Abstract
Expression of NK cell markers identifies pro-inflammatory T cell subsets in the liver and intestinal immune compartments. Specifically, CD161 is expressed on Th17 cells which play an important role in the regulation of mucosal inflammation. In this study, we characterized human peripheral blood CD161+ T cells as an effector population partially resembling a gut T cell phenotype. CD161+ CD4+ T cells express the gut-associated TNF family member, LIGHT, and respond to crosslinking of DR3, a receptor to another gut-associated cytokine, TL1A. Robust IFN-γ production in response to DR3 signaling correlated with enhanced expression of surface DR3 on CD161+ T cells and co-stimulation with IL12 and IL18. CD161+ T cell effector function was directly demonstrated by activation of responder monocytes in co-culture leading to CD40 upregulation and CD14 downregulation. CD161+ T cells reciprocally responded to activated monocytes, inducing expression of activation marker, CD69, and production of IL2 and IFN-γ, further demonstrating effective CD161+ T cell cross-talk with monocytes. Finally, CD161 defined a subset of T cells that co-express CD56, a second NK marker. Our findings implicate human CD161+ T cells in gut-associated signaling mechanisms, and suggest a monocyte mediated effector function in mucosal inflammation.
Keywords: cytokines, inflammation, inflammatory bowel disease, LIGHT, mucosa, T lymphocytes, TL1A
Introduction
Immune responses in the intestinal compartment are tightly regulated to tolerate the heavy antigenic load characteristic of the gut [1]. Studies in human and mouse demonstrated that perturbations of both the innate and adaptive immune system can result in mucosal inflammation [1], and pointed to a complex, multi-gene pathology [2]. Contrary to the periphery, most intestinal T cells express CD45RO, which reflects antigen-driven differentiation [3]. However, TCR response to antigen-mediated stimulation is attenuated, suggesting gut specific mechanisms of T cell signaling and activation, which may be antigen independent [3]. In inflammatory bowel disease (IBD), tolerance to intestinal antigens is breached [1], and an aberrant Th1 and Th17 T cell response was implicated [1], specifically in Crohn’s Disease (CD) [1].
Signaling via the TNF family of cytokines plays a critical role in mammalian biology and mediates pro-inflammatory T cell responses [4]. Targeting soluble TNF and TNF expressing T cells can benefit a subset of CD [5], and ulcerative colitis (UC) patients [6]. However, the partial success of blocking TNF in IBD emphasizes the complexity of mucosal immune regulatory mechanisms, and prompted investigation of other ligands in the TNF family including LIGHT and TL1A [4, 7]. LIGHT, TNF and LTαβ form an integrated signaling network mediating multiple immune functions and regulating inflammation [7, 8]. LIGHT signals via two members of the TNF receptor family, herpesvirus entry mediator (HVEM, TNFRSF14), and lymphotoxin (LT)-β receptor (LTβR; TNFRSF3) [9, 10], which also binds the LTαβ heterotrimer involved in the development and organization of peripheral lymphoid tissue [11]. LTβR is broadly expressed on stromal and myeloid cells, whereas HVEM is expressed on lymphocytes [10] and epithelial cells [12]. While HVEM serves as a pro-inflammatory receptor mediating LIGHT signaling, it also triggers an anti-inflammatory signal likely acting as a ligand for the B- and T-Lymphocyte Attenuator (BTLA, CD272) [13]. Similar to LIGHT, TL1A is a TNF super-family member, linked to mucosal immune regulation. TL1A signals via death domain receptor 3 (DR3, TN-FRSF25), which is expressed on T cells following activation by anti-CD3 or by IL-12 and IL-18 [14]. DR3 mediates a pro-inflammatory T cell signal leading to NF-κB and cIAP-2 activation, and inducing IFN-γ production and cell proliferation [15]. Both LIGHT and TL1A are bound by decoy receptor 3 (DcR3, TNFRSF6B), a soluble decoy receptor with an inhibitory function [8, 14], which was implicated in mucosal inflammation [16].
Transgenic expression of LIGHT (TNFSF14) by T cells induced severe intestinal inflammation [17, 18], and inhibition of LIGHT signaling with a chimeric LTbR-Fc decoy receptor ameliorated inflammation in the CD4+ CD45Rhigh T cell transfer model of colitis [19]. In humans, LIGHT is constitutively expressed on gut associated T cells, and expression levels are enhanced in inflamed intestinal mucosa [7, 20], thus implicating mucosal T cells in LIGHT pro-inflammatory function. Similarly, TL1A and DR3 expression is elevated on macrophages and T cells in the inflamed mucosa [21, 22], TL1A-DR3 signaling was implicated in the pathology of two murine models of ileal inflammation [23], neutralizing antibodies to TL1A inhibited 2 murine models of chronic colitis [24], and constitutive expression of TL1A in mice lead to ileal inflammation and gut fibrosis [25, 26]. Genetic analysis mapped the human LIGHT locus within the MHC paralogous region [27], which is contains a candidate susceptibility locus for CD [28]. Genome-wide association studies linked genetic variants of the TL1A gene with CD in Japanese patients, in several European cohorts, in Jewish patients, and in pediatric patients [26].
T cells are pivotal in mucosal immune mechanisms mediated by LIGHT and TL1A. Pro-inflammatory and regulatory T cells, in particular T cells expressing NK markers, have been described in the human gut [29]. While traditional NKT cells are scarce in humans [30], expression of NK markers on mature T cells is intriguing since CD56 and CD161 expression is primarily limited to early stages of T cell ontogeny and lost during thymic maturation. Our analysis [31], as well as others [32] demonstrated that mature T cells expressing NK markers constitute a significant subset, both in the periphery, and in the mucosal compartment. Mucosal CD161+ T cells express pro-inflammatory cytokines [33], and effector T cells can express CD56 in the gut [31], and liver [34]. Moreover, CD161 expression was reported on CD4+ Th17 T cells, which play an important role in the regulation of gut inflammation [35]. A functionally distinct, polyclonal and not CD1 restricted phenotype [36], thus suggest a role for T cells expressing NK markers in adaptive immunity.
In this study, we examined gut-associated T cells expressing CD56 and CD161 in the peripheral blood and linked CD161 expression with enhanced LIGHT expression and responsiveness to a TL1A-DR3 signal as molecular mechanisms that could mediate effector function in the gut mucosa. We directly demonstrated enhanced CD161+ T cell activation of monocytes and a reciprocal response of CD161+ T cells to activation by monocytes, suggesting effective monocyte-T cell cross-talk. Robust effector function and enhanced signaling via LIGHT and TL1A-DR3 suggests a role for CD161+ T cells in the pathology of intestinal inflammation.
Materials and methods
Human subjects and specimen procurement
Blood leukocytes were obtained by venipuncture from healthy adult volunteers. Procedures for subject recruitment, informed consent, and specimen procurement were in accordance with protocols approved by the Institutional Review Board (IRB 3358) for Human Subject Protection of the Cedars-Sinai Medical Center.
