Abstract
The prorenin receptor (PRR) is highly expressed in podocytes, but its role in the maintenance of podocyte function is unknown. Here we generated podocyte-specific PRR-knockout mice and found that these animals died between 2 to 3 wk after birth. Within 14 d, PRR-knockout mice developed nephrotic syndrome, albuminuria with podocyte foot-process fusion, and cytoskeletal changes. Podocyte-specific PRR deletion also led to disturbed processing of multivesicular bodies and enrichment of autophagosomal (LC3) and lysosomal (LAMP2) markers, indicating a functional block in autophagosome-lysosome fusion and an overload of the proteasomal protein-degradation machinery. In vitro, PRR knockdown and pharmacologic blockade of vacuolar H+-ATPases, which associate with the PRR, increased vesicular pH, led to accumulation of LC3-positive and LAMP2-positive vesicles and altered the cytoskeleton. Taken together, these results suggest that the PRR is essential for podocyte function and survival by maintaining autophagy and protein-turnover machinery. Furthermore, PRR contributes to the control of lysosomal pH, which is important for podocyte survival and cytoskeletal integrity.
Glomerular diseases lead to a progressive impairment of renal function and account for the majority of end-stage kidney diseases. Within the glomerulus, podocytes build a complex framework. They tightly envelope the glomerular capillaries with their foot processes and are responsible for maintaining the renal filtration barrier. Podocyte injury disrupts their actin cytoskeleton, leading to foot process effacement and molecular reorganization of the slit diaphragm accompanied by urinary protein loss.1 Moreover, recent studies indicate that podocyte damage is a key determinant of proteinuric glomerular diseases and the loss of podocytes predicts disease progression.2,3 However, the mechanisms responsible for podocyte maintenance and well-being are not yet fully elucidated.
The prorenin receptor (PRR/ATP6ap2) is located on the X chromosome and encodes for a 37-kD single transmembrane protein, which is ubiquitously expressed in mammals. Besides the central nervous system, heart, intestine, and germ-line organs, PRR is highly expressed in the kidney, especially in podocytes, implying an important cell-specific function.4–6 The extracellular domain binds renin and prorenin, leading to increased catalytic activity of these molecules and subsequent local angiotensin (Ang) II production.5 Moreover, prorenin and renin can activate ERK1/2 and p38 MAP kinase signaling via the PRR, independent of Ang II.5,7
Initially, the PRR was reported to be a newly discovered renin-angiotensin system component.5 Hirose et al. demonstrated that PRR gene polymorphisms are associated with hypertension in Japanese men and suggested that PRR has a role in BP regulation.8 Ichihara et al. found that PRR blockade with a so-called handle-region peptide ameliorated cardiac and renal damage in several rodent models,9 although others were not able to confirm these findings.10 The specificity of the PRR-blocking peptide is also unclear. Several groups failed in their attempts to generate PRR-null mice, possibly because PRR-deficient embryonic stem cells do not form chimeras after blastocyst injection.6 Similarly, PRR inhibition by RNA interference or morpholinos in Caenorhabditis elegans11 and zebrafish12 yields embryos that die before the end of embryogenesis, indicating an essential, but still unknown, cellular function for PRR. The generation of conditional knockout mice circumvents the embryonic lethality and permits studying the pathophysiological role of PRR. Surprisingly, Ichihara et al. demonstrated that cardiomyocyte-specific PRR deletion resulted in early mortality due to disturbed cardiac tissue integrity,13 in sharp contrast to the organ-protective effect of the putative PRR-blocking handle region peptide.9 This finding suggests that the observed effects of PRR deletion were independent of the renin-angiotensin system.
Remarkably, a 8.9-kD fragment of the PRR is associated with the vacuolar H+-ATPase (v-ATPase).14 These ATP-dependent proton pumps are involved in numerous cellular processes, including membrane trafficking, protein degradation, and coupled transport of small molecules.15 Interestingly, recent data suggest a functional correlation between the PRR and intravesicular acidification, leading to impaired Wnt signaling.16 The role of PRR in podocytes for maintaining renal function is not known. Here we demonstrate that podocyte-specific PRR inactivation leads to severe glomerular damage, renal failure, and death by the age of 3 wk. Our study also suggests that the PRR may be generally essential for protein turnover, cytoskeletal integrity, and autophagy homeostasis.
RESULTS
Podocyte PRR Deletion Causes Nephrotic Syndrome and Acute Kidney Injury
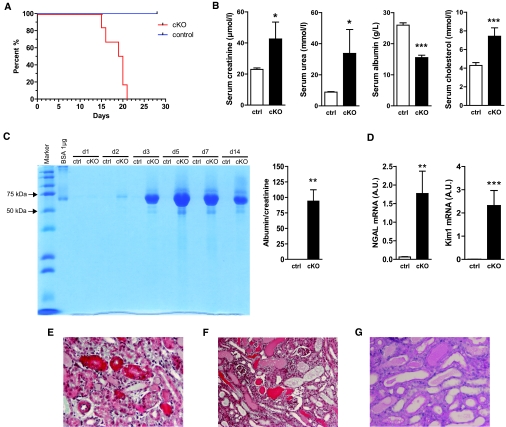
Generation of conditional podocyte-specific knockout mice (cKO; Cre+/o x PRRflox/Y) is shown in detail in Supplemental Figure 1. Conditional KO mice were born at normal Mendelian frequencies. Inactivation of the PRR gene resulted in premature death of cKO mice between 2 to 3 wk after birth (Figure 1A). The cKO mice developed nephrotic syndrome with increased levels of serum creatinine, serum urea, and serum cholesterol, as well as decreased serum albumin measured by postnatal age day 14 (Figure 1B). Moreover, albuminuria occurred already at day 2 in cKO (Figure 1C), indicating severe filtration barrier impairment and podocyte damage. At day 14, albumin-creatinine ratio was dramatically increased in cKO (Figure 1C). Furthermore, neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule (Kim)-1 were 28-fold and 258-fold increased, respectively (Figure 1D). Histologic analysis at postnatal day 14 revealed massive protein enrichment in proximal tubular epithelial cells (Figure 1E), protein casts in tubules, tubular dilation (Figure 1F), and loss of brush borders (Figure 1G).
Figure 1.
Conditional PRR deletion (cKO) leads to congenital nephritic syndrome and acute kidney injury. (A) Kaplan-Meier survival curve of control (ctrl) and conditional knockout (cKO) animals (n = 12 control and n = 6 cKO mice were analyzed). (B) Serum creatinine at age day 14 was doubled, serum urea was tripled, serum albumin was decreased by 40%, while serum cholesterol was increased by 70% (n = 10 control and n = 5 cKO mice). (C) Urine SDS PAGE (Coomassie) shows marked albuminuria of cKO mice at age 2, 3, 5, 7, and 14 d (two μL of urine from each mouse and albumin standards were loaded). Urine albumin-to-creatinine ratio measured at day 14 was significantly increased in cKO animals. (D) Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (Kim1) were markedly upregulated in cKO kidneys (E) Masson trichrome staining of kidney sections show tubular protein accumulation, (F) cast formation with tubular dilation, as well as (G) dilated tubules with flattened epithelium (Periodic Acid-Schiff stain; n = 10 control and n = 5 cKO mice). Magnification: ×20 in E and G; ×10 in F.
PRR Deletion in Podocytes Impairs Vacuolar Acidification Leading to Abnormal Cytoskeleton Organization and Cell Death
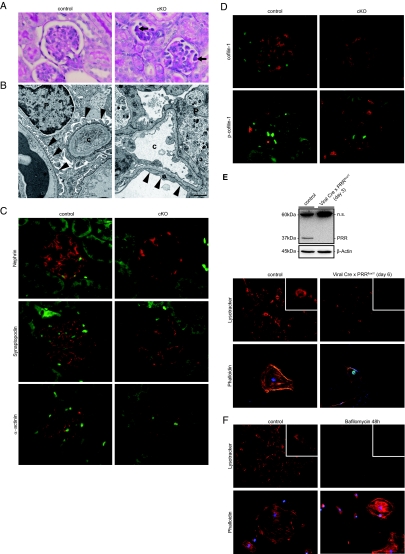
To further study the severe kidney damage observed, we performed closer analysis of renal glomeruli. Histologic analyses at day 14 demonstrated that glomeruli from cKO mice showed PAS-positive enrichment indicating protein deposition in podocytes without major changes in glomerular mesangial expansion or glomerular vascular pathology (Figure 2A). Electron microscopy (EM) demonstrated that podocytes from cKO mice showed foot processes effacement (Figure 2B), a hallmark of glomerular injury leading to proteinuria.17 There were no major alterations of glomerular basement membrane diameter observed. The early onset proteinuria accompanied by podocyte foot process fusion prompted us to analyze podocyte-specific markers and cytoskeletal dynamics. In glomeruli from control mice, nephrin staining demonstrated a linear pattern along the glomerular basement membrane corresponding to its localization in the slit diaphragm. Glomeruli from cKO mice showed sharply reduced nephrin expression (Figure 2C). Synaptopodin is present in completely differentiated podocytes and modulates the actin-based shape and motility. Synaptopodin expression was also dramatically reduced in cKO glomeruli (Figure 2C). We next analyzed the expression of α-actinin, an actin-associated protein that links the slit diaphragm to the actin cytoskeleton. The distribution of α-actinin was significantly reduced in cKO glomeruli (Figure 2C). Actin-binding proteins regulate assembly and disassembly of actin filaments. Cofilin belongs to the ADF/cofilin family and has a 70% sequence homology to the actin-depolymerizing factor (ADF). Cofilin-1 is important for maintaining podocyte architecture.18 In its phosphorylated (inactive) state, cofilin-1 is not able to bind to actin, which consequently leads to impaired actin filament severing. In cKO glomeruli, we observed increased cofilin-1 phosphorylation, without distinct changes in total cofilin-1 (Figure 2D), indicating a regulatory influence of the PRR on cytoskeleton assembly and disassembly.
Figure 2.
PRR deletion leads to podocyte damage and cytoskeletal rearrangement by impairment of vesicular acidification. (A) Periodic Acid-Schiff stain shows glomuerlar protein accumulation (arrows). (B) EM shows normal epithelial cells and foot processes in control animals. cKO mice developed foot process fusion (c = capillary, p = podocyte). (C) Nephrin, synaptopodin, and α-actinin were absent or sharply reduced in cKO glomeruli. (D) Cofilin is an actin-binding protein. Its appearance is normal in cKO podocytes. However, its phosphorylation was markedly increased in cKO compared with control podocytes. (E) Cultured floxed podocytes treated with Cre recombinase adenoviral vector showed decreased PRR protein; increased vesicular pH, indicated by the decreased lysotracker signal; and altered cytoskeletal rearrangement, indicated by phalloidin staining compared with LacZ-treated controls. Lysotracker and phalloidin staining in red; nuclear Cre staining in green. (F) The same pattern occurred by treating control cells with the vacuolar ATPase inhibitor bafilomycin A1. Magnification: ×40 in A, C, and D; ×10 in E and F (Lysotracker); ×40 in E and F (Phalloidin). Insert shows a representative single cell. Scale bars: (B) 1 μm.
The v-ATPase plays a pivotal role in the control of cellular and intracellular vesicle pH.15 Zebrafish embryo mutants that lack PRR/ATP6ap2 and v-ATPase subunits display similar phenotypes,12 suggesting a possible functional link between the proteins. Recently, the PRR was implicated in vesicular acidification.13 However, the role of v-ATPases for podocyte function and physiology is unknown. We speculated that dysregulation of vesicular pH in podocytes might lead to podocyte malfunction with abnormal cytoskeleton organization. We treated primarily isolated floxed podocytes with adenoviral Cre recombinase, resulting in decreased PRR protein expression (Figure 2E), and then studied pH and cytoskeleton alterations. As hypothesized, we found that Cre-positive podocytes showed increased vesicular pH, indicated by a loss of the Lysotracker signal, which subsequently resulted in an altered cytoskeleton arrangement and podocyte morphology (Figure 2E). To underscore the notion that pH alterations lead to rearrangements of actin cytoskeleton, we treated immortalized normal murine podocytes with the potent v-ATPase inhibitor, bafilomycin A1. As expected, v-ATPase inhibition also resulted in a loss of Lysotracker signal and morphologic changes that were similar to those observed in our PRR-inactivated podocytes (Figure 2F). These findings support the idea that the PRR is essential for v-ATPase function in podocytes and that disturbances in v-ATPase activity lead to cytoskeletal disruption.
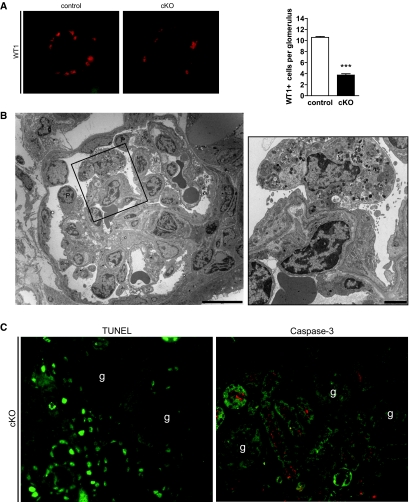
Besides changes in podocyte morphology, we discovered a massive loss of Wilms tumor (WT)-1 positive cells (Figure 3A) indicating podocyte cell death. Interestingly, EM showed cell destruction by cell lysis, with massive vacuole formation consistent with a nonapoptotic cell death (Figure 3B). Furthermore, our in vivo glomerular analysis did not identify TUNEL-positive cells or evidence of caspase-3 activation, whereas these markers were detectable in tubules of cKO (Figure 3C). Blocking of vesicular acidification with bafilomycin in cultured podocytes also resulted in enhanced cell death (Supplemental Figure 2), which could only marginally inhibited by a potent pan-caspase inhibitor q-VD-OPh.19 These observations support the conclusion that apoptosis was not the main mechanism leading to cell death induced by impaired vesicular acidification in our experiments. Our EM data, the lack of DNA fragmentation, and absent caspase activation in PRR-deficient podocytes, as well as in cells subjected to v-ATPase inhibition, all point to cell necrosis rather than apoptosis as the cause for podocyte loss.
Figure 3.
Deletion of PRR leads to podocyte cell death. (A) In cKO glomeruli, the number of Wilms tumor protein (WT1)-positive nuclei was markedly reduced compared with control cells. (B) EM showed cKO podocyte (p = podocyte) cytolysis. (C) Immunofluorescence of cKO kidney sections showed massively enhanced DNA fragmentation, measured by TUNEL assay in tubular epithelial cells, whereas no fragmentation occurred in glomeruli (marked with “g”). Furthermore, staining for cleaved caspase-3 was only measurable in epithelial cells and not in glomeruli (n = 5 animals were analyzed). Magnification: ×40 in A and C. Scale bars: (B left) 10 μm; (B right) 2 μm.
PRR Deletion Impairs Autophagy
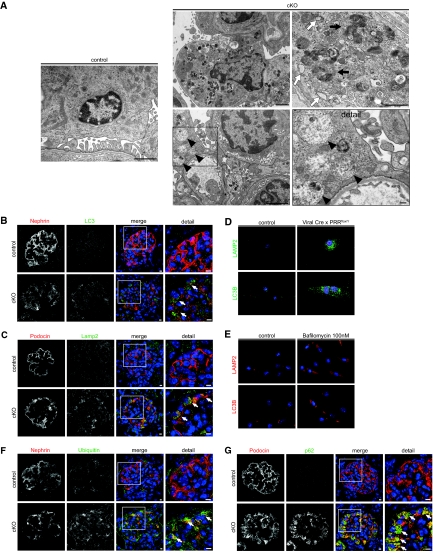
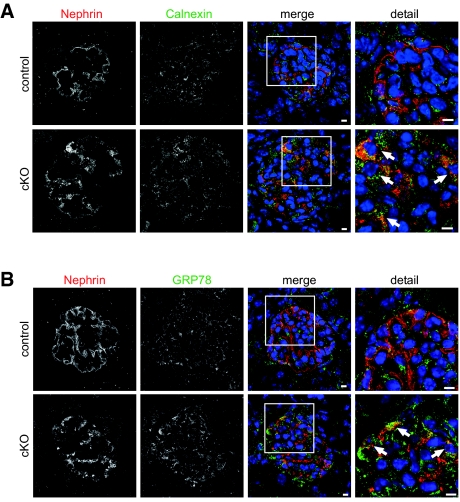
Autophagy is an essential cellular mechanism that has been shown to fulfill relevant functions for podocyte integrity and physiology.20 The digestion of proteins in this system is dependent on correct intravesical pH. We therefore investigated the influence of PRR deletion on autophagy. EM revealed extensive vacuolar degeneration of podocyte cell bodies with an accumulation of multivesicular bodies, lysosomes, and autophagolysosomes (Figure 4A). Many of the vacuoles consisted of expanded endoplasmic reticulum (ER) lumens (Figure 4A). Further analysis of glomeruli showed an enrichment of the autophagosomal marker LC3 (Figure 4B) and the lysosomal marker LAMP2 (Figure 4C) in podocytes of cKO, indicating a processing defect of autophagosomes and lysosomes. To further elucidate the causal role of PRR and v-ATPase activity on autophagy impairment, we inactivated primary floxed podocytes by adenoviral Cre recombinase and treated cultured murine podocytes with bafilomycin A1. In both cases, we showed excessive accumulation of LC3-positive and LAMP2-positive vesicles (Figure 4, D and E). In cKO glomeruli, we observed enhanced formation of ubiquitinated protein (Figure 4F) and a dramatic accumulation of p62/SQSTM1 (Figure 4G), an ubiquitin-binding scaffold protein. The accumulation of p62 in cKO particularly suggests an abnormal accumulation of toxic proteins and organelles, which might overwhelm any compensatory attempt of the proteasome degradation pathway to counter-regulate the podocyte disturbance. A mechanism to compensate for damaged protein overload is endoplasmic reticulum (ER) stress, which might have led to irreversible podocyte injury and loss. This state-of-affairs distinguishes podocytes from other renal cells that are able to proliferate. We found that in PPR-deficient podocytes, dilated ER lumens stained positive for calnexin indicating increased ER stress (Figure 5A), which was confirmed by the increased detection of the ER stress marker GRP78 (Figure 5B).
Figure 4.
Conditional PRR deletion (cKO) impairs autophagy and protein degradation by inhibiting vesicular acidification. (A) EM shows healthy podocytes and foot processes. EM of 14-d cKO mouse podocytes shows massive vacuolar accumulation with multivesicular bodies (black arrowheads), rough ER (white arrows), and lysosomes (black arrows). Immunofluorescence shows accumulation of (B) autophagy marker LC3B and (C) the lysosomal marker LAMP2 in cKO podocytes (arrows indicate positively stained podocytes). (D) In vitro, treatment of PRR floxed podocytes for 6 d with Cre recombinase adenoviral vector led to accumulation of LC3B- and LAMP2-positive structures; controls were treated with adenoviral LacZ (n = 3 independent experiments). Cre positivity was confirmed by costaining (red signal) with an appropriate antibody. (E) Similar results were obtained by treatment of murine podocytes with bafilomycin A1 or vehicle (DMSO) for 48 h at the indicated concentration. (F) Ubiquitin and (G) the autophagy adaptor protein p62 were strongly upregulated in cKO podocytes. Scale bars: 100nm (A, detail) 1 μM (A, any others); 5μm (B, C, F, G). Magnification: ×40 in D; ×20 in E.
Figure 5.
PRR deletion leads to increased ER stress in podocytes. At day 14 postnatal, cKO podocytes showed increased staining for (A) the ER marker calnexin and (B) GRP78 (arrows indicate podocytes stained positive for GRP78). Scale bars: 5 μm.
DISCUSSION
We provide the first evidence that podocyte-specific PRR knockout mice develop congenital nephrotic syndrome and begin to die at day 14. We could show that the PRR is essential for podocyte survival by maintaining the protein turnover machinery, indicating a functional relationship between the PRR, vacuolar acidification, and the malfunction of autophagy. Podocyte-specific deletion of the PRR impairs vesicular acidification, leading to an abnormal cytoskeleton organization as well as impaired lysosomal processing, defective autophagocytosis with the generation of dysfunctional autophagolysosomes—as supported by p62 accumulation—ER stress, and impaired multivesicular body disposition.
Our data support a fundamental role of the PRR in cellular physiology, since its deletion in podocytes causes loss of cell function, cell integrity, and leads to an early death from renal failure rather than a role in modulating the renin-angiotensin system. Further evidence for an Ang II-independent PRR function stems from a recent study by Cruciat et al. demonstrating that the PRR is essential for canonical Wnt/β-catenin signaling.16 Nevertheless, we believe that the severe phenotype in our podocyte-specific PRR knockout mice is probably not related to a defect in Wnt/β-catenin signaling, since podocyte-specific β-catenin knockout mice do not develop obvious renal damage without additional injury models, nor do they die prematurely.21
In our study, we found that cKO animals develop proteinuria, which is correlated with massive foot process effacement. These results support a functional link between PRR deletion and cytoskeleton alterations, which may be related to impaired v-ATPase function. Supporting this notion is the recent finding that v-ATPase subunit C binds to and induces cross-linking of actin filaments.22 Furthermore, cofilin-1 deficient mice show persistent proteinuria and impaired podocyte architecture.18 Podocytes are highly differentiated cells with a very complex cellular architecture, resembling that found in neurons.23 The PRR is also highly expressed in the nervous system.5 Interestingly, the first description of the PRR in human disease did not involve its role in the renin-angiotensin system but demonstrated an exon-4 splice mutation in the PRR gene that was linked to mental retardation.24 A growing body of evidence, largely from neurodegenerative diseases, supports a link between cellular transport defects and altered actin cytoskeletal organization.25 Moreover, phosphorylation (inactivation) of cofilin in hippocampal neurons leads to dystrophic morphologic changes, as well as to cell death, and appears to be a potential mechanism of neuronal dystrophy in Alzheimer's disease.26 Thus, it is tempting to speculate that cofilin in podocytes could also be, at least in part, responsible for cytoskeleton rearrangements and foot process effacement, due to its enhanced phosphorylation/inactivation.
In eukaryotes, protein degradation is mainly orchestrated by two major mechanisms, namely, the ubiquitin-proteasome system and macroautophagy (autophagy).27 Autophagy is an essential and evolutionarily conserved cellular process that capacitates the cell to get rid of superfluous or damaged organelles and misfolded cytosolic proteins, and is a central biologic pathway that functions to promote health.28 Autophagy represents the major lysosomal degradation pathway and, unlike proteasomal degradation, is believed to be largely nonspecific. Autophagy involves the sequestration of cytoplasmic regions within an enveloping double-membrane structure to form an autophagosome. Damaged proteins and organelles are delivered to the lysosome for pH-dependent degradation by lysosomal hydrolases.27 Disturbances in either protein degradation system ultimately leads to impaired elimination of toxic and malfunctioning proteins, which can lead to cellular disintegration and disease.27
We provide first evidence that the PRR is essential for podocyte survival by maintaining protein degradation machinery, even in early renal development. In general, postmitotic podocytes are particular dependent on fine-tuned protein degradation mechanisms. Recently, Hartleben et al. showed that podocytes exert high levels of autophagy under basal conditions.20 Furthermore, the group showed that loss of autophagosome-forming capability in podocytes leads to impaired podocyte function and glomerular disease in aging mice.20 Atg5 is a protein specifically required for autophagy. Atg5-deficient mice developed a late onset of impaired autophagy leading to mild glomerular disease.20 In contrast, we already identified an early onset of failed autophagy in our cKO mice, which may be due to the deficiency of the PRR already in embryonic stages of the developing kidney in our model. We observed a functional relationship between the PRR, vacuolar acidification, and a malfunction of autophagy. Several mechanisms could have contributed to the severe phenotype, including impaired lysosomal processing; defective autophagocytosis with the generation of dysfunctional autophagolysosomes, as supported by p62 accumulation; and finally, a disturbed processing of multivesicular bodies. In this context, evidence emerges that cytoplasmic endosomes execute functions that may also maintain intracellular signaling cascades. As recently demonstrated, multivesicular bodies are involved in Wnt signaling by inactivating Glykogen Synthase Kinase 3 (GSK-3) through incorporation, a crucial step in the canonical Wnt pathway.29
Furthermore, our results may go beyond the demonstration of a link between impaired autophagy, cytoskeleton dysfunction, and podocyte cell death. Lee et al. recently demonstrated that Alzheimer's-disease-related protein presenilin-1 is essential for v-ATPase targeting to lysosomes, lysosome acidification, and proteolysis during autophagy. Presenilin-1 mutations lead to pathogenic protein accumulation and neuronal cell death.30 In our study, we were not able to elucidate the precise mechanism involved in how the PRR affects v-ATPase function. Nevertheless, we speculate that the PRR could be involved in the trafficking of v-ATPase to lysosomes, and therefore for lysosome acidification and proteolysis during autophagy. We propose a new model of podocyte survival and protection, where the PRR affects intravesicular acidification and protects cells against disturbed protein degradation or protein turnover due to impaired autophagy. We suggest that PPR deficiency leads to intensified ER stress, massive accumulation of unprocessed proteins, and finally, podocyte death. We observed features similar to those found in neurodegenerative diseases or with advanced aging. Our findings underscore the importance of the PRR in maintaining intravesicular acidification and, most likely, v-ATPase function in developing podocytes. Impaired v-ATPase function leads to impaired autophagy and podocyte death. Together with the findings by Hartleben and colleagues,20 our results suggest that impaired protein degradation is an important pathologic process in podocytes.
CONCISE METHODS
Mice
Female mice, in which loxP sites flanked exon 2 of the PRR/ATP6ap2 loxP gene, were bred with male mice expressing the Cre recombinase under control of the podocin promoter. Since the PRR gene is located on the X chromosome, the resulting male Cre+/o x PRRflox/Y mice (Supplemental Figure 1) represent homozygous podocyte-specific PRR knockout mice (cKO). Male Cre+/o littermates (control) were used to exclude the possibility that Cre-mediated effects were responsible for phenotypic differences. All mice were from a pure C57BL/6 background. Local authorities approved the studies and American Physiologic Society guidelines for animal care were followed.
Genotyping, Serum, Protein, and RNA Analysis
See supplemental online data for detailed protocols on genotyping procedures, measurement of serum parameters, Western blotting, immunofluorescence, and real-time PCR analysis.
Electron Microscopy
Small pieces of the renal cortex were fixed with 4% formaldehyde/2.5% glutaraldehyde in 0.1 M cacodylate buffer for 3 d. Samples were stained with 1% OsO4 for 2h, dehydrated in a graded ethanol series and propylene oxide, and embedded in Poly/BedR 812 (Polysciences, Inc., Eppelheim, Germany). Ultrathin sections were contrasted with uranyl acetate and lead citrate, and examined with a FEI Morgagni electron microscope. Digital images were taken with a Morada CCD camera and the iTEM software (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
Glomeruli Isolation and Primary Cell Culture
Glomerular isolation and primary cell culture were performed as described previously.31,32 Briefly, mice were anesthetized, the kidneys were perfused initially with ice-cold PBS via the abdominal aorta, and afterwards with Dynabeads (diameter 4.5 μm; Invitrogen, Karlsruhe, Germany). Kidneys were removed, minced, and digested with collagenase A (Roche, Grenzach, Germany). Digested kidney tissue was further sieved through a 100-μm cell mesh with intermittent PBS. Using a magnet catcher and a subsequent washing procedure, the Dynabead-containing glomeruli were purified until an approximate purity of 95% determined microscopically (Zeiss Axiovert, Germany) was achieved.
Glomeruli were transferred into a collagen I-coated T75 cell culture flask and cultured in primary cell culture medium for 4 to 5 d (37 °C, 5% CO2) until glomeruli were surrounded by a small halo of primary cells. In passage one, glomeruli and cells were trypsinized (0.05% trypsin, 0.02% EDTA in PBS), and glomeruli containing magnetic particles and excessive magnetic particles were removed via magnet catcher. Cells were centrifuged at 1100 RPM for 5 min to remove excessive trypsin, and primary cells were transferred to a collagen I-coated T75 cell culture flask with primary cell culture medium. Percentage of primary podocyte cells was approximately 80% (confirmed microscopically). With each passage, primary cells were transferred to a collagen I-coated T75 cell culture flask with primary cell culture medium. Cells were split once confluence reached approximately 70%. Passage 3 of each primary podocyte cell line (PRRflox/Y or WT) was used for viral transduction (for details, please see the supplemental data).
Immortalized Podocyte Cell Culture
Dr. Peter Mundel (Harvard University, Boston, Massachusetts) generously provided immortalized murine podocytes. Immortalized murine podocyte were cultured as described.33 Briefly, podocytes were grown under permissive temperature in the presence of IFN-γ 10 U per ml on type I collagen. To induce differentiation, the cells were maintained at 37 °C without IFN-γ for 10 to 14 d.
Statistics
Data are presented as means ± SEM. Statistically significant differences in mean values were tested by two-tailed t tests. A value of P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Petra Berkefeld, Blanka Duvnjak, Ilona Kamer, May-Britt Köhler, Jutta Meisel, and Astrid Schiche for their excellent technical assistance. The Deutsche Forschungsgemeinschaft (MU1467/3 to 1), the Novartis Foundation, and the Excellence Initiative of the German Federal and State Governments (EXC 294 to TBH) all supported this work. The authors are not aware of interest conflicts.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Prorenin Receptor: What's in a Name,” on pages 2141–2143.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H: Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med 86: 629–635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sihn G, Rousselle A, Vilianovitch L, Burckle C, Bader M: Physiology of the (pro)renin receptor: Wnt of change? Kidney Int 78: 246–256, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Saris JJ, 't Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH: Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, Sugimoto K, Katsuya T, Ohkubo T, Hashimoto J, Rakugi H, Takahashi K, Imai Y: Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: The Ohasama study. Am J Hypertens 22: 294–299, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen G, Muller DN: The biology of the (pro)renin receptor. J Am Soc Nephrol 21: 18–23, 2010 [DOI] [PubMed] [Google Scholar]
- 11. WormBase web site, http://www.wormbase.org, release WS206, October 4, 2009
- 12. Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N: Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A 101: 12792–12797, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H: The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schagger H: Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Forgac M: Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 17. D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB: Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem 285: 22676–22688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL: Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8: 345–352, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vitavska O, Merzendorfer H, Wieczorek H: The V-ATPase subunit C binds to polymeric F-actin as well as to monomeric G-actin and induces cross-linking of actin filaments. J Biol Chem 280: 1070–1076, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ramser J, Abidi FE, Burckle CA, Lenski C, Toriello H, Wen G, Lubs HA, Engert S, Stevenson RE, Meindl A, Schwartz CE, Nguyen G: A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet 14: 1019–1027, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Maloney MT, Bamburg JR: Cofilin-mediated neurodegeneration in Alzheimer's disease and other amyloidopathies. Mol Neurobiol 35: 21–44, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Heredia L, Helguera P, de Olmos S, Kedikian G, Sola Vigo F, LaFerla F, Staufenbiel M, de Olmos J, Busciglio J, Caceres A, Lorenzo A: Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: A potential mechanism of neuronal dystrophy in Alzheimer's disease. J Neurosci 26: 6533–6542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizushima N, Levine B, Cuervo AM, Klionsky DJ: Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine B, Kroemer G: Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM: Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA: Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141: 1146–1158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sitek B, Potthoff S, Schulenborg T, Stegbauer J, Vinke T, Rump LC, Meyer HE, Vonend O, Stuhler K: Novel approaches to analyse glomerular proteins from smallest scale murine and human samples using DIGE saturation labelling. Proteomics 6: 4337–4345, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Potthoff SA, Sitek B, Stegbauer J, Schulenborg T, Marcus K, Quack I, Rump LC, Meyer HE, Stuhler K, Vonend O: The glomerular proteome in a model of chronic kidney disease. Proteomics Clin Appl 2: 1127–1139, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Shankland SJ, Pippin JW, Reiser J, Mundel P: Podocytes in culture: Past, present, and future. Kidney Int 72: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.