Abstract
Renin-expressing cells modulate BP, fluid-electrolyte homeostasis, and kidney development, but remarkably little is known regarding the genetic regulatory network that governs the identity of these cells. Here we compared the gene expression profiles of renin cells with most cells in the kidney at various stages of development as well as after a physiologic challenge known to induce the transformation of arteriolar smooth muscle cells into renin-expressing cells. At all stages, renin cells expressed a distinct set of genes characteristic of the renin phenotype, which was vastly different from other cell types in the kidney. For example, cells programmed to exhibit the renin phenotype expressed Akr1b7, and maturing cells expressed angiogenic factors necessary for the development of the kidney vasculature and RGS (regulator of G-protein signaling) genes, suggesting a potential relationship between renin cells and pericytes. Contrary to the plasticity of arteriolar smooth muscle cells upstream from the glomerulus, which can transiently acquire the embryonic phenotype in the adult under physiologic stress, the adult juxtaglomerular cell always possessed characteristics of both smooth muscle and renin cells. Taken together, these results identify the gene expression profile of renin-expressing cells at various stages of maturity, and suggest that juxtaglomerular cells maintain properties of both smooth muscle and renin-expressing cells, likely to allow the rapid control of body fluids and BP through both contractile and endocrine functions.
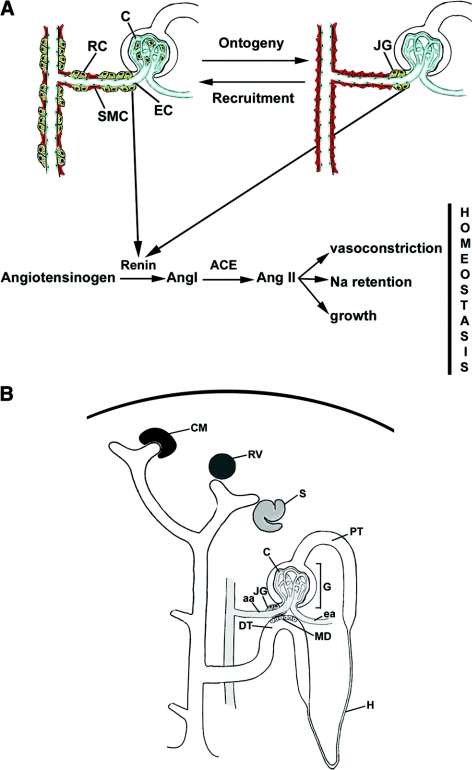
Renin-expressing cells are crucial in the control of BP and fluid-electrolyte homeostasis.1 In the adult mammal, renin cells are located in the afferent arteriole at the entrance to the glomerulus, thus their name, juxtaglomerular (JG) cells (Figure 1). However, during embryonic life, renin cells are distributed throughout the intrarenal arterial tree and inside the glomeruli. With maturation, renin cells differentiate into smooth muscle cells and become restricted to a few cells in the classical JG localization in the adult (Figure 1).2 If an adult animal is subjected to manipulations that threaten BP and fluid-electrolyte homeostasis, there is an increase in the number of renin cells along the preglomerular arteries and inside the glomerulus, resembling the embryonic pattern (Figure 1). This “recruitment” of renin cells is achieved by the retransformation of arteriolar smooth muscle and mesangial cells to the renin phenotype.3 We hypothesized that maturing, adult, and recruited cells express a unique set of genes characteristic of the renin cell phenotype, which is, in turn, vastly different from other cell types in the kidney. Although understanding the genetic regulatory network that governs the identity of renin cells is of fundamental biologic and medical relevance, several problems prevented investigators from achieving this goal: (1) renin cells are very few in number (0.01 to 0.001% of the total kidney cell mass), (2) it had been almost impossible to isolate renin cells to purity, (3) in culture, renin cells stop making renin after 48 to 72 h, and (4) there had been no markers that could identify these cells independently of renin.
Figure 1.