PBMC isolation and cell subset purification
Peripheral blood mononuclear cells (PBMC) were isolated from uncoagulated blood by standard Ficoll-Hypaque density gradient centrifugation. Monocytes were enriched by negative selection on a magnetic bead column using the Monocyte Isolation Kit II (Miltenyi Biotec) and preparations were routinely >90% pure as determined by esterase stain (Sigma-Aldrich). Monocytes were cultured in RPMI 1640 containing 2 mM glutamine and 25 mM HEPES buffer (Mediatech) supplemented with 10% FBS, and antibiotics. CD3+ T cell subsets (CD56+/– and/or CD161+/–) and NK cells were purified or depleted from PBMC by flow cytometry (MoFlow, Dakocytomation, Fort Collins, CO) gating on viable CD3+, lymphocyte size cell subsets. Purity of enriched populations was consistently greater than 99% for the gated markers with less than 0.5% of depleted lymphocyte subsets remaining when reanalyzed by flow cytometry (FACScan, Becton Dickinson, or Cyan, Dakocytomation).
Cell culture
Lymphocytes were cultured at 0.25–1×106 cells/ml in RPMI 1640 containing 2 mM L-glutamine and 25 mM HEPES buffer (Mediatech, Inc., Herndon, VA), supplemented with 10% heat inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA), 50 mg/ml gentamycin (Omega Scientific, Tarzana, CA), with additional 0.25 mg/ml amphotericin B (Gemini Bio-products, Woodland, CA). Where indicated, lymphocytes were stimulated by 40 ng/ml Phorbol 12-Myristate 13-Acetate (PMA) and 1 mg/ml Ionomycin (Sigma); or by antibody crosslinking of cell surface CD2 used at 0.4
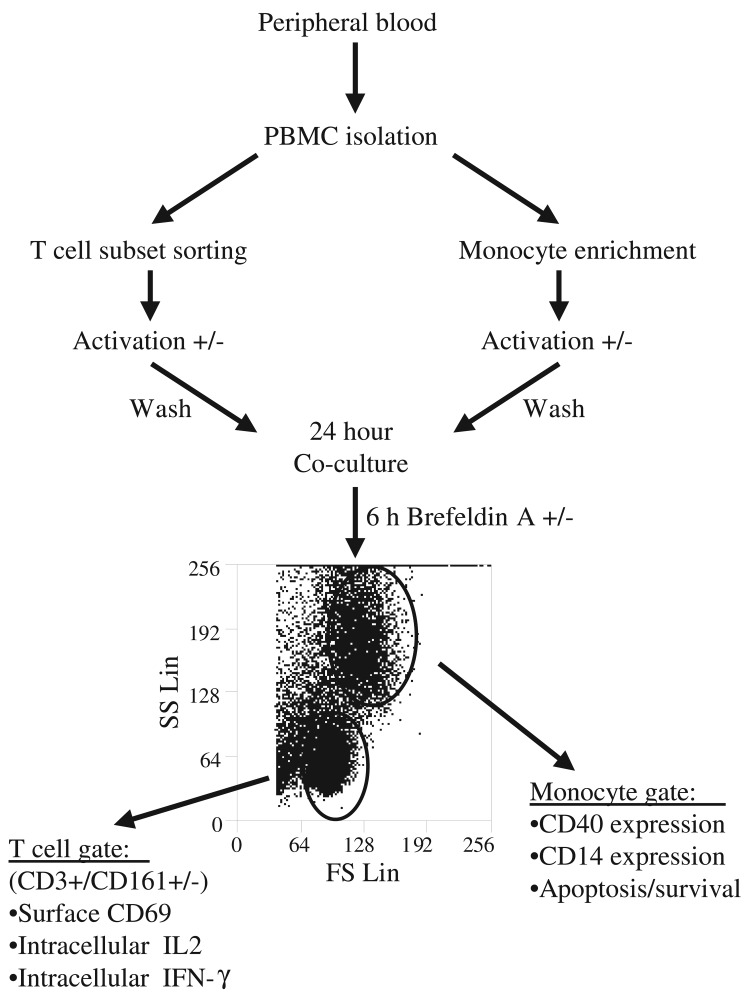
µg/ml. In order to permit sensitive evaluation of monocyte responses and to avoid the significant monocyte activation and limited survival that is associated with standard culture methods, we excluded charged culture platforms and employed opaque polypropylene flat bottom culture tubes (Corning Incorporated, Corning, NY) for monocyte cultures. Where indicated, monocytes were stimulated by 40 ng/ml PMA in the absence of Ionomycin, or by LPS (InvivoGen, 100 ng/ml) for 6 hours. For co-culture experiments, effector T cells or monocytes were washed thrice in culture media to deplete the stimulant prior to co-culture with responder cells and a total of 106 cells/ml of media were co-cultured at 1:1 Effector to responder cell ratio.
Antibody reagents
Anti-DR3 mAb (clone F05) was generated at Teva Biopharmaceuticals (Rockville, MD) ([19]. Isotype- or species-specific control antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Gem1A.1 is an anti-human LIGHT combinatorial Ab containing VH and V chains generated from a BALB/c mouse immunized with soluble r LIGHT by Gemini Biolabs (La Jolla, CA) [27]. Anti-CD40 mAb EA5 clone was a gift of Dr. C. D. Benjamin, Biogen, Inc., Cambridge, MA. The ascites was purified over a protein G column and quantified by ELISA. Additional chromophore-conjugated antibodies specific for human CD3, CD4, and CD8, CD14, CD161, IL2 and IFN-γ were from Caltag (Burlingame, CA), and anti-human CD56 was from Beckman-Coulter (Fullerton, CA).
Cell staining and flow cytometry
For intracellular cytokine analysis, cells were incubated in the presence of Golgi inhibitor, Brefeldin A (Calbiochem, La Jolla, CA), for the last 5-6 hours of culturing. Cells were then washed and stained for surface markers as above, followed by light fixation and permeablization in the presence of anti-cytokine, or isotype control antibodies using the Fix and Perm, intracellular staining kit (Caltag, Burlingame, CA). Cells were then washed, fixed in 1% paraformaldehyde and stabilized at 4°C for 16-20 hours prior to flowcytomeric analysis. Non-specific staining by control isotypes or staining of unstimulated cells was subtracted from percentage staining for each cell subset to determine specific mean fluorescence.
Flow cytometric analysis included at least 2×104 events on a FACScan (Becton Dickinson) or Cyan (Dakocytomation) and analyzed with the respective CellQuest or Summit software. Percentages of cytokine producing or surface marker expressing cells represent the fraction of total the total cell subset defined.
Statistical analysis
The Student t-test was applied when comparing frequencies of cytokine producing cells.
