, Javadian E. Visceral leishmaniasis of jackals and dogs in northern Iran. Trans R Soc Trop Med Hyg. 1982;76(6):756–7. doi: 10.1016/0035-9203(82)90100-6. [DOI] [PubMed] [Google Scholar]
- 19.Mahjouri E. Historical Geography of Mazandaran Province. Tehran: Geographical Organization Press; 2001. [Google Scholar]
- 20.Harith A, Salappendel RJ, Reiter I, Knapen F, Korte P, Huigen E, Kolk RHG. Application of a direct agglutination test for detection of specific anti- leishmania antibodies in the canine reservoir. J Clin Microbiol. 1989;27:2252–7. doi: 10.1128/jcm.27.10.2252-2257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motazedian H, Karamian M, Noyes HA, Ardehali S. DNA extraction and amplification of Leishmania from archived, Giemsa-stained slides, for the diagnosis of cutaneous leishmaniasis by PCR. Ann Trop Med Parasitol. 2002;96:31–34. doi: 10.1179/000349802125000484. [DOI] [PubMed] [Google Scholar]
- 22.Fakhar M, Motazedian MH, Hatam GR, Asgar Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 2008;102:577–83. doi: 10.1179/136485908X337526. [DOI] [PubMed] [Google Scholar]
- 23.Mohebali M, Edrissian GHH, Nadim A, Hajjaran H, Akhoundi B, Hooshmand B, Zarei Z, Arshi S, Mirsamadi N, Manouchehri-Naeini K, Mamishi S, Sanati AA, Moshfe AA, Charehdar S, Fakhar M. Application of direct agglutination test (DAT) for the diagnosis and seroepidemiological studies of visceral leishmaniasis in Iran. Iranian J Parasitol. 2006;1:15–25. [Google Scholar]
- 24.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diag Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S. New developments in diagnosis of Leishmaniasis. Indian. J Med Res. 2006;123:311–330. [PubMed] [Google Scholar]
- 26.Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102. doi: 10.1016/s0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 27.Maurya R, Singh RK, Kumar B, Salotra P, Rai M, Sundar S. Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure. J Clin Microbiol. 2005;43:3038–3041. doi: 10.1128/JCM.43.7.3038-3041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao CH, Wang JY, Yang YT, Bao YF. Study on PCR method for detecting the asymptomatic infection of Leishmania infantum Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24(2):92–96. [PubMed] [Google Scholar]
- 30.Cascio A, Calattini S, Colomba C, Scalamonga C, Galazzi M, Pizzuto M, Camilli R, Gramiccia M, Titone L, Corbellino M, Antinori S. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatrics. 2002;109:1–5. doi: 10.1542/peds.109.2.e27. [DOI] [PubMed] [Google Scholar]
- 31.Moshfe A, Mohebali M, Edrissian GhH, Zarei Z, Akhoundi B, Kazemi B, Jamshidie Sh, Mahmoodi M. Seroepidemiological study on canine visceral leishmaniasis in Meshkin-Shahr District, Ardabil Province, northwest of Iran during 2006–2007. Iranian J Parasitol. 2008;3:1–10. [Google Scholar]
- 32.Ashford DA, David JR, Freire M, David R, Sherlock I, Eulalio MC, Sampanio DP, Badaro R. Studies on control of visceral leishmaniasis impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyge. 1998:28–59. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 33.Kumar PV, Vasei M, Sadeghipour A. Visceral leishmaniasis: bone marrow biopsy findings. J Pediatr Hematol Onchol. 2007;29(2):77–80. doi: 10.1097/MPH.0b013e31803076a8. [DOI] [PubMed] [Google Scholar]
- 34.Asgari Q, Fakhar M, Motazedian MH, Cheraqali F, Banimostafavi E. Visceral leishmaniasis, an alarming rate of misdiagnosis. Iranian Red Crescent Med J. 2007;95(1):45–7. [Google Scholar]
- 35.Sharma U, Redhu NS, Mathur P, Singh S. Re-emergence of visceral leishmaniasis in Gujarat, India. J Vect Borne Dis. 2007;44(3):230–2. [PubMed] [Google Scholar]