As the kidney matures, renin cells are restricted to the classical juxtaglomerular localization. (A) Top: Schematic representation of the distribution of renin cells (RC depicted in yellow) during early development (left) and the progressive restriction in the location of RCs to the JGA (right) during kidney ontogeny. If an adult animal is subjected to a condition that threatens BP and/or fluid-electrolyte homeostasis, there is a recruitment of RCs along the afferent and interlobular arterioles, and within the glomeruli—by dedifferentiation of smooth muscle cells (SMC, red) and glomerular mesangial cells to renin-expressing cells—in a pattern resembling the embryonic and fetal stages of renin distribution. Bottom: Classical depiction of the circulating renin angiotensin system and its acknowledged functions. EC, endothelial cell depicted in blue; C, capillaries; Ang I, angiotensin I; Ang II, angiotensin II; ACE, angiotensin converting enzyme; Na, sodium. (B) Development of the nephron and its vasculature. Upon induction by the dividing ureteric tip, the mesenchymal cells around it condensate to form the cap mesenchyme (CM), which, upon differentiation, evolves into the renal vesicle (RV) the s-shaped body (S), glomerulus (G), and the proximal tubule (PT). The JG cells and arterioles originate from a separate group of mesenchymal precursors that differentiate in situ to form the afferent and efferent arterioles, (aa) and (ea), respectively. The distribution of renin cells in the immature kidney shown on the left is extensive along the aa, interlobular arterioles, and arcuate arteries. H, loop of Henle; MD, macula densa; DT, distal tubule.
To circumvent those problems and address the aforementioned hypothesis, we used a well-characterized Ren1c-YFP mouse that faithfully expresses YFP in renin cells throughout development and in response to physiologic challenges.4 We isolated YFP + cells from the kidney of newborn and adult mice, and from adult mice subjected to a physiologic challenge that elicits the retransformation of arteriolar smooth muscle cells (aSMCs) to the renin phenotype, and compared their gene profiles to those from multiple cell types of the nephron at various stages of development (Figure 1B).5 Finally, to define whether the set of genes expressed by the renin cell located at the pole of the glomerulus—the bonafide adult JG cell—is different from the set of genes expressed by other renin cells, we developed a single cell isolation and amplification procedure that allowed us to uncover the expression profile of the classical JG cell.
RESULTS
Data from 48 Affymetrix Mouse Gene 1.0 ST arrays, representing 16 different kidney samples in biologic triplicate, using Nugen RiboSpia target amplification technology, were analyzed with GeneSpring software. The samples included FACS purified renin expressing cells from newborns, adults, and adults treated with captopril. Genes with elevated expression in renin cells were sequentially screened for fold change versus total kidney cortex, Welch ANOVA (P < 0.05), yielding 1051 probesets. Further screening for fold enrichment, compared with a virtual kidney cortex made by combining the individual compartment expression data, resulted in a list of 92 probesets showing elevated expression in adult renin cells (see Concise Methods for details and Supplementary Table 1 for complete gene lists of the 1051 and 92 gene sets).
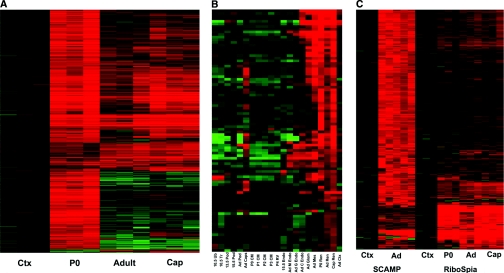
The heat map of Figure 2A provides an overview of the gene expression pattern of P0, adult, and captopril-treated (recruited) adult cells. The bulk of differentially expressed genes was associated with the newborn renin cells. Further analysis showed that most of these genes were related to the highly proliferative state of P0 cells, and included genes involved in cell division and DNA synthesis. A full list of genes differentially expressed in P0 and adult renin cells is shown in Supplementary Table 2. Interestingly, newborn cells express a significant number of factors (Reelin, Angiopoietin 2, tetraspanins, Lpar4, integrins, Notch receptors [Figure 4, I and L] and ligands) known to be involved in angiogenesis. The heatmap of Figure 2B compares renin cells with other cells in the kidney. As expected, P0, adult, and captopril-treated adult cells are the most closely related. The captopril treatment of adults resulted in a more P0-like gene expression signature, reflecting the increased number of cells expressing renin along the kidney vasculature as it occurs during development. Renin cells also show significant gene expression similarities to mesangial cells, endothelial cells, and, to a lesser extent, the renal capsule. For complete gene lists with associated heat maps see Supplementary Tables 1 through 3.
Figure 2.
