Abstract
The prorenin receptor is an accessory subunit of the vacuolar H+-ATPase, suggesting that it has fundamental functions beyond activation of the local renin-angiotensin system. Podocytes express the prorenin receptor, but its function in these cells is unknown. Here, podocyte-specific, conditional, prorenin receptor-knockout mice died of kidney failure and severe proteinuria within 4 weeks of birth. The podocytes of these mice exhibited foot process effacement with reduced and altered localization of the slit-diaphragm proteins nephrin and podocin. Furthermore, the podocytes contained numerous autophagic vacuoles, confirmed by enhanced accumulation of microtubule-associated protein 1 light chain 3-positive intracellular vesicles. Ablation of the prorenin receptor selectively suppressed expression of the V0 c-subunit of the vacuolar H+-ATPase in podocytes, resulting in deacidification of intracellular vesicles. In conclusion, the prorenin receptor is important for the maintenance of normal podocyte structure and function.
The prorenin receptor (PRR) has been identified as a receptor for renin and prorenin. Activation of the PRR is involved both in the production of local angiotensin I from angiotensinogen as a result of nonproteolytic conformational changes in prorenin and in intracellular signaling. We have demonstrated that these two pathways contribute to the development of kidney damage in diabetes mellitus1–4 and essential hypertension.5 On the basis of these lines of evidence, deletion of the PRR had been expected to be beneficial and protective against end-organ damage. Recently, however, we uncovered a role for the PRR as an accessory subunit for vacuolar H+-ATPase (V-ATPase), ATP6 accessory protein 2 (ATP6AP2). This finding implies that PRR plays essential roles in fundamental cellular functions as a proton pump subunit, as well as the cell surface receptor for renin and prorenin.6
Within the kidneys, podocytes are highly specialized postmitotic cells. Podocytes, together with endothelial cells and the glomerular basement membrane (GBM), maintain the filtration barrier and the normal structure of the glomerular capillary, are involved in the remodeling of the GBM and the endocytosis of filtered proteins, and counteract intracapillary hydrostatic pressure.7–9 Podocyte dysfunction results in the development of proteinuria and a number of glomerular diseases. The PRR is also expressed in podocytes, where it is involved in both tissue angiotensin II production and PRR-mediated intracellular signaling.10,11 Previously, we demonstrated that overexpression of human PRR in rats caused slowly progressive proteinuria and glomerular sclerosis, which suggests that PRR-mediated signaling is involved in the development of glomerular diseases.12 However, the physiologic role of the PRR in podocytes has not yet been fully understood. In this study, we show that PRR/ATP6AP2 is essential for the maintenance of normal structure and function in murine podocytes.
RESULTS
To investigate the role of the PRR in podocytes, Atp6ap2-floxed mice were crossed with mice that expressed the Cre recombinase under the control of the podocyte-specific podocin (NPHS2) promoter.13 The resulting podocyte-specific conditional PRR-knockout (CKO) mice were born at the expected Mendelian frequency, without any gross renal anomalies noted in the newborn mice, although podocyte-specific ablation of Atp6ap2 inevitably resulted in lethal renal failure, with mice dying within 4 weeks of birth (Figure 1A). Twenty-four-hour urine collection on postnatal day (P) 21 revealed that, compared with wild-type (WT) mice, CKO mice developed significantly greater proteinuria (3.01 ± 5.00 versus 85.13 ± 29.67 mg/mg creatinine, respectively; n = 10; P < 0.001) and albuminuria (0.10 ± 0.06 versus 94.17 ± 19.42 mg/mg creatinine, respectively; n = 10; P < 0.001). Consistent with these findings, blood chemistry analysis on P21 demonstrated that, compared with WT mice, CKO mice had significantly lower serum total protein (2.87 ± 0.28 versus 1.59 ± 0.51 g/dl, respectively; n = 10; P < 0.001), lower serum albumin (2.54 ± 0.21 versus 1.25 ± 0.30 g/dl, respectively; n = 10; P < 0.001), higher total cholesterol (45.2 ± 11.9 versus 165.1 ± 34.7 mg/dl, respectively; n = 10; P < 0.001), and higher serum creatinine (0.14 ± 0.04 versus 0.56 ± 0.18 mg/dl, respectively; n = 10; P < 0.001), indicating that the CKO mice were suffering from nephrotic syndrome and consequently died of renal failure (Figure 1B).
Figure 1.