Results
CD161 defines T cells consistently expressing CD56 in culture
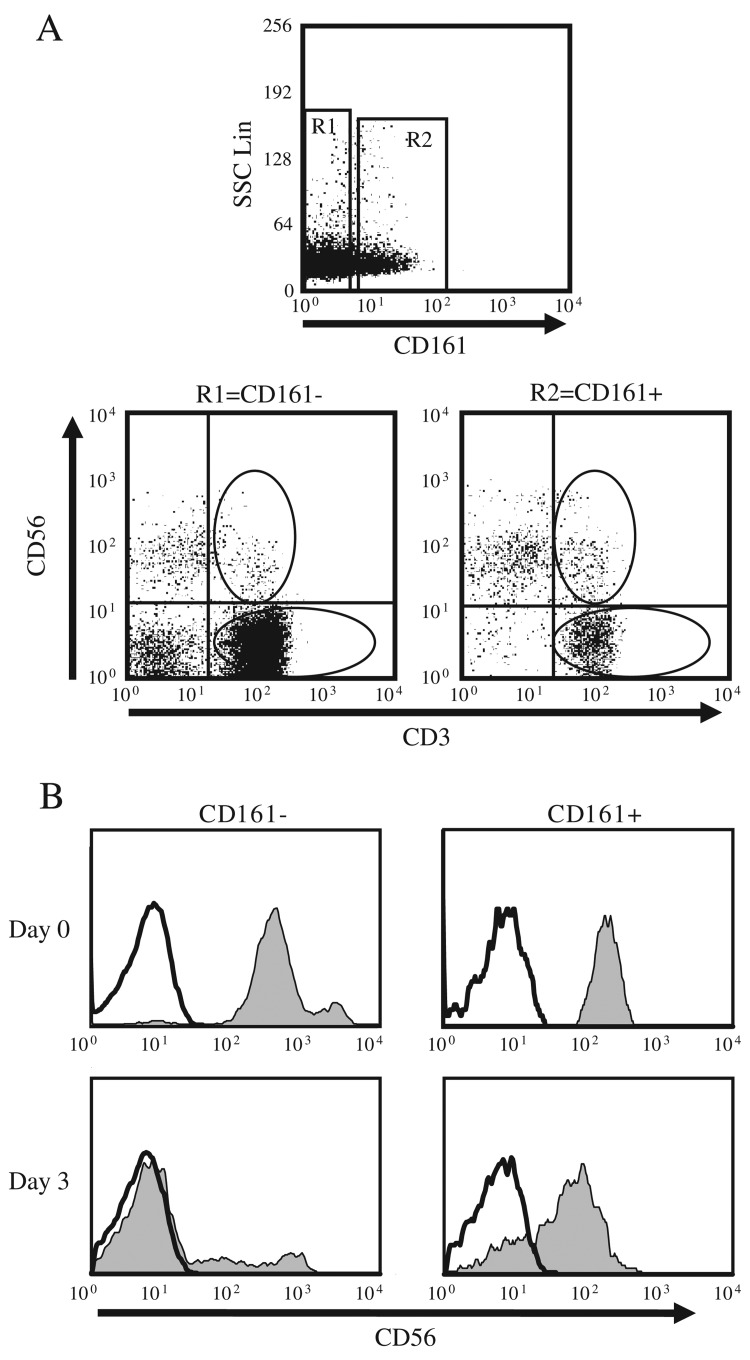
NK cell surface markers, CD161 and CD56, can be expressed on T cell subsets that express pro-inflammtory cytokines in the intestinal mucosa or liver immune compartments [29, 33, 34]. CD56+ T cells activate responder T cell proliferation and cytokine production in vitro [31], and several studies proposed extrathymic selection of this cell population [37–39]. However, possible changes in temporal CD161 or CD56 expression have not been analyzed when using these markers to define primary T cell subsets [29, 31, 33, 34]. We used flow cytometry sorting to isolate primary CD3+/CD56+/CD161+, CD3+/CD56–/CD161+, CD3+/CD56+/CD161–, and CD3+/CD56–/CD161– T cells from PBMC preparations, and assessed variation in CD56 and CD161 protein expression over time (Figure 1). Surface CD161 and CD56 protein expression was ascertained over 3 days by a repeat surface immunostain and flow cytometric analysis. We observed significant downregulation of CD56 expression on CD161– T cells by day 3, while expression persisted almost exclusively on CD161+ T cells (Fig. 1B). In contrast, expression of CD161 was constant on sorted T cells over time, and expression levels of both CD56 or CD161 as well as cell survival rates did not significantly differ following short-term activation (6–18 h) of these T cell subsets (data not shown). These data validated CD161 as a constitutive cell marker for a subset of T cells and demonstrated that CD161+ T cells coexpressing CD56 are more likely to express CD56 for several days, a critical consideration for studies investigating CD56+ T cell effector function in vitro [31].
Fig. 1.
DC56 is persistently expressed only on T cells co-expressing CD161. Lymphocytes were isolated from human peripheral blood and surface stained with fluorescent conjugated anti-CD3, anti-CD161 and anti-CD56 antibodies, followed by flow cytometric analysis. A. Human CD3+ T cell subsets were purified from PBL preparations by flow cytometry sorting based on CD3, CD56 and CD161 staining characteristics. Gated viable, lymphocyte size, scatter plots are shown for representative samples, indicating staining profiles and sort gates. B. Sorted CD161+/CD56+, CD161–/CD56+, CD161+/CD56– and CD161–/CD56– were cultured separately and CD56 staining histograms are shown for the CD56+ (Shaded) and CD56– (unshaded) staining in sorted populations prior to culture (top) or upon re-staining on day 3 (Bottom). Data are representative of at least five experiments
CD161+ T cells preferentially express the gut-associated TNF family member, LIGHT
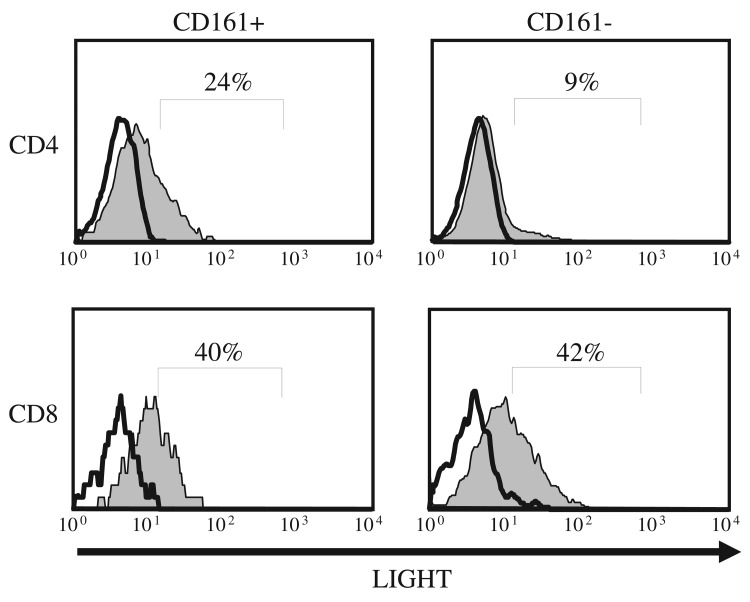
CD161+ T cells are frequently found in the intestinal mucosa and release pro-inflammatory cytokines [29]. LIGHT, a cytokine in the TNF family, mediates inflammation specifically in the gut compartment when expressed on T cells [17, 18], and enhanced LIGHT expression was reported on human lamina propria T cells and on gut-associated T cells in the periphery [20]. Hence, we tested whether CD161 expression on peripheral blood (PB) T cells defines a gut-associated subpopulation of cells that preferentially express LIGHT. We compared surface LIGHT protein expression on CD161+ and CD161– primary human T cells by immunostaining and flow cytometry, following in vitro activation with phorbol ester (PMA) and an ionophor (Ionomycin). LIGHT was predom inantly expressed on CD4+ T cells coexpressing CD161 when compared with CD4+/CD161– T cells (Fig. 2). By contrast, although CD8+ T cells express higher levels of LIGHT [7], surface LIGHT protein expression did not differ between the CD8+/CD161+ and CD8+/CD161– T cell populations (Fig. 2). In contrast to peripheral small bowel homing CCR9+ T cells where LIGHT is constitutively expressed [20], we did not detect significant levels of membrane LIGHT protein on resting CD161+ T cells, suggesting that CD161 may define a population of CD4+ T cells with enhanced potential for LIGHT expression following activation, and that LIGHT expression is not secondary to an activated state in this cell subset.