The transcriptome of renin cells is vastly different from any other renal cell type. (A) Heatmap of 1051 probesets, showing differential expression in adult total kidney cortex (Ctx) and renin expressing cells from newborn (P0), adult, and captopril-treated adults (Cap). Each horizontal line represents one probeset expression, with red showing high-level expression. (B) Heatmap of renin cell-enriched genes, comparing across multiple cell types. Developmental times are given (E10.5, E13.5, Adult, P0, P1, P2, P3, P4, E15.5), as are tissue compartment (Ub, ureteric bud; Tr, trunk; Pod, podocyte; CM, cap mesenchyme; RV, renal vesicle; Endo, endothelial cells; M Endo, medullary endothelial cells; G Endo, glomerular endothelial cells; C Endo, cortical endothelial cells; Glom, glomerulus; Mes, mesangial cells; Ren, renin-expressing cells; Cap Ren, renin cells from captopril-treated adult; Ctx, total renal cortex). (C) Heatmap of 369 most specific adult renin cell genes, derived from both single cell analysis (SCAMP) and RiboSpia analysis. Ctx, total kidney cortex; Ad, adult renin cells; P0, newborn renin cells; Cap, adult captopril-treated renin cells. See also Supplementary Figure 1 and Supplementary Tables 1, 2, and 3.
Figure 4.
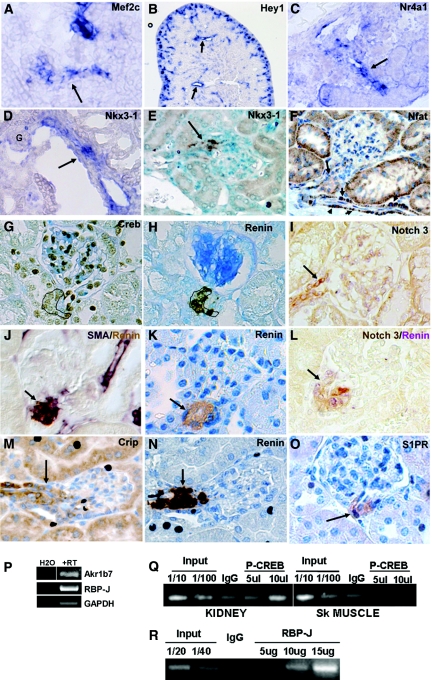
Genes identified in the JG cell signature are expressed in JG cells and vessels. (A–D) In situ hybridization in newborn kidneys. (A) Mef2c expression in developing vessel (arrow). (B) Hey1 is expressed in the vessels (arrows) and in glomeruli of the nephrogenic zone. (C) Nr4a1 expression in an afferent arteriole (arrow). (D) Nkx3–1 is expressed in JG cells and afferent arteriole (arrow, G, glomerulus). (E) Immunostaining shows that Nkx3–1 localizes to JG cells and the afferent arteriole in kidney of a captopril-treated adult (arrow). (F–O) Immunostaining in adult kidneys. (F) Nfat is expressed in JG cells (arrow), vessels (short arrows), and some tubules. (G) Creb is expressed in renin-expressing JG cells (outline). (H) Consecutive section of (G) showing renin expression (outline). (I) Notch3 (brown) is expressed in JG cells. (L) Double staining for Notch3 (brown) and renin (purple) in a consecutive section of (I) shows coincidence of expression. Arrows in (I) and (L), JG cells. (J) Costaining for αSMA (purple) and renin (brown) shows expression of αSMA in JG cells as well as afferent arterioles. (K) Renin expression in JG cells for comparison with (J) Arrows in (J) and (K), JG cells. (M, N) Crip1 localizes to renin expressing JG cells as well as some tubules. Arrows in (M) and (N), JG cells. (O) S1P receptor is expressed in JG cells (arrow). (P) RT-PCR of RNA extracted from FACS isolated YFP+ cells confirms the expression of Akr1b7 and RBP-J mRNAs. (Q) Chromatin immunoprecipitation shows enrichment of phopspho-Creb at the cAMP responsive element in the renin enhancer in renin-expressing kidney cortex cells but not in skeletal muscle cells, which do not express renin. (R) RBP-J is enriched at the RBP-J element in cells from primary cultures of kidney arterial smooth muscle cells.