Podocyte-specific ablation of Atp6ap2 inevitably caused renal failure. (A) Kaplan-Meier survival curves showing lethality within 4 weeks of birth in the conditional prorenin receptor-knockout (CKO) group. (B) Biochemical analysis of urine and plasma showing renal failure and severe renal protein wasting in CKO mice on postnatal day (P) 21. Cr, creatinine; TP, total protein; TC, total cholesterol; ns, not significant. The data are the means ± SD (n = 10). **P < 0.001 versus wild-type (WT) control.
Histologic examination of kidneys from CKO mice on P1 revealed that obvious anomalies were absent compared with kidneys from WT mice (Figure 2). On P7, focal glomerular changes comprising mesangial cell proliferation were evident in kidneys from CKO mice. On P14, diffuse glomerular changes, consisting of expansion of the mesangium matrix, segmental sclerosis, hypertrophic changes to parietal epithelial cells without crescent formations, and mild tubulointerstitial changes, such as tubular casts and dilation, were observed in kidneys from CKO mice. In addition, some of the podocytes appeared to be vacuolated. On P21, glomeruli from CKO mice exhibited diffuse and global mesangial sclerosis. Tubulointerstitial changes compatible with changes secondary to severe proteinuria were also evident, including tubular dilation, tubular atrophy with disappearance of the brush border, and extensive tubular casts. In contrast, the arteries and arterioles appeared to be intact, without endothelial dysfunction or any infiltration of inflammatory cells.
Figure 2.
Podocyte-specific deletion of the prorenin receptor caused glomerular sclerosis. Light microscopic examination of kidneys was performed from wild-type (WT) and conditional prorenin receptor-knockout (CKO) mice on postnatal day (P) 1, P7, P14, and P21 using periodic acid-Schiff and periodic acid-silver methenamine staining. No differences were evident on P1. On P7, focal glomerular changes, consisting of the proliferation of mesangial cells, appeared in kidneys from CKO mice. On P14, diffuse glomerular changes, such as proliferation of the mesangial matrix, segmental sclerosis, hypertrophic changes of parietal epithelial cells without crescent formations, and mild tubulointerstitial changes, such as tubular casts and dilation, were observed in kidneys from CKO mice. On P21, kidney glomeruli from CKO mice revealed diffuse and global mesangial sclerosis. Tubulointerstitial changes, including tubular dilation, tubular atrophy with disappearance of the brush border, and extensive tubular casts were prominent. Scale bars, 100 μm (left panel), 20 μm (center and right panels). Arrowheads indicate tubular casts. The asterisks indicate mesangial expansion and sclerosis.
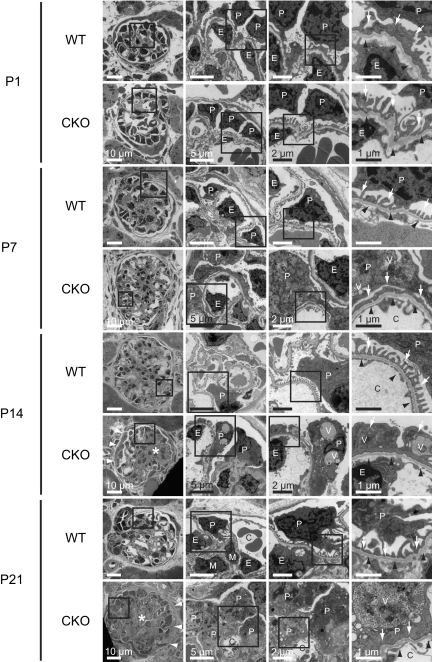
Electron microscopic examination of kidneys from CKO mice on P1 showed that there were no obvious differences compared with WT mice (Figure 3). As early as P7, kidneys from CKO mice were found to contain prominently enlarged podocytes with extensive foot process effacement and actin filament aggregation. A number of electron-dense autophagic vacuoles containing partially digested cellular components were observed in the cytoplasm of these podocytes. In addition, mesangial matrix expansion was noted in CKO glomeruli, as was appreciated in the light microscopic examination. Ultimately, on P21, the capillary lumen of kidneys from CKO mice collapsed because of the presence of highly vacuolated podocytes and mesangial expansion. Compared with kidneys from WT mice, kidneys from CKO mice exhibited significantly hypertrophic podocytes (podocyte size, 19.2 ± 5.6 versus 76.8 ± 27.7 μm2, respectively; P < 0.001). The thickness of glomerular basement membranes was similar between WT and CKO mice (0.18 ± 0.03 versus 0.19 ± 0.02 μm, respectively; P = 0.768), without any electron-dense deposits in any region of the glomeruli.
Figure 3.
