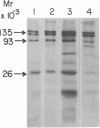
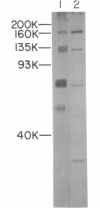
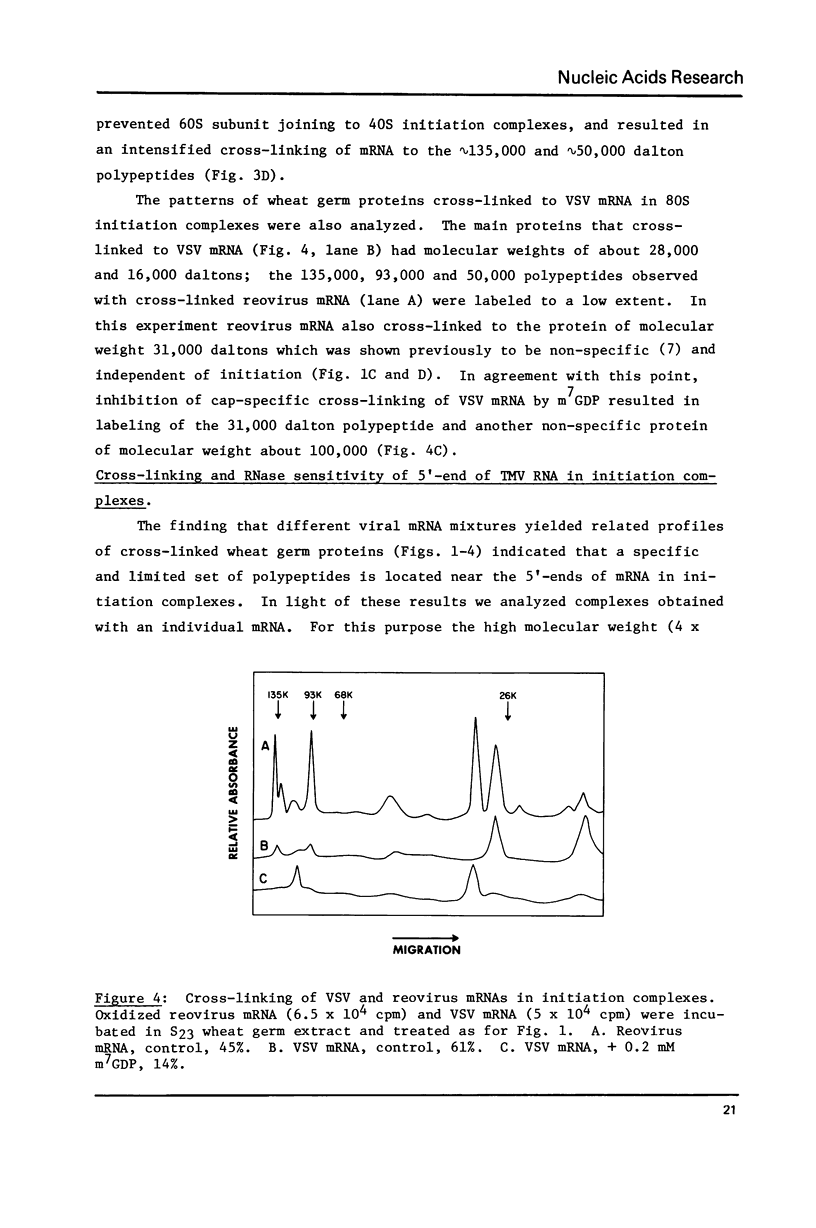
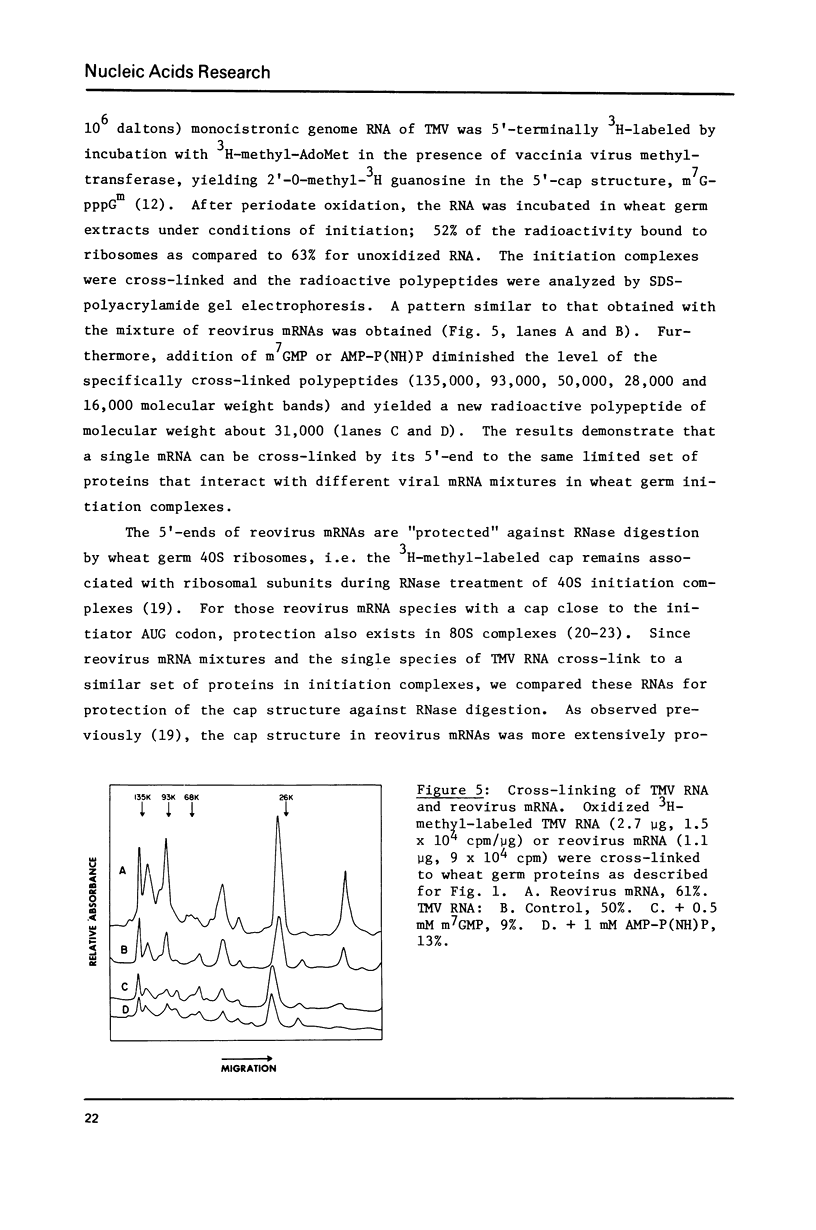
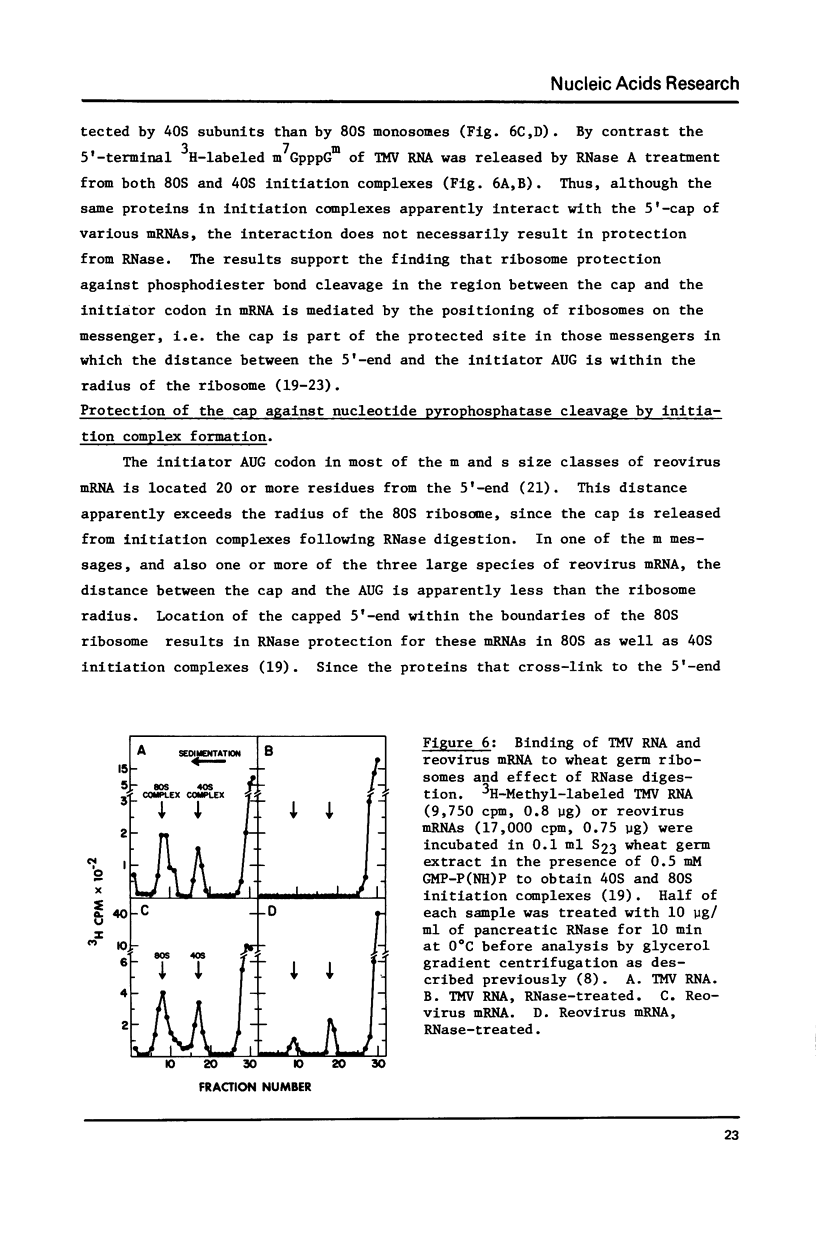
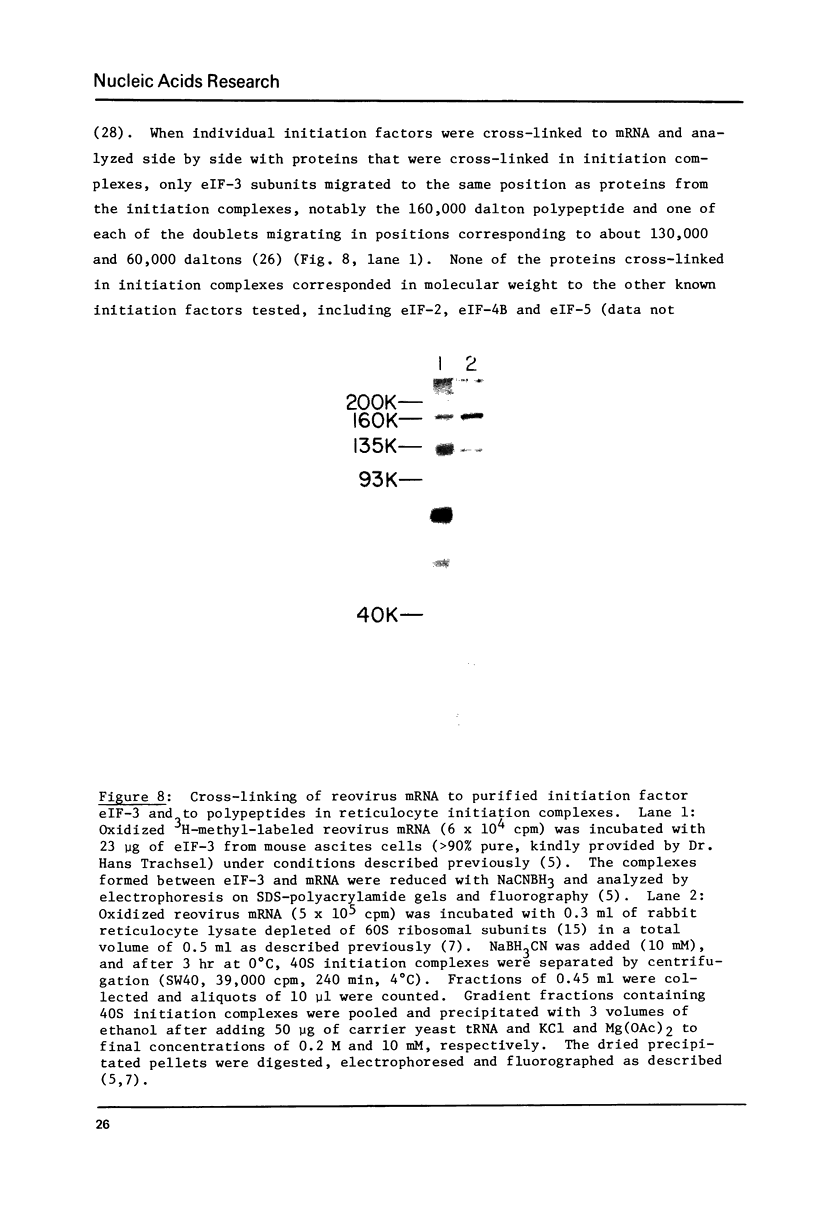
Abstract
A variety of 5'-3H-methyl-labeled, oxidized viral mRNAs were used as probes for detecting in wheat germ initiation complexes proteins that interact with, and can be cross-linked to, the 5'-cap structure. A limited and reproducible set of specific proteins was obtained with the different mRNAs. The binding of these proteins to the 5'-end of mRNA apparently results in protection against nucleotide pyrophosphatase digestion of the cap even in initiation complexes in which the 5'-end is susceptible to pancreatic RNase digestion. Cross-linked proteins from mammalian initiation complexes comigrated with several of the subunits of similarly treated eIF-3. A model for cap binding protein interaction with mRNA cap during initiation of translation is suggested.
Full text
PDF


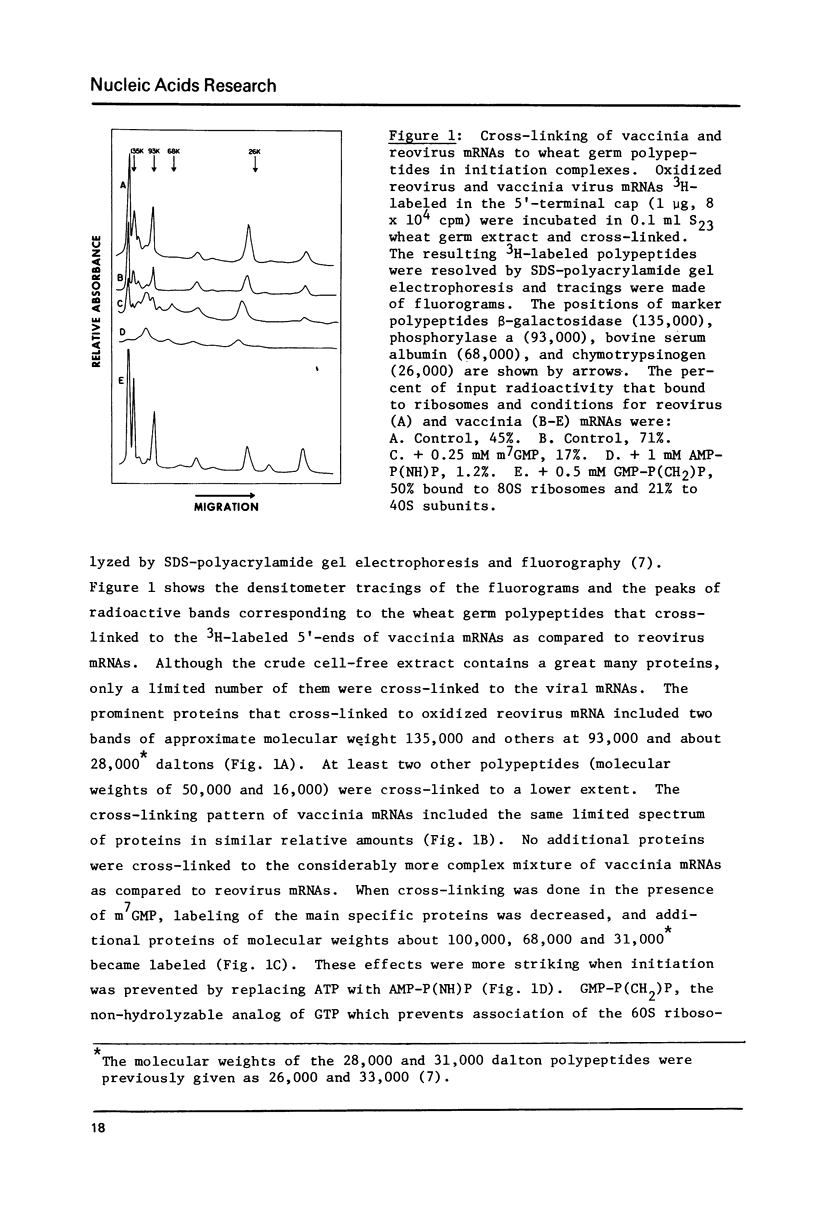

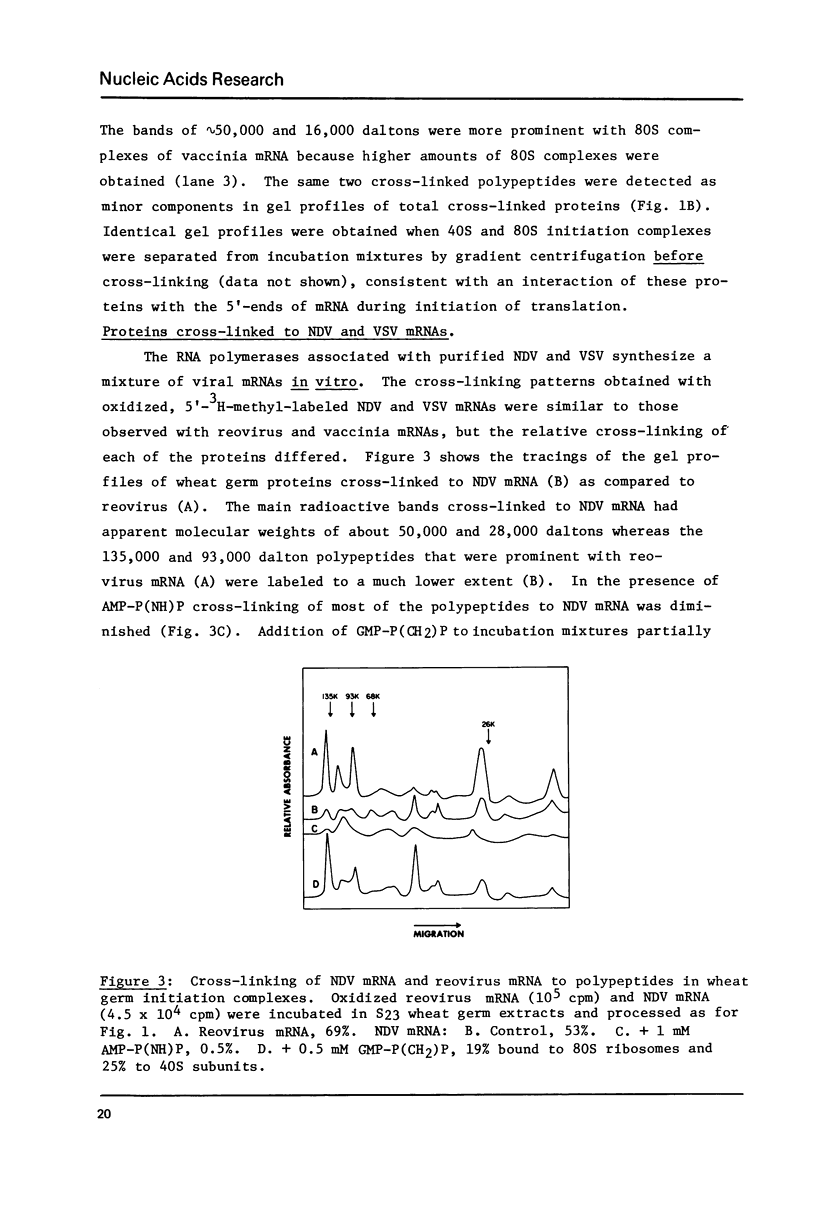





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Effect of 5'-terminal structure and base composition on polyribonucleotide binding to ribosomes. J Mol Biol. 1976 Jul 5;104(3):637–658. doi: 10.1016/0022-2836(76)90126-1. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Methylation of messenger RNA of Newcastle disease virus in vitro by a virion-associated enzyme. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2611–2615. doi: 10.1073/pnas.72.7.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Elson N. A., Anderson W. F. Initiation of globin synthesis: assays. Methods Enzymol. 1974;30:101–127. doi: 10.1016/0076-6879(74)30014-6. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freienstein C., Blobel G. Nonribosomal proteins associated with eukaryotic native small ribosomal subunits. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3392–3396. doi: 10.1073/pnas.72.9.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno M., Vázquez D. Initiation of translation with native 40-S ribosomal subunits. Eur J Biochem. 1978 Feb 1;83(1):169–178. doi: 10.1111/j.1432-1033.1978.tb12081.x. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G. A., Traugh J. A., Croser E. A., Traut R. R. Ribosomal proteins from rabbit reticulocytes: number and molecular weights of proteins from ribosomal subunits. J Mol Biol. 1975 Apr 15;93(3):391–404. doi: 10.1016/0022-2836(75)90285-5. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Rosen H., Israeli R. Translational control: recognition of the methylated 5' end and an internal sequence in eukaryotic mRNA by the initiation factor that binds methionyl-tRNAfMet. Proc Natl Acad Sci U S A. 1978 Feb;75(2):650–654. doi: 10.1073/pnas.75.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Nucleotide sequences of 5'-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977 Sep 29;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of ribosome-protected fragments from reovirus messenger RNA. J Biol Chem. 1976 Jul 25;251(14):4259–4266. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences and properties of two ribosome binding sites from the small size class of reovirus messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6895–6908. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences of two 5'-terminal ribosome-protected fragments from reovirus messenger RNAs. J Mol Biol. 1977 May 5;112(1):75–96. doi: 10.1016/s0022-2836(77)80157-5. [DOI] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Peterson D. T., Merrick W. C., Safer B. Binding and release of radiolabeled eukaryotic initiation factors 2 and 3 during 80 S initiation complex formation. J Biol Chem. 1979 Apr 10;254(7):2509–2516. [PubMed] [Google Scholar]