The transcriptome of the bonafide JG cell is likely to differ from other renin cells. Although FACS isolation provides excellent purity, the renin cell is quite rare, making purification challenging, and there are reports that cells outside of the JGA can produce renin, even in the normal adult.6,7 To insure purity of the JG cell, we developed a single cell amplification procedure (SCAMP) that allowed us to obtain the gene profile of five individual YFP positive cells isolated from the JG poles of sieve-purified glomeruli from Ren1c-YFP mice (see Concise Methods for a brief description of the JG cell isolation, RNA amplification, and microarray analysis, and Supplemental Methods for details.). The excellent reproducibility (Pearson correlation coefficients: 0.86 to 0.94) and high sensitivity of SCAMP data in comparison with two commercial systems, Nugen RiboSpia OneDirect and Miltenyi uMACS SuperAmp, are shown in Figure 2C, Supplementary Figure 1, and the Supplemental Methods. To provide a uniform amplification chemistry baseline for comparison, we also used SCAMP to amplify aliquots of RNA from total adult kidney cortex. The resulting array data were screened, requiring raw signal of at least 125 (giving 10,260 probesets) and FC greater than 2.5, Welch t test, P < 0.05, and concordance between the RiboSpia and SCAMP microarray datasets, arriving at an adult JG cell gene expression signature of 369 genes. The heatmap (Figure 2C) shows that the two target amplification strategies, SCAMP and RiboSpia, gave reproducible, overlapping, yet distinctive, gene lists, with some probe sets clearly amplified better by one procedure than the other. The complete gene list for the 369-gene set is provided in Supplementary Table 1. Complete lists of biologic processes, molecular functions, regulatory transcription factors, candidate target genes, and regulatory microRNAs are included in Supplementary Table 3.
Genes with Renin-Cell-Enriched Transcripts
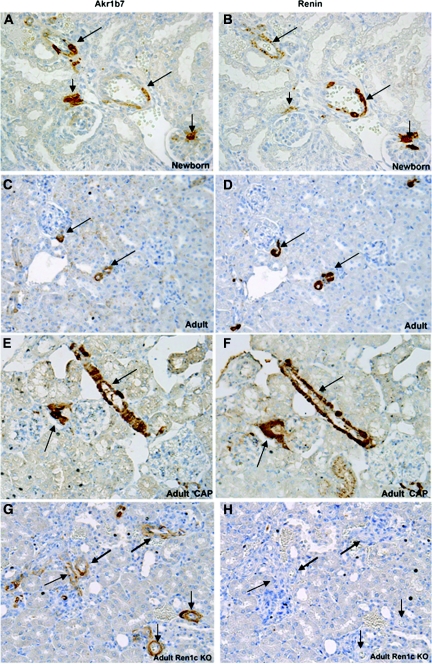
As expected, renin mRNA showed the highest enrichment in adult renin cells, 41-fold (FACS-RiboSpia data, raw signal approximately 11,000) when compared with total kidney cortex, providing an important positive control. The second highest was aldose-keto-reductase (Akr1b7), 23-fold enrichment. AKR1B7 localizes to JG cells, renal arterioles, and the glomerular mesangium in the newborn kidney, it becomes restricted to the JG cells in the adult, and it is re-expressed in afferent aSMCs that underwent retransformation to renin-expressing cells in the captopril-treated adult (Figure 3, A, C, and E). Thus, AKR1B7 follows the pattern of renin expression (Figure 3, B, D, and F) throughout development and in response to a homeostatic challenge. Interestingly, in animals with homozygous deletion of the renin gene,8 AKR1B7 was maintained in those cells that would have normally expressed renin (Figure 3G), indicating that AKR1B7 does not depend on renin expression for its expression and confirming that AKR1B7 serves as an invaluable new independent marker of renin cells. Among the adult renin cell-specific expressed genes, it is interesting to note the high expression levels of a select subgroup, including, in addition to renin and Akr1b7, the genes Rgs5, Crip1, ATP1b2, Syne2, Plac9, Myh11, Jph2, Myo18, and Mgp.
Figure 3.
Akr1b7 expression is an independent marker of renin cells. Immunostaining for Akr1b7 (A, C, E, G) and renin (B, D, F, H), in consecutive sections, shows colocalization of Akr1b7 in renin cells in (A, B) newborn kidney. Short arrow, JG cells; long arrows, arterioles and larger vessel. (C, D) Adult kidney. Arrows, JG cells. Akr1b7 marks renin cells at all stages of development. (E, F) Adult kidney from an animal administered low sodium diet + captopril stimulates re-expression of renin and Akr1b7. Arrows, JG cells and afferent arteriole. Akr1b7 is expressed in the same pattern as renin. (G, H) Ren1c knockout kidney:Akr1b7 marks renin cells even although the cells are unable to make renin. Arrows in (H) indicate JG cells and afferent arterioles that are expressing Akr1b7 in (G).