The prorenin receptor-deleted podocytes developed foot process effacement and intracellular vacuoles. Electron microscopic examination of kidneys was performed from wild-type (WT) and conditional prorenin receptor-knockout (CKO) mice on postnatal day (P) 1, P7, P14, and P21. On P1, there were no obvious differences between WT and CKO mice. As early as P7, kidneys from CKO mice were found to contain prominently enlarged podocytes with extensive foot process effacement and actin filament aggregation. Numerous electron-dense autophagic vacuoles containing partially digested cellular components were observed in the cytoplasm of these podocytes. Mesangial matrix expansion was also noted in CKO glomeruli. Ultimately, on P21, the capillary lumen of kidneys from CKO mice had collapsed because of the presence of highly vacuolated podocytes and mesangial expansion. Basement membranes were intact without any electron-dense deposits in any of the glomeruli. Scale bars, 10 μm (left panels), 5 μm (left center panels), 2 μm (right center panels), and 1 μm (right panels). C, glomerular capillary; E, endothelial cells; P, podocytes; V, vesicular structure. Arrows indicate foot processes. White arrowheads indicate hypertrophic change of parietal epithelial cells. Black arrowheads indicate glomerular basement membrane. Asterisks indicate mesangial expansion and sclerosis.
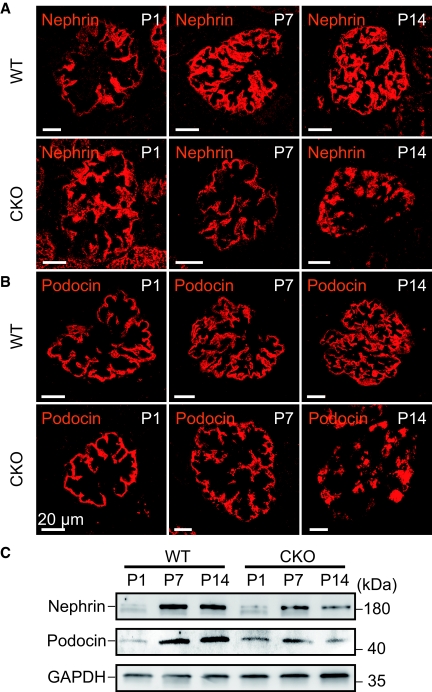
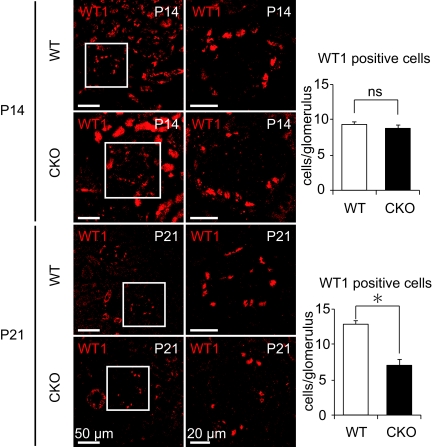
Consistent with the electron microscopic findings of podocytes in kidneys from CKO mice, immunofluorescence staining and immunoblotting analyses revealed a time-dependent reduction in the expression of nephrin and podocin, in addition to altered expression patterns for both proteins (Figure 4, A through C). Initially, immunofluorescence staining for nephrin and podocin, slit-diaphragm proteins crucial for filtration barrier function whose defects are associated with human congenital nephrotic syndrome,14,15 was positive along the GBM. However, over the course of development in CKO mice, the expression of nephrin and podocin was reduced and seen only in the cytosol of podocytes. Immunohistochemical analysis of glomeruli from CKO mice revealed that, on P14, the number of cells positive for WT1, a nuclear marker for podocytes, was similar to that in WT mice, but that by P21 the number of WT1-positive cells was significantly less in CKO compared with WT mice. These observations imply increased podocyte cell death or detachment from the GBM (Figure 5).
Figure 4.
Reduced expression and altered localization of slit-diaphragm proteins nephrin and podocin in the podocyte-specific prorenin receptor deleted kidney. (A and B) Immunofluorescent staining of nephrin (A) and podocin (B) was performed on postnatal day (P) 1, P7, and P14 in wild-type (WT) and conditional prorenin receptor-knockout (CKO) mice. Over the course of development, the localization of nephrin and podocin shifted from the glomerular basement membrane to podocyte cell bodies in glomeruli from CKO mice. (C) Nephrin and podocin expression were significantly decreased in kidneys from CKO mice on P1, P7, and P14. Scale bars, 20 μm.
Figure 5.