Fig. 2.
LIGHT is preferentially expressed on CD4+ T cells co-expressing CD161. PBL were isolated from a healthy donor blood and cultured for 18 hours in the presence of PMA and Ionomycin. Cells were surface stained with anti-CD3, anti-CD4/8, anti-CD161 and anti-LIGHT antibodies and analyzed by flowcytometry. Stimulated (shaded) and unstimulated (unshaded) histogram plots are shown for live CD3+ cells gated for CD161, and CD4/8 expression as indicated. Data are representative of at least five experiments
CD161+ T cells preferentially respond to signaling through the gut-associated TNF receptor family member, DR3
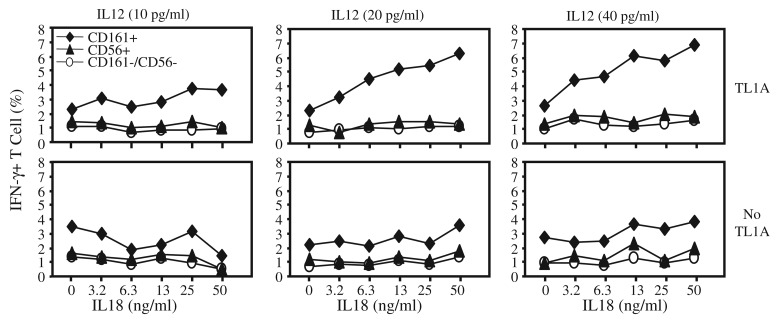
To further assess the role of peripheral CD161+ T cells in molecular immunoregulatory mechanisms specific to the gut compartment, we evaluated T cell responses to signaling through DR3, a receptor for the gut-associated cytokine, TL1A. T cell signaling via TL1A-DR3 requires co-treatment with IL12 and IL18 [4, 40], hence, varying requirement for these cytokines in the CD161+ and CD161– T cell subsets were also ascertained. T cells were cultured in the presence of varying concentrations of IL12 and IL18, and then stimulated by DR3 crosslinking using agonistic anti-DR3 antibodies. T cell response was measured as a function of IFN-γ production by intracellular immunostaining and flow cytometry. CD161+ T cells were the primary subset producing IFNg following DR3 crosslinking, and a significant fraction of CD161+ T cells produced IFNγ even at rel atively low concentrations of IL12 and IL18 (Fig. 3). Selective IFNγ production suggested that CD161 expression marks a specific T cell subset that are characterized by enhanced responsiveness to DR3 signaling at lower levels of co-stimulation with IL12 and IL18. The fact that CD161– CD56+ T cells were not induced to produce IFNγ following DR3 stimulation indicated that enhanced CD161+ T cell response is DR3 specific and not a reflection of preferential TH1 like character or a mature phenotype, since we previously showed that a similar fraction of CD56+ T cells can produce IFNγ as CD161+ T cells [31].
Fig. 3.
CD161 defines a T cell population responsive to TL1A-DR3 signal. Isolated PBL were stimulated with anti-DR3 antibodies for 18 hours followed by 6 hours in the presence of Golgi inhibitor, Brefeldin A. Cells were then surface stained with anti-CD3, anti-CD56 and anti-CD161 antibodies and intracellularly immunostained for IFNγ. Plots show percent of viable CD3+ gated T cells expressing INFγ following activation with anti-DR3 (top) or isotype control (bottom) antibodies in the presence of titrated exogenous IL18 and IL12. Spontaneous cytokine production by unstimulated cells was consistently less than 2%. Data are representative of at least five experiments
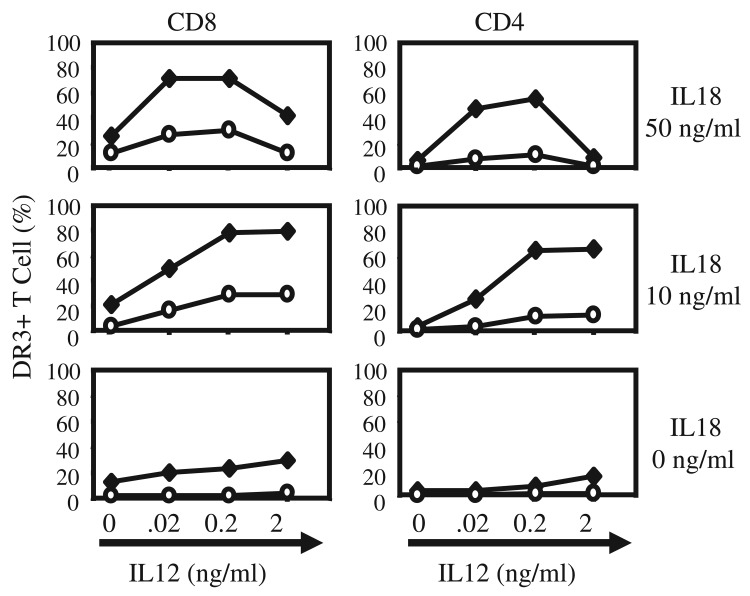
IL12 and IL18 facilitate TL1A-DR3 signaling by upregulating DR3 expression on the cell membrane of T and NK cells [41]. To determine whether enhanced expression of DR3 on CD161+ T cells reduces the requirement for IL12 and IL18 costimulation, we stimulated PB T cells with varying concentrations of IL12 and IL18, and measured surface DR3 protein expression on T cell subsets by immunostaining and flow cytometry. CD161+ T cells induced surface DR3 expression at lower concentrations of IL12 and IL18 than CD161– T cells, with up to 80% of CD161+ T cells staining positive for DR3 (Fig. 4). Enhanced expression of DR3 on CD161+ T cells was consistent with enhanced response to DR3 stimulation at low concentrations of IL12 and IL18 (Fig. 3). Interestingly, compared to CD4+ T cells, higher frequency of DR3 expression was detected on CD8+ T cells regardless of CD161 expression, suggesting that CD161 better associates a CD4+ T cell population with DR3 signaling, in agreement with enhanced LIGHT expression on CD4+/CD161+ T cells (Figs 2 and 4).
Fig. 4.
Surface DR3 protein expression is preferentially induced on CD161+ T cells at low IL12/IL18 concentrations. Isolated PBL were cultured for 24 hours in the presence of varying concentrations of exogenous IL12 and IL18, and then immunostained for surface CD3, CD8/4, CD161 and DR3 expression. Plots show percent of viable CD3+ gated CD161+ (♦) and CD161– (o) T cells expressing DR3, which did not bind the DR3 isotype control. Data are representative of at least three experiments
Effector CD161+ T cells stimulate monocyte activation
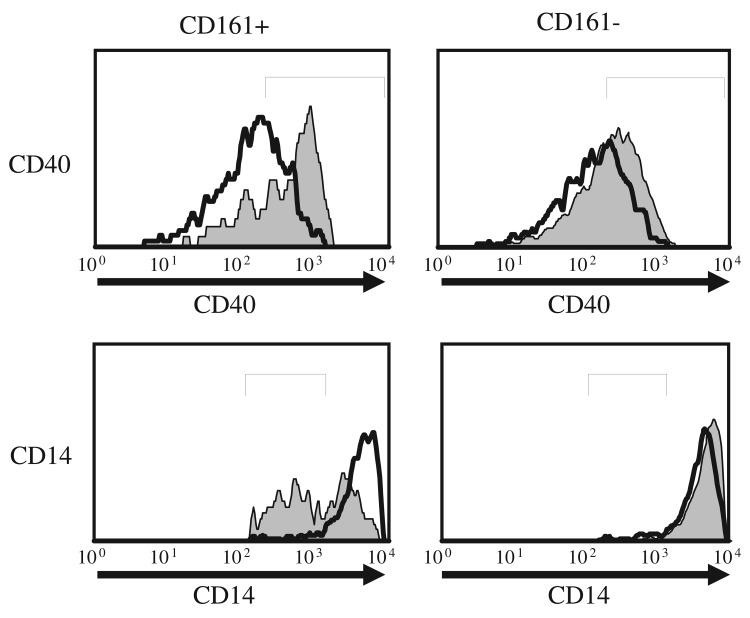
DR3 mediated induction of cytokine expression suggested an effector function for CD161+ T cells. To determine whether DR3 signaling potentiates effector function in CD161+ T cells, we used a recently established in vitro co-culture system to evaluate effector-responder T cell interactions (Fig. 5) [31]. Candidate effector T cell subsets were purified by flow sorting from human PBL preparation and stimulated using agonistic anti-DR3 antibodies or isotype control in the presence of IL12 and IL18 for 18 hours. Following rigorous washing of stimulants, activated effector cells were co-culture with responder autologous monocyte preparations at an optimal 1:1 ratio for 24–48 hours [31]. To ascertain responder cell activation, co-cultured cells were surface immunostained and analyzed by flowcytometry gating on a CD3– and a non-apoptotic monocyte size/density profile [42]. CD40 expression was measured as a sensitive indicator of monocyte activation [43]. We observed signif icant CD40 upregulation on monocytes co-cultured with DR3 activated CD161+ T cells, but only marginal change in CD40 levels on monocytes co-cultured with similarly activated CD161– T cells (Fig. 6). In addition, CD14 down regulation was assessed as surrogate marker of monocyte cell membrane fluidity and active shedding of CD14 protein, which provide early measures of monocyte activation [44]. CD14 surface staining was significantly compromised on monocytes following co-culture with DR3 activated CD161+ T cells, while no changes in CD14 expression were detected following co-culture with CD161– T cells (Fig. 6). Loss of CD14 expression can also be associated with apoptosis [42], however no significant differences were detected in survival or the frequency of apoptotic monocytes in co-culture with either CD161+ or CD161– T cells (<25% by Annexin V and 7AAD staining).
Fig. 5.
Experimental setup for the analysis of effector–responder interaction in monocyte-T cell co-cultures. Monocytes were isolated from human leukocyte preparations by negative selection using magnetic beads and T cell subsets were purified by flow sorting (>99% pure by flow cytometric reanalysis). Effector cells were then activated as indicated, washed and co-cultured with responder cells at 1:1 ratio for 24 hours. Responder T cell or monocyte activation was determined as a function of cytokine and surface protein expression profiles evaluated by flow cytometric analysis gating on the responder cell subset
Fig. 6.
DR3 stimulated CD161+ T cells activate primary autologous monocytes. Flow sorted CD3+/CD161+, or CD3+/CD161– T cells to be tested as effectors cells were activated with anti-DR3 antibodies (shaded) or isotype control (unshaded) in the presence of IL12 (0.2 ng/ml) and IL18 (10 ng/ml) for 24 hours, washed and co-cultured with monocytes as responder cells for an additional 24 hours. Co-cultured cells were analyzed by flow cytometry and histograms show gated monocytes stained for CD40 (top) or CD14 (bottom). Data are representative of at least three experiments
CD161+ T cells preferentially respond to stimulation by activated monocyte
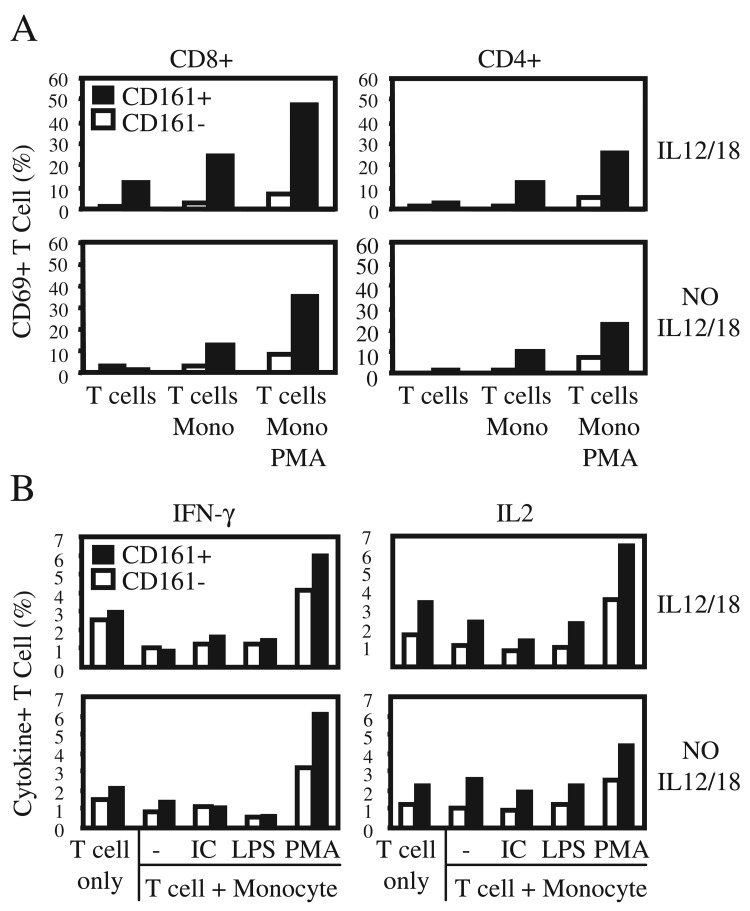
CD161+ T cell mediated activation of monocytes suggested effective T cell-monocyte cross-talk favoring the CD161+ T cell subset. We examined whether CD161+ T cells are also preferentially responsive to a reciprocal antigen independent activation by monocytes in the same co-culture system (Fig. 5). Following co-culture with PMA activated monocytes, T cells were analyzed by immunostaining and flow cytometry gating on CD3+, viable lymphocyte size/density cells. We measured activation of responder CD161+ or CD161– T cells as a function of activation marker upregulation on the cell surface (CD69), or cytokine expression by intracellular immunostaining (IL2 and IFNγ). Co-cultured CD161+ T cells up-regulated CD69 expression (Fig. 7A), and expressed pro-inflammatory cytokines, IL2 and IFNγ more frequently than CD161– T cells (Fig. 7B). Monocytes activated with PMA induced responder T cell expression of CD69 more efficaciously in both CD4+ and CD8+ T cells CD161 subsets (Fig. 7A), in agreement with enhanced DR3 expression requiring lower concentrations of IL12 and IL18 in both CD4+/CD161+ and CD8+/CD161+ T cells (Fig. 4).
Fig. 7.
CD161+ T cells are preferentially activated by monocytes. PBL were co-cultured with activated monocytes for 24 hours in the presence (top) or absence (bottom) of IL12 and IL18. A. CD4+ and CD8+ T cell activation was analyzed as a function of surface staining for CD69 by flow cytometry, following co-culture with PMA activated monocytes. B. T cells were co-cultured with monocytes pre-stimulated by PMA, and T cell activation measured as a function intracellular staining for IFNγ and IL2 following 5 hours of culture in the presence of Golgi maturation inhibitor, Brefeldin A. Bar graphs represent percent positive cells in CD3+/CD161+ (closed), and CD3+/CD161– (open) gates. Data are representative of a minimum of three experiments
Discussion
Human T cells expressing CD161 are present in the intestinal immune compartment and express pro-inflammatory cytokines, thus suggesting an effector function [29], likely in the Th17 context [35, 45]. Our findings link the CD161+ T cell population to the LIGHT and TL1A signaling circuits as potential molecular mechanisms that mediate gut mucosal inflammation. We previously reported enhanced LIGHT expression in lamina propria T cells [7], as well as constitutive expression and elevated levels in inflamed mucosal tissue [20]. Interestingly, our data indicated that on peripheral CD4+ T cells, LIGHT is primarily expressed on the CD161+ subset, whereas LIGHT was detected on CD8+ cells irrespective of CD161 status (Fig. 2). Enhanced LIGHT expression on CD4+/CD161+ T cells suggests a gut associated phenotype and is in agreement with enhanced LIGHT expression on a second peripheral CD4+ T cell subset which expresses the small bowel homing chemokine receptor, CCR9 [20]. Cell type specific gene transfer studies indicate that LIGHT expressed on T cells can induce intestinal inflammation [17, 18], a function mediated either directly via a pro-inflammary LIGHT-HVEM signal [10, 46], or via a LIGHT-LTβR apoptotic signal compromising epithelial barrier function [12]. Hence, enhanced LIGHT expression on CD161+ T cells and the high frequency of these cells in the intestinal mucosa could lead to higher levels of LIGHT protein in the gut and mediate inflammation preferentially in the mucosal immune compartment.
TL1A is produced by activated monocytes and DC [40, 47, 48], and induces Th1-like cytokine expression on some T cells by signaling through its receptor, DR3 [41]. Our data indicate that peripheral CD161+ T cells are a predominant subset producing IFNγ in response to a TL1A-DR3 signal (Fig. 3). Although, CD161+ T cells share mature phenotypic characteristics with the CD45RO including enhanced IFNγ production [29], our data suggests that CD161 marks a unique and independent cell population. TL1A responsiveness cannot just be attributed to a mature phenotype (i.e. CD45RO expression), since CD161– T cells do not respond to TL1A mediated stimulation despite frequent CD45RO expression in this subset. Additionally, CD56+ T cells, a mature population composed predominantly of IFNγ producing cells, failed to respond to DR3 signaling (Fig. 3). Failure of CD56+ T cells to respond to a TL1A-DR3 signal is consistent with the absence of CD56 expression on small bowel homing CD4+/CCR9+ T cells [49], a population that does respond to DR3 signaling [50].
Interestingly, CD161+ T cell response to DR3 stimulation was potentiated by significantly lower levels of IL12 and IL18 when compared to non-enriched T cell preparations (40 and 4 fold lower concentrations for IL12 and IL18, respectively) [41]. Dependence on lower IL12 and IL18 concentrations suggests that CD161+ T cells could mediate TL1A-DR3 signaling function early in a mucosal inflammatory response prior to substantial increase in cytokine levels. Enhanced DR3 expression may explain a lower requirement for IL12 and IL18, since priming with these cytokines has been suggested to induce T cell expression of DR3 [14, 41]. We demonstrated enhanced DR3 expression specifically on CD161+ T cells, with up to 80% of CD161+ T cells expressing DR3 at lower IL12 and IL18 concentration than required for CD161– cells, while some CD161+ T cells expressed DR3 even in the complete absence of IL12 and IL18. These observations suggest that TL1A-DR3 signal efficacy is plausibly enhanced on CD161+ T cells via mechanisms regulating DR3 protein expression at the cell surface. By contrast, the LIGHT receptor, HVEM, is constitutively expressed on all T cells, and induced expression of LIGHT appears pivotal in regulating the LIGHT-HVEM signaling circuit [7, 20, 31]. Taken together, our data indicate that CD161+ T cells define a predominant subset expressing LIGHT and responding to DR3 signal, suggesting a central role for these cells in inflammatory mechanisms mediated by the LIGHT-HVEM and TL1A-DR3 signaling circuits. Consequently, elevated CD161+ T cell frequency in the intestine may explain the tissue specificity of LIGHT and TL1A signaling in the mucosal compartment.
T cells expressing NK markers, CD161+ and CD56+ produce an array of cytokines implicating a pro-inflammatory effector function [29, 34, 35]. We directly demonstrate that CD161+, but not CD161– T cells, pre-stimulated via TL1A-DR3 signaling, can activate monocytes in vitro (Figs 6 and 7). Increased expression of activation marker CD40 suggested monocyte induction of transcriptional mechanisms [51], and decreased cell surface CD14 is consistent with increased membrane fluidity and rates of receptor internalization or shading [44]. However, monocyte activation by the CD161+ subset is intriguing since a complementary population of T cells expressing CD56 did not activate monocytes, but were more efficacious at activating responder T cells via T–T interaction [31]. Thus, CD161 and CD56 expression may identify T cells subsets with different effector functions.
Efficacious monocyte-T cell cross-talk mechanisms were further illustrated by the reciprocal activation of CD161+ T cells in co-culture with monocytes, which induced cytokine production and activation marker expression primarily on CD161+ T cells. Our autologous monocyte-T cell co-culture experiments do not include saturated antigen mediated TCR stimulation following T cell priming and expansion in vivo, and hence, observed monocyte-T cell cross-talk likely reflects antigen independent mechanisms of T cell activation such as LIGHT-HVEM or TL1A-DR3 signaling. Antigen independent T cell activation is consistent with attenuation of TCR signaling, a characteristics unique to the mucosal compartment [52]. Both, LIGHT and TL1A are primarily expressed by monocytes, thus, enhanced LIGHT-HVEM or TL1A-DR3 signal under low IL12 and IL18 concentrations may augment monocyte-CD161+ T cell cross-talk. Although, additional mechanisms likely contribute to monocyte-T cell interactions, since blocking LIGHT or TL1A in co-culture did not selectively inhibit monocyte activation (data not shown).
NK markers such as CD161 or CD56 are expressed early in lymphoid cell development prior to the T-NK split, but lost during T cell selection and maturation in the thymus. Expression on T cell subsets associated with the mucosal immune compartment is noteworthy and may provide clues to T cell differentiation and trafficking in the intestines. Notably, effector Th17 T cells were suggested to originate from a CD4+/CD161+ T cell subset [45], and CD161 can directly induce T cell expansion when bound by the Proliferation-Induced Lymphocyte-Associated Receptor (PILAR) [51]. Extensive exposure to antigen at the intestinal mucosa is consistent with the mature (CD45RO) and non-proliferative phenotypes, which are primary hallmarks of intestinal T cells [3, 52], shared by CD161+ [29] and CD56+ T cells [31, 34]. Antigen specificity repertoires unique to the gut compartment have been described, which suggested mucosal T cell differentiation and selection independent of the thymus as a mechanism likely to play a role in the pathology of IBD [53]. A study reported “leakage” of T cell progenitors from the thymus to the gut as a novel mechanism for thymus independent T cell selection [54]. More specifically, CD56 expression was reported on CD7+/CD3– T cell progenitors in the gut which are capable of differentiating into mature T cells [37], and a second study proposed extra thymic development of CD56+ T cells in studies of human neonate cord blood [38]. Hence, the occurrence of fully differentiated T cells expressing CD161 or CD56 in association with the gut is consistent with the concept of thymus independent differentiation and an independent mucosal T cell repertoire [39].
Non-exclusive co-expression of CD161 and CD56, and loss of CD56 only from the CD161– T cell subset suggest that these markers define overlapping, yet possibly distinct subsets of T cells (Fig. 1). Functional analysis further supports a distinct immunoregulatory role for T cells expressing either CD161 or CD56. For instance, CD56+ T cells more effectively activate responder T cells via T–T interactions [31], while our data demonstrate efficacious reciprocal activation between CD161+ T cell and monocytes (Figs 6 and 7). Likewise, CD161+, but not CD56+ T cells, expressed higher levels of surface LIGHT and responded to TL1A-DR3 mediated signaling at low IL12 and IL18 concentrations (Fig. 2). In addition, we have previously shown that CD56+ T cells exhibit compromised proliferation potential compared to CD161+ T cells [31]. Although likely defining functionally distinct populations, both CD161+ and CD56+ T cells are localized to the intestinal immune compartment and express pro-inflammatory cytokines [29, 31, 34]. Similarly either CD161+ or CD56+ T cells were sufficient in mediating CD2 induction of a peripheral T cell proliferative response [31], thus linking both subsets with the gut associate CD2 signaling pathway [3].
In conclusion, our work links CD161+ T cells with the gut associated LIGHT and TL1A signaling circuits, thus implicating this T cell population in the mucosal immune compartment. Our data suggests regulation of DR3 expression as a determinant of enhanced TL1A responsiveness in CD161+ T cells, and we demonstrate efficient activation of monocytes by CD161+ T cells. These findings suggest that CD161+ T cells may play an important role in pathologic gut inflammation. Further analysis of CD161+ T cells, in particular with TL1A and LIGHT signaling mechanisms, will better our understanding of mucosal immune regulation and may lead to novel therapeutic approaches in the treatment of IBD.
Acknowledgments
We are grateful to Patricia Lin for help with flow cytometry and Cindy Ting for critical reading of this manuscript. This work was supported in part by grants from the National Institutes of Health F32DK10139 (OC), DK056328 (SRT) and R37AI33068 (CFW).
Contributor Information
O. Cohavy, 1Inflammatory Bowel & Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
D. Q. Shih, 1Inflammatory Bowel & Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
T. M. Doherty, 1Inflammatory Bowel & Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
C. F. Ware, 2Division of Molecular Immunology, La Jolla Institute for Allergy and Immunology, San Diego, CA 92121, USA.
S. R. Targan, 1Inflammatory Bowel & Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
References
- 1.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008 Jan 21;14(3):390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008 Dec;10(6):568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targan SR, Deem RL, Liu M, Wang S, Nel A. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol. 1995 Jan 15;154(2):664–675. [PubMed] [Google Scholar]
- 4.Shih DQ, Michelsen KS, Barrett RJ, Biener-Ramanujan E, Gonsky R, Zhang X, Targan SR. Insights into TL1A and IBD pathogenesis. Adv Exp Med Biol. 2011;691:279–288. doi: 10.1007/978-1-4419-6612-4_29. [DOI] [PubMed] [Google Scholar]
- 5.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 6.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005 Dec 8;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 7.Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004 Jul 1;173(1):251–258. doi: 10.4049/jimmunol.173.1.251. [DOI] [PubMed] [Google Scholar]
- 8.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001 Feb 23;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998 Jan;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 10.Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997 May 30;272(22):14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 11.Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998 Jul;9(1):59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 12.Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC, Cohen GH, Eisenberg RJ, Ware CF. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000 May 12;275(19):14307–14315. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006 Sep;6(9):671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 14.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002 Mar;16(3):479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 15.Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003 Oct 3;278(40):39251–39258. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]
- 16.Fayad R, Brand MI, Stone D, Keshavarzian A, Qiao L. Apoptosis resistance in ulcerative colitis: high expression of decoy receptors by lamina propria T cells. Eur J Immunol. 2006 Aug;36(8):2215–2222. doi: 10.1002/eji.200535477. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001 Dec 1;167(11):6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y, Tamada K, Chen L, Fu YX. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001 Dec;108(12):1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay F, Browning JL, Lawton P, Shah SA, Comiskey M, Bhan AK, Mizoguchi E, Terhorst C, Simpson SJ. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998 Dec;115(6):1464–1475. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 20.Cohavy O, Zhou J, Ware CF, Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol. 2005 Jan 15;174(2):646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 21.Bamias G, Martin C, 3rd, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, Pizarro TT, Wei P, Cominelli F. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003 Nov 1;171(9):4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 22.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, Targan SR. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004 Jul;112(1):66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, Pizarro TT, Cominelli F. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, Braun J, Targan SR. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008 Aug;135(2):552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meylan F, Song YJ, Fuss I, Villarreal S, Kahle E, Malm IJ, Acharya K, Ramos HL, Lo L, Mentink-Kane MM, Wynn TA, Migone TS, Strober W, Siegel RM. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011 Mar;4(2):172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih DQ, Barrett R, Zhang X, Yeager N, Koon HW, Phaosawasdi P, Song Y, Ko B, Wong MH, Michelsen KS, Martins G, Pothoulakis C, Targan SR. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011 Jan 11;6(1):e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001 Nov 1;167(9):5122–5128. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]
- 28.Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB, Bitton A, Williams CN, Greenberg GR, Cohen Z, Lander ES, Hudson TJ, Siminovitch KA. Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet. 2000 Jun;66(6):1863–1870. doi: 10.1086/302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Keeffe J, Doherty DG, Kenna T, Sheahan K, O'Donoghue DP, Hyland JM, O'Farrelly C. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol. 2004 Aug;34(8):2110–2119. doi: 10.1002/eji.200424958. [DOI] [PubMed] [Google Scholar]
- 30.Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O'Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003 Aug 15;171(4):1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 31.Cohavy O, Targan SR. CD56 marks an effector T cell subset in the human intestine. J Immunol. 2007 May 1;178(9):5524–5532. doi: 10.4049/jimmunol.178.9.5524. [DOI] [PubMed] [Google Scholar]
- 32.Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA. Comparative studies of CD3- and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol. 1991 Sep;136(2):486–495. doi: 10.1016/0008-8749(91)90369-m. [DOI] [PubMed] [Google Scholar]
- 33.Iiai T, Watanabe H, Suda T, Okamoto H, Abo T, Hatakeyama K. CD161+ T (NT) cells exist predominantly in human intestinal epithelium as well as in liver. Clin Exp Immunol. 2002 Jul;129(1):92–98. doi: 10.1046/j.1365-2249.2002.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999 Aug 15;163(4):2314–2321. [PubMed] [Google Scholar]
- 35.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009 Mar 16;206(3):525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999 Jan;60(1):20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 37.Gunther U, Holloway JA, Gordon JN, Knight A, Chance V, Hanley NA, Wilson DI, French R, Spencer J, Steer H, Anderson G, MacDonald TT. Phenotypic characterization of CD3-7+ cells in developing human intestine and an analysis of their ability to differentiate into T cells. J Immunol. 2005 May 1;174(9):5414–5422. doi: 10.4049/jimmunol.174.9.5414. [DOI] [PubMed] [Google Scholar]
- 38.Musha N, Yoshida Y, Sugahara S, Yamagiwa S, Koya T, Watanabe H, Hatakeyama K, Abo T. Expansion of CD56+ NK T and gamma delta T cells from cord blood of human neonates. Clin Exp Immunol. 1998 Aug;113(2):220–228. doi: 10.1046/j.1365-2249.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loza MJ, Luppi P, Kiefer K, Martin ES, Szczytkowski JL, Perussia B. Human peripheral CD2-/lo T cells: an extrathymic population of early differentiated, developing T cells. Int Immunol. 2005 Sep;17(9):1213–1225. doi: 10.1093/intimm/dxh298. [DOI] [PubMed] [Google Scholar]
- 40.Shih DQ, Kwan LY, Chavez V, Cohavy O, Gonsky R, Chang EY, Chang C, Elson CO, Targan SR. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur J Immunol. 2009 Nov;39(11):3239–3250. doi: 10.1002/eji.200839087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, Wei P, Targan SR. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004 Jun 1;172(11):7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 42.Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels HG. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997 Oct 1;159(7):3178–3188. [PubMed] [Google Scholar]
- 43.Mahanonda R, Sa-Ard-Iam N, Charatkulangkun O, Promsudthi A, Schifferle RE, Yongvanichit K, Pichyangkul S. Monocyte activation by Porphyromonas gingivalis LPS in aggressive periodontitis with the use of whole-blood cultures. J Dent Res. 2004 Jul;83(7):540–545. doi: 10.1177/154405910408300706. [DOI] [PubMed] [Google Scholar]
- 44.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996 May;64(5):1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008 Aug 4;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrop JA, Reddy M, Dede K, Brigham-Burke M, Lyn S, Tan KB, Silverman C, Eichman C, DiPrinzio R, Spampanato J, Porter T, Holmes S, Young PR, Truneh A. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998 Aug 15;161(4):1786–1794. [PubMed] [Google Scholar]
- 47.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007 Apr 1;178(7):4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 48.Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Takayama T, Kitazume MT, Okamoto S, Koganei K, Sugita A, Kanai T, Hibi T. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn's disease. Inflamm Bowel Dis. 2010 Apr;16(4):568–575. doi: 10.1002/ibd.21124. [DOI] [PubMed] [Google Scholar]
- 49.Papadakis KA, Landers C, Prehn J, Kouroumalis EA, Moreno ST, Gutierrez-Ramos JC, Hodge MR, Targan SR. CC chemokine receptor 9 expression defines a subset of peripheral blood lymphocytes with mucosal T cell phenotype and Th1 or T-regulatory 1 cytokine profile. J Immunol. 2003 Jul 1;171(1):159–165. doi: 10.4049/jimmunol.171.1.159. [DOI] [PubMed] [Google Scholar]
- 50.Papadakis KA, Zhu D, Prehn JL, Landers C, Avanesyan A, Lafkas G, Targan SR. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005 Apr 15;174(8):4985–4990. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]
- 51.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Engle XA, Rigby WF, Pioli PA, Guyre PM, Conejo-Garcia JR. PILAR is a novel modulator of human T-cell expansion. Blood. 2008 Aug 15;112(4):1259–1268. doi: 10.1182/blood-2007-12-130773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abreu-Martin MT, Targan SR. Regulation of immune responses of the intestinal mucosa. Crit Rev Immunol. 1996;16(3):277–309. doi: 10.1615/critrevimmunol.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 53.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995 Dec;102(3):448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol. 2006 Jan;7(1):76–82. doi: 10.1038/ni1293. [DOI] [PubMed] [Google Scholar]