RGS5 (regulator of G protein signaling 5) is a potent GTPase-activating protein for Giα and Gqα, which is expressed in vascular smooth muscle and has been considered as a marker for pericytes, which express RGS5 at high levels.9 Rgs5 is expressed sevenfold higher in renin cells (FACS data) compared with total kidney cortex, and it is known to be expressed by the smooth muscle cells of the developing kidney arterioles and by mesangial cells in the adult animal.9 The RGS5 expression pattern overlaps, in fact, with the expression patterns of PDGF-RB and renin,10,11 suggesting a potential relationship between pericytes and the renin cell.
Cysteine-rich intestinal protein (CRIP1) is also highly expressed in JG cells. The localization of CRIP1 to renin cells is shown in Figure 4M. Crip1 belongs to the LIM domain/double zinc finger protein family, which, in the gut, controls zinc transport. CRIP 1 may play a different role in JG cells, as a novel stress response factor whereby CRIP1 may provide a survival advantage. High levels of CRIP1 have been correlated with the differentiation of cells to the myofibroblast cell lineage.12 CRIP1 protein has also been implicated in muscle differentiation,13 suggesting that CRIP1 may be involved in the maintenance of the smooth muscle phenotype of the JG cell.
The Smooth Muscle Signature of Newborn and Adult Renin Cells
Newborn and adult renin cells express numerous genes characteristic of the smooth muscle phenotype (Table 1). In addition to alpha smooth muscle actin (α-SMA, Figure 4J), Rgs5, Rgs2, smooth muscle myosin heavy chain (SM-MHC, Myh11), calponin (Cnn1), and transgelin (SM22α) are also highly represented in renin cells. These findings corroborate previous work demonstrating a lineage relationship between renin-expressing cells and SMCs of the renal arterioles.3,14 An interesting feature is the expression of other muscle genes normally thought to be exclusively expressed in skeletal and/or cardiac muscle. These include dystrophin (DMD), phospholamban, and NKX2.3 (transcription factor involved in myocardial development), MYO18B, and PDLIM 3 (an actinin-associated LIM protein whose deficiency causes dilated cardiomyopathy). Although the function of these genes in the JG cell is unexplored, it is possible that their roles in morphogenesis and contractility of skeletal and cardiac muscle are conserved in the JG cell.
Table 1.
Expression of smooth muscle genes in renin cells during development
| Gene | Adult Renin Cells |
Newborn Renin Cells |
||
|---|---|---|---|---|
| CM | RV | CM | RV | |
| Renin | 81.9 | 76.0 | 72.5 | 67.3 |
| α-SMA | 4.9 | 4.7 | 13.0 | 12.2 |
| SM-MHC | 8.2 | 8.7 | 20.3 | 21.5 |
| SM22α | 3.1 | 2.2 | 13.7 | 9.7 |
| Calponin 1 | 2.1 | 2.6 | 15.9 | 19.6 |
| RGS5 | 34 | 25.8 | 33 | 25.1 |
| RGS2 | 4.2 | 4.1 | 11.3 | 11.1 |
CM, cap mesenchyme; RGS2, regulator of G-protein signaling 2; RGS5, regulator of G protein signaling 5; RV, renal vesicle; αSMA, alpha smooth muscle actin; SM-MHC, smooth muscle myosin heavy chain; SM22α, transgelin. The expression of the indicated genes is presented as fold enrichment over CM or RV.
Expression of Genes Related to Calcium Control of Renin Release
Renin-expressing cells depend on calcium signaling for renin synthesis and release. Calcium regulation of JG cells is different from other endocrine cells, with the exception of parathyroid cells that also respond to increase in intracellular calcium with a decrease in hormone secretion. This inverse relationship between calcium and renin release has been termed the calcium “paradox.” Some of the genes expressed by renin cells involved in calcium homeostasis are shown in Figure 5B and Table 2. Our renin cells have a profusion of calcium, potassium, and sodium channels, and other transporters that connect extracellular signals with the cell's milieu. It remains to be determined how the integrated activity of these channels control the precise amounts of renin released to the circulation.
Figure 5.