The significant podocyte loss by the prorenin receptor deletion. Immunofluorescent staining of kidneys was performed from wild-type (WT) and conditional prorenin receptor-knockout (CKO) mice on postnatal day (P) 14 and P21 for the podocyte nuclear marker WT1. The number of WT1-positive podocytes in glomeruli from CKO mice was similar to that in WT mice on P14 but decreased significantly in glomeruli from CKO mice on P21, probably reflecting increased cell death or detachment from glomerular basement membrane of podocytes. Scale bars, 50 μm (left panels), 20 μm (right panels). Graphed data show the means ± SD. *P < 0.05 compared with WT control.
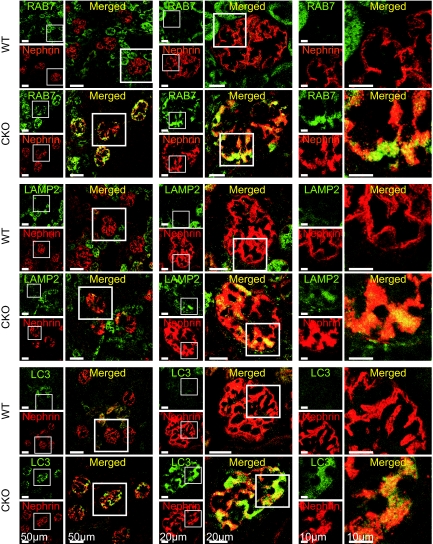
On P14, the affected glomeruli from CKO mice were positive for the late endosomal/lysosomal markers RAB7 and lysosomal-associated membrane protein 2 (LAMP2). In addition, glomeruli from CKO mice showed increased expression of microtubule-associated protein 1 light chain 3 (LC3), reflecting the accumulation of autophagosomes (Figure 6). Intriguingly, these markers were colocalized with nephrin in CKO mice, indicating that the localization of these vesicles corresponds with the podocytes.
Figure 6.
Autophagosomes in the prorenin receptor-null podocytes. Immunofluorescent staining of kidneys was performed from wild-type (WT) and conditional prorenin receptor-knockout (CKO) mice on postnatal day (P) 14 for the late endosome and lysosome marker RAB7, lysosomal-associated membrane protein 2 (LAMP2), and the autophagosome marker light chain 3 (LC3). Glomeruli from CKO mice showed increased expression for RAB7, LAMP2, and LC3. Note that these markers are colocalized with nephrin, meaning that these markers correspond with vacuoles of podocytes. Scale bars, 50 μm (left panels), 20 μm (center panels), and 10 μm (right panels).
On the basis of these findings, the phenotype observed in CKO mice was expected to be explained by the function of PRR as ATP6AP2, which is associated with V-ATPase, rather than as a receptor for renin and prorenin. The V-ATPase is a large multisubunit complex consisting of a V1 segment, which is responsible for ATP hydrolysis, and a V0 segment located on the membrane that is responsible for the translocation of the proton from the cytosol to the organelle lumen or extracellular space. Thus, the V1V0 complex maintains an acidic luminal environment in intracellular vesicular compartments.16,17 The V-ATPase is found in various subcellular compartments, such as the trans-Golgi network, secretory vesicles, sorting endosomes, late endosomes, and lysosomes.18 V-ATPase-dependent acidification of organelles facilitates protein sorting, membrane fusion and trafficking, receptor-mediated endocytosis, and lysosomal protein degradation. Recently, we showed that the PRR is required for V-ATPase function.6 To examine the cellular mechanism underlying the podocytopathy in CKO mice in this study, we examined the role of the ATP6AP2 protein in the function of the V-ATPase using conditionally immortalized human cultured podocytes.