- Richards K., Guilley H., Jonard G., Keith G. Leader sequence of 71 nucleotides devoid of G in tobacco mosaic virus RNA. Nature. 1977 Jun 9;267(5611):548–550. doi: 10.1038/267548a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Sugimura T. Enzyme cleaving the 5'-terminal methylated blocked structure of messenger RNA. FEBS Lett. 1976 Jun 1;65(2):254–257. doi: 10.1016/0014-5793(76)80492-9. [DOI] [PubMed] [Google Scholar]
- Smith K. E., Henshaw E. C. Binding of Met-tRNAf to native 40 S ribosomal subunits in Ehrlich ascites tumor cells. J Biol Chem. 1975 Sep 10;250(17):6880–6884. [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Nonspecific effect of m7GMP on protein-RNA interactions. J Biol Chem. 1978 Oct 10;253(19):6630–6632. [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J., Ricciardi R. P., Rubin M., Goodman R. M. Analysis of terminal structures of RNA from potato virus X. Nucleic Acids Res. 1978 Jul;5(7):2501–2512. doi: 10.1093/nar/5.7.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Two methyltransferase activities in the purified virions of vesicular stomatitis virus. J Virol. 1977 Dec;24(3):786–793. doi: 10.1128/jvi.24.3.786-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. A., Sadnik I., Scheinbuks J., Moldave K. Studies on native ribosomal subunits from rat liver. Purification and characterization of a ribosome dissociation factor. Biochemistry. 1977 May 17;16(10):2221–2230. doi: 10.1021/bi00629a028. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Braun L., Staehelin T. Purification of seven protein synthesis initiation factors from Krebs II ascites cells. Biochim Biophys Acta. 1979 Feb 27;561(2):484–490. doi: 10.1016/0005-2787(79)90156-4. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]