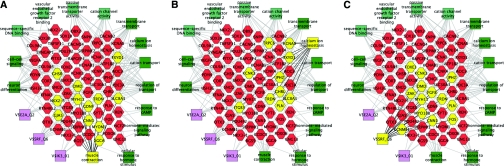
Gene network characteristic of the adult renin cell. (A) Renin cell genes known to be involved in muscle contraction. Some of the 369 adult renin cell signature set of genes are shown in the center hexagons, with surrounding rectangles representing biologic processes (dark green), molecular functions (light green), and transcription factors (violet), with associated genes connected by lines. Genes involved in muscle contraction are highlighted in yellow. (B) Genes involved in calcium homeostasis are highlighted in yellow. (C) Transcription factors (SRF, E2A, IK3) and the smooth muscle phenotype. SRF candidate target genes are highlighted in yellow. See also Supplementary Table 3.
Table 2.
Expression of genes related to Ca2 + control of renin synthesis and release
| Gene | Name | Function |
|---|---|---|
| KCNA3 (Kcna3) | Potassium voltage-gated channel, shaker-related subfamily, member 3 | Mediates the voltage-dependent potassium ion permeability of excitable membranes and also comprises the Ca2+-activated Slo (actually 7-TM) and the Ca2+-activated SK subfamilies |
| JPH2 (Jph2) | Junctophilin 2 | Approximates the L calcium channels and the ryanodine receptor |
| PLN (Pln) | Phospholamban | Inhibits sarcoplasmic reticulum Ca2+-ATPase |
| TRP6 (Trpc6) | Transient receptor potential cation channel, subfamily C, member 6 | Activated by G-protein coupled receptors; important for the Ca++ response to adrenergic stimulation and may integrate systemic responses conveyed by the renal nerves, which, in turn, are modulated by the regulation of intracellular Ca++ |
| TRDN (Trdn) | Triadin | Releases calcium from the sarcoplasmic reticulum triggering muscle contraction through Ca++-induced Ca++ release |
| FXYD1 (Plm) | Phospholemman FXYD domain-containing ion transport regulator 1 | Plasma membrane protein that possesses channel activity; may also regulate Na,K-ATPase activity and modulate ion transport in extraglomerular mesangial cells and JG cells in response to changes in macula densa NaCl concentrations (tubuloglomerular feedback) |
| HRC (Hrc) | Histidine rich calcium binding protein | Luminal sarcoplasmic reticulum protein that may be a target of the MEF2c transcription factor linking the smooth muscle specification with the regulation of intracellular Ca++ |
| MGP (Mga) | Matrix gla protein | Extracellular matrix protein that may act as a buffer preventing wide variations and/or excessive free Ca++ accumulation in the JG cell microenvironment |
| PTP4A3 (Ptp4a3) | Protein tyrosine phosphatase type IV, A3 | Inhibits AngII induced Ca++ mobilization and may play a role in the regulation of the negative feedback for renin release caused by AngII |
| SFRP2 (Sfrp2) | Secreted frizzled-related protein 2 | Soluble modulator of Wnt signaling, binds to Wnt-4, and may regulate Wnt-4 signaling during kidney development; also a stimulator of angiogenesis via calcineurin-NFAT pathway and may be important for the maintenance of the JG cell's bivalent -contractile and endocrine–phenotype |
Transcriptional Control of the Smooth Muscle Phenotype
Analysis of the promoter regions of the genes coordinately expressed in the adult renin cell for the presence of evolutionarily conserved transcription factor binding sites (TFBS) allowed the identification of candidate regulators. Three factors—SRF, E2A (Tcf3), and IK3—gave the strongest statistics. A brief description of these TFs is provided in Table 3. Figure 5C shows the predicted SRF targets in the renin cell. SRF binds to the serum response element of target genes and, together with GATA 4 and NKX2.5, directs early cardiac development. Several members of the NKX family (NKX2.3 to 2.5 and NKX3.1, Figure 5C and Figure 4, D and E) are expressed in JG cells, and, together with SRF, may contribute to the development and maintenance of their smooth muscle character. SRF is also involved in the development of vascular SMCs. Together with Myocardin (Myocd, 1051 gene list in Table S1), SRF directs transcription of SMC genes. Mice lacking Myocd have serious vascular defects and die before E10.5.15
Table 3.