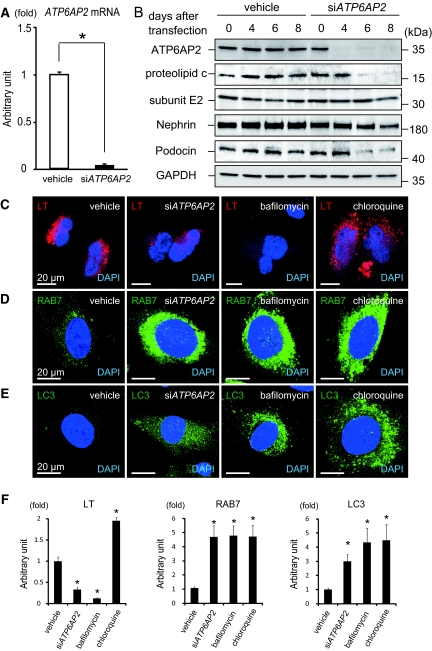
In vitro quantitative polymerase chain reaction (PCR) analysis 3 days after short interference RNA (siRNA) knockdown of ATP6AP2 in human cultured podocytes revealed that >90% of ATP6AP2 mRNA were missing from podocytes compared with levels in podocytes that had been treated with scrambled siRNA (vehicle; Figure 7A). Western blot analyses revealed that levels of the membrane-bound V0 c-subunit were significantly decreased in podocytes after ATP6AP2 siRNA transfection compared with levels in vehicle-treated podocytes (Figure 7B and Supplementary Figure 2A). In contrast, ATP6AP2 siRNA transfection had no effect on levels of the V1 E2-subunit. This is consistent with previous findings in mouse embryonic fibroblasts.6 It has been demonstrated that the conditionally immortalized human podocyte cell line expresses nephrin and podocin.19 In this study, nephrin and podocin expressions were reduced in podocytes after ATP6AP2 siRNA treatment (Figure 7B and Supplementary Figure 2, B and C). To examine the acidification of intracellular vesicular compartments, LysoTracker staining was performed. The LysoTracker is enriched in low-pH compartments or autophagic vacuoles, whereas decreased staining reflects high-pH compartments. Human cultured podocytes were treated with ATP6AP2 siRNA, as well as bafilomycin and chloroquine, pharmacologic inhibitors of the intracellular acid environment. Both ATP6AP2 siRNA and bafilomycin decreased LysoTracker staining, whereas chloroquine induced accumulation of LysoTracker (Figure 7, C and F). This observation is in agreement with previous studies demonstrating that chloroquine induces a transient increase in the staining of acidic vesicles with LysoTracker.20,21 Podocytes treated with ATP6AP2 siRNA, bafilomycin, and chloroquine had increased expression of RAB7 and LC3 (Figure 7, D through F). Together, these findings suggest that the loss of PRR affects the function of the V-ATPase, thereby compromising intracellular acidification and affecting the general structure and function in podocytes.
Figure 7.
ATP6AP2 is indispensable for the assembly of the vacuolar H+-ATPase (V-ATPase). (A) In human cultured podocytes, the expression of ATP6AP2 mRNA is reduced after transfection of ATP6AP2 short interference RNA (siATP6AP2). Graphed data show the means ± SD. *P < 0.05 compared with vehicle control. (B) Protein levels of ATP6AP2 and the c-subunit of the V0 segment of V-ATPase are significantly decreased in cultured podocytes after transfection of ATP6AP2 siRNA. Transfection of ATP6AP2 siRNA had no effect on the E2-subunit of V1. Note that nephrin and podocin expressions were reduced in podocytes after ATP6AP2 siRNA treatment. (C) Defective acidification in podocytes after knockdown of the ATP6AP2 gene and treatment with bafilomycin and chloroquine. The nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). (D and E) RAB7-positive vacuoles (D) and light chain 3 (LC3)-positive autophagic vacuoles (E) after treatment with ATP6AP2 siRNA, bafilomycin, and chloroquine. Scale bars, 20 μm. (F) Quantitative analysis of LysoTracker, RAB7, and LC3-positive vacuoles after treatment with vehicle, ATP6AP2 siRNA, bafilomycin, and chloroquine. Graphed data show the means ± SD. *P < 0.05 compared with vehicle control.
DISCUSSION
This study demonstrated both in vitro and in vivo that deletion of the PRR in podocytes resulted in a reduction in slit-diaphragm proteins and the accumulation of intracellular vacuoles because of disturbances to V-ATPase-mediated intracellular vesicular acidification, which culminated in severe proteinuric kidney disease and renal failure. On the basis of these observations, the PRR is essential for filtration barrier function as well as trafficking and degradation systems in murine podocytes.
The slit diaphragm undergoes permanent renewal processes to protect its stability in response to changes in filtration pressure. This would require constant reorganization of the podocyte foot process and renewal of slit-diaphragm components.22 Therefore, protein sorting and degradation events are important for the maintenance of cellular function in podocytes. Although the link between the V-ATPase and slit-diaphragm proteins in podocytes has not been investigated thus far, previous studies have demonstrated the physiologic relevance of V-ATPase activity in podocytes. For example, the V-ATPase a1-subunit has been demonstrated in podocytes, with the vesicles in podocyte foot processes associated with endocytosis and exocytosis.23 In addition, IgG that accumulates at the glomerular filtration barrier is actively taken up into the cytosol of podocytes by Fcγ receptor-mediated active endocytosis, one of the most important cellular processes associated with V-ATPase, such that the barrier is not blocked by filtered IgG. This implies that endocytic trafficking is active inside podocytes under physiologic conditions.24 Nephrin, a structural component of the glomerular slit diaphragm, is a single transmembrane-spanning receptor and also undergoes endocytosis, which affects slit diaphragm integrity.22,25,26 In this study, reduction and altered staining patterns of nephrin and podocin would be due to a general defect in a secretory system as a cause of the podocyte disorder. Accumulation of intracellular vesicles in podocytes clearly argues into this direction and does not imply a specific defect in trafficking of slit-diaphragm proteins as a cause of the disease. Thus, the changes in nephrin and podocin expressions induced by the PRR deletion may reflect general effects of the PRR on the health of the podocytes. Collectively, V-ATPase is crucial for fundamental cellular processes within podocytes, such as protein sorting, membrane fusion and trafficking, endocytosis, and lysosomal protein degradation.
There is recent evidence that V-ATPase-mediated acidification and PRR are essential for Wnt signaling27 and/or planar cell polarity signaling.28 The Wnt signaling is obviously important for kidney morphogenesis.29 In podocytes, the planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution.30 On the other hand, the Wnt signaling promotes podocyte dysfunction and albuminuria,31 which is ameliorated by paricalcitol.32 In this study, there was no obvious abnormality in development, polarity, or morphogenesis observed in CKO mice kidney. Future studies should address the issue of whether the PRR affects the Wnt signaling in podocytes.
In this study, ablation of the PRR in podocytes resulted in severe proteinuric podocyte disease with various glomerular changes, such as mesangial expansion, foot process effacement, capillary collapse, and expansion of parietal epithelial cells without thickening of the GBM and electron-dense deposits. Thus, the podocytes seem to have an effect on the development of surrounding glomerular cells, and the PRR in podocytes is essential for glomerular function.
Diseases that are caused by podocyte damage or dysfunction are termed podocytopathies.7,33 It has been proposed that podocytopathies be classified histologically into four categories on the basis of podocyte numbers and the pattern of glomerular morphology as follows: (1) minimal change disease (no change in podocyte number); (2) focal segmental glomerulosclerosis (podocyte detachment/death); (3) mesangial sclerosis (low podocyte proliferation); and (4) capillary collapse (high podocyte proliferation). The podocytopathy observed in PRR knockout mice corresponds histologically to mesangial sclerosis, although it could also be explained by focal segmental glomerulosclerosis with respect to the reduced number of podocytes and by the capillary collapse caused by the prominently enlarged podocytes. Thus, there may be a link between the PRR in podocytes and the development of podocytopathy in humans.
In conclusion, the deficiency of the PRR in murine podocytes resulted in disruption of the filtration barrier and accumulation of intracellular vesicles caused by V-ATPase dysfunction. The regulation of slit-diaphragm proteins, the membrane trafficking/degradation system by the PRR, and the clinical relevance of the PRR in human podocytopathy require further investigation in future studies.
CONCISE METHODS
Animals
All of the animal experiments were reviewed and approved by the institutional animal care and use committee at Keio University School of Medicine.
Generation of Podocyte-specific Atp6ap2 CKO Mice
The generation of Atp6ap2-floxed mice has been described in detail elsewhere.6 Briefly, in this study, Atp6ap2-floxed mice were bred with mice that expressed the Cre recombinase under the control of the podocyte-specific podocin (NPHS2) promoter, provided by Dr. Susan Quaggin (University of Toronto, Toronto, Canada).13 The resulting Atp6ap2lox/Y;NPSH2-Cre+/0 mice represent podocyte-specific Atp6ap2 CKO mice. Control male mice consisted of WT (NPHS2+/0;Atp6ap2+/Y) mice, as well as littermates that were heterozygous for either Atp6ap2 or NPHS2 (NPSH2+/0;Atp6ap2+/Y), thereby excluding Cre- and flox-mediated toxicity as the basis for phenotypic disparity (Supplementary Figure 1A). On P14, podocyte-specific knockout of the PRR was confirmed by double immunostaining of PRR and the podocyte-specific marker synaptopodin, which revealed no PRR expression where synaptopodin was positive in CKO mice (Supplementary Figure 1B).
Urine and Plasma Collection
On P20, urine was collected from mice over a 24-hour period using metabolic cages. In addition, blood samples were obtained from diethyl ether-anesthetized mice before death. The blood samples were mixed with EDTA and then centrifuged at 1000 × g for 10 minutes at 4°C to obtain plasma. Total protein was determined by the Biuret method, albumin levels were determined using the bromocresol green method, and creatinine or total cholesterol levels were determined using enzyme methods.
Histology
Kidneys were fixed overnight in 10% formalin at 4°C, dehydrated with 70% ethanol, mounted in paraffin, and sectioned at 5 μm. Kidney sections were stained with hematoxylin and eosin, periodic acid-Schiff, and periodic acidsilver-methenamine.1 Low- and high-magnification fields of views were analyzed using a BIOREVO (BZ-9000; Keyence, Japan).
For immunostaining, kidneys were embedded in OCT compound (Miles Laboratories, Elkhart, IN) and stored frozen. Sections (4 μm) were mounted on gelatin-coated slides and were stained immunochemically as described previously.34 The immunofluorescence of cultured cells was determined as described previously.35 Podocytes were observed 8 days after the transfection of ATP6AP2 siRNA and 12 hours after treatment with chloroquine and Carl Zeiss, Oberkochen, Germany). For the podocyte counts, we used selected glomerular cross-sections and counted WT1-positive cell nuclei as described previously.36 Image J software (National Institute of Health, Bethesda, MD) was used to quantify WT1 positive cells.
For transmission electron microscopy, the tissues were minced; fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M cacodylate buffer; and prepared according to standard protocols. Electron microscopy was performed on sections prepared using an RMC MT6000 ultramicrotome and visualized with the Hitachi H7500 electron microscope and the 2K × 2K Gatan CCD camera. For podocyte size measurements, the area of each glomerular profile was measured using the Image J software. The total podocyte area per glomerulus was divided by the measured number of podocytes per glomerulus to give an estimation of podocyte size. GBM thickness was measured at five points on each photomicrograph.
Antibodies
Antibodies against V-ATPase subunits and RAB7 were as described previously.37–40 Other primary antibodies used were monoclonal antibodies against LAMP2 (Developmental Studies Hybridoma Bank, University of Iowa), a GAPDH monoclonal antibody (Cell Signaling Technology, Danvers, MA), an ATP6AP2 polyclonal antibody (pAb: R&D Systems, Minneapolis, MN), an LC3 pAb (a gift from Dr. Komatsu, Tokyo Metropolitan Institute of Medical Science), a synaptopodin pAb (Synaptic Systems, Goettingen, Germany), a WT1 pAb (Santa Cruz Biotechnology, Santa Cruz, CA), a nephrin pAb produced in guinea pigs (Progen, Heidelberg, Deutschland), a podocin pAb (Santa Cruz Biotechnology), a nephrin pAb produced in rabbits, and a podocin pAb (a gift from Dr. Kawachi, Institute of Nephrology, Niigata University Graduate School of Medical and Dental Sciences). Fluorescent dye or enzyme-linked secondary antibodies were obtained from Jackson ImmunoResearch.
Gene Expression
Total RNA was purified using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For reverse transcription-PCR, total RNA was used for reverse transcription using random hexamer primers. Quantitative real-time PCR was performed with the ABI Prism 7700 sequence detection system (Applied Biosystems, Carlsbad, CA). Predesigned gene-specific primer and probe sets (TaqMan Gene Expression Assays) were used. 18 S ribosomal RNA was amplified as an internal control.
Western Blotting
Protein lysate preparation and immunoblotting procedures were performed as described previously.41 Total lysates were applied (10 to 20 μg/lane) on 5 to 20% gradient polyacrylamide gels (Daiich Pure Chemicals, Tokyo, Japan) and then transferred to polyvinylidene difluoride membranes with a pore size of 0.2 μm. Immunoblots were prepared using the aforementioned antibodies. DMEM, MEM, and FBS were obtained from Invitrogen. The protein blot was developed with an ECL detection kit (GE Healthcare, Little Chalfont, UK), and images were obtained using an image capture system (model LAS3000 luminoimager; Fujifilm, Tokyo, Japan). Band intensities were measured and analyzed with Image Gauge Software (Fujifilm).
Podocyte Culture
The conditionally immortalized human podocyte cell line was developed by transfection with the temperature-sensitive SV40 T-gene and the telomerase gene, provided by Dr. Moin A. Saleem (University of Bristol, UK).19 The podocytes were proliferated at 33°C and were differentiated after transfer to 37°C for >14 days. For experiments, the cells between passages 6 and 10 were seeded onto cell plates and were cultured at 37°C, under 5% CO2 in standard RPMI 1640 medium (Sigma, Tokyo, Japan) containing 10% fetal calf serum (Life Technologies, Tokyo, Japan) or 1 mg/ml endotoxin-free bovine serum albumin (Sigma) and insulin-transferrin sodium selenite supplement (Sigma) for at least 14 days until the cells had differentiated and were exhibiting an arborized morphology. After the podocytes had been incubated for 14 days at 37°C under 5% CO2, they were used for experimentation.11
siRNA Oligonucleotides and Transfection
siRNA oligonucleotides against the human ATP6AP2 gene, as well as control siRNA, were purchased from Thermo Scientific and Ambion, respectively. Transfection of these siRNA oligonucleotides was achieved using Lipofectamine RNAiMAX reagent (Invitrogen).
LysoTracker Analysis
The immunofluorescence of cultured cells was determined as described previously.35 To label acidic organelles, the cells were incubated with LysoTracker (Molecular Probes) for 30 minutes, before being fixed with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4).
Statistics
The data are presented as the means ± SD. The significance of differences between two mean values was evaluated by two-tailed unpaired t test. Multiple comparisons involving more than three groups were made using ANOVA. P < 0.05 was considered significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors thank Tomofusa Migita and Toshihiro Nagai for their excellent technical assistance. H.I. is a core member of the Global Center of Excellence for Human Metabolomics Systems Biology, Ministry of Education, Culture, Sports, Science and Technology. This work was supported by a Grant 22390171 from the Ministry of Education, Culture, Sports, Science and Technology (to A.I.). Y.O. and K.K. contributed equally to this work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Prorenin Receptor: What's in a Name,” on pages 2141–2143.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H: Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens 2: 332–340, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T: Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950–1961, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H: Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J Am Soc Nephrol 18: 2054–2061, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Nakagawa T, Nishiyama A, Kawachi H, Shimizu F, Inagami T: Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J Am Soc Nephrol 17: 2495–2503, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H: The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M, Hahnel B: A role for podocytes to counteract capillary wall distension. Kidney Int 45: 369–376, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Tryggvason K, Wartiovaara J: Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H: The (pro)renin receptor and the kidney. Semin Nephrol 27: 524–528, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Sakoda M, Ichihara A, Kurauchi-Mito A, Narita T, Kinouchi K, Murohashi-Bokuda K, Saleem MA, Nishiyama A, Suzuki F, Itoh H: Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. Am J Hypertens 23: 575–580, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H: Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Forgac M: Structure and properties of the coated vesicle (H+)-ATPase. J Bioenerg Biomembr 24: 341–350, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Sun-Wada GH, Wada Y, Futai M: Vacuolar H+ pumping ATPases in luminal acidic organelles and extracellular compartments: common rotational mechanism and diverse physiological roles. J Bioenerg Biomembr 35: 347–358, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Forgac M: Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G: Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25: 1025–1040, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G: Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22: 3927–3936, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M: CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem 285: 25285–25295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A, Villa A, Heikkila E, Soliymani R, Boucherot A, Cohen CD, Kretzler M, Nitsche A, Ripamonti M, Malgaroli A, Pesaresi M, Forloni GL, Schlondorff D, Holthofer H, D'Amico G: Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 20: 976–978, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M: Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol 20: 1269–1276, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Babayeva S, Zilber Y, Torban E: Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol 300: F549–F560, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barisoni L, Schnaper HW, Kopp JB: Advances in the biology and genetics of the podocytopathies: Implications for diagnosis and therapy. Arch Pathol Lab Med 133: 201–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, Takehara Y, Kato O, Makino S, Ogawa S, Fukuda K: Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation 116: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sun-Wada GH, Imai-Senga Y, Yamamoto A, Murata Y, Hirata T, Wada Y, Futai M: A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J Biol Chem 277: 18098–18105, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura N, Yamamoto A, Wada Y, Futai M: Syntaxin 7 mediates endocytic trafficking to late endosomes. J Biol Chem 275: 6523–6529, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M: Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 275: 8760–8765, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Nakamura N, Sun-Wada GH, Yamamoto A, Wada Y, Futai M: Association of mouse sorting nexin 1 with early endosomes. J Biochem 130: 765–771, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Sun-Wada GH, Wada Y, Futai M: Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct 28: 455–463, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Sano M, Tokudome S, Shimizu N, Yoshikawa N, Ogawa C, Shirakawa K, Endo J, Katayama T, Yuasa S, Ieda M, Makino S, Hattori F, Tanaka H, Fukuda K: Intramolecular control of protein stability, subnuclear compartmentalization, and coactivator function of peroxisome proliferator-activated receptor gamma coactivator 1alpha. J Biol Chem 282: 25970–25980, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.