Transcriptional control of the smooth muscle phenotype
| Gene | Name | Function |
|---|---|---|
| E2A (Tcf3) | Transcription factor 3 | Basic helix-loop-helix transcription factor critical for lineage commitment, differentiation, and survival of lymphocytes, pancreatic, muscle, and neural cells |
| IK3 (Ikzf3) | IKAROS family zinc finger 3 | Zinc finger DNA-binding protein that regulates lymphocyte commitment and differentiation |
| MYOCD (Myocd) | Myocardin | Expressed in renin cells and, together with SRF, directs transcription of SMC genes |
| NFAT (Nfatc4) | Nuclear factor of activated T-cells, cytoplasmic 4 | Involved in vascular patterning and angiogenesis and required for pericytes to coat the vessel wall |
| NKX (Nkx2.3, Nkx2.4, Nkx2.5, Nkx3.1) | NKX-homeodomain factor | Critical regulators of organ development; in JG cells; together with SRF, they may contribute to the development and maintenance of their smooth muscle character |
| SRF (Srf) | Serum response factor | Member of the MADS box (MCM1, agamous, Defficiens, and SRF) superfamily of transcription factors; together with GATA 4 and NKX2.5, it directs early cardiac development and is involved in the development of vascular SMCs |
E2A transcription factors are critical for lineage commitment, differentiation, and survival of lymphocytes, muscle, and neural cells.16,17 Ikaros (IK3) encodes a Zn++ finger DNA-binding protein that regulates lymphocyte commitment and differentiation.
Another transcription factor, NFATc4 (1051 gene list in Supplementary Table 1 and Figure 4F) is of particular interest because of its connection to calcium (see Supplementary Table 4 for the list of all transcription factors). Increase in intracellular Ca++ leads to activation of calcineurin, which dephosphorylates NFAT, which, in turn, is translocated to the nucleus, where it binds the promoter of target genes. Null mutations of NFATc4 and NFATc3 in mice result in defects in vascular patterning and angiogenesis, and NFAT is required for pericytes to coat the vessel wall.18 Given its predicted target genes, NFAT may play a central role in integrating calcium signals with transcriptional control of the smooth muscle phenotype in JG cells.
Transcriptional Control of the Renin Phenotype
To better understand the regulation of the renin phenotype, we analyzed the combination of expressed TFs in the adult renin-expressing cells and the evolutionarily conserved TFBS in the renin promoter and enhancer. We first used GeneSpring to combine the single-cell and FACS-based datasets, requiring minimum raw expression of 400, and elevated expression in renin cells compared with cortex, yielding 118 renin-cell-enriched transcription factors (Supplementary Table 4).
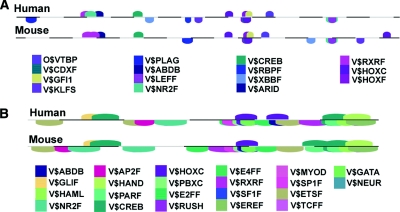
We also specifically examined regulation of the renin promoter. Genomatix was used to identify the TFBS with conserved sequence and spacing between human and mouse proximal promoters, with repeat sequences removed (Figure 6A). There was a striking concordance between the two datasets, with most of the conserved TFBS having corresponding expressed TFs in the renin cell. The corresponding TFs are shown in Table 4.
Figure 6.
Localization of binding sites conserved in mice and humans for transcription factors enriched in JG cells. (A) The renin promoter and (B) the renin enhancer. See also Supplementary Table 4.
Table 4.
Transcription factors in the renin proximal promoter and enhancer
| Gene | Name | Function |
|---|---|---|
| O$VTBP (Tbp) | TATA binding protein | Binds to the TATA box ∼35bp upstream of the transcription start in many genes and forms part of the RNA polymerase II preinitiation complex |
| V$ABDB (Hoxd10) | Homeobox D10 | Member of the Abd-B homeobox family; expressed in the developing limb buds and involved in differentiation and limb development; mutations in this gene have been associated with Wilm's tumor |
| V$AP2F (Tcfap2) | Activator Protein-2 | Mediates transcriptional activation of the CYP11A1 gene by interacting with Sp1 |
| V$ARID (Arid5b) | AT rich interactive domain 5B | Transcriptional control of lymphocyte differentiation |
| V$CDXF (Cdx1) | Vertebral caudal-related homeodomain 1 | Regulates intestine-specific gene expression and enterocyte differentiation. Cdx1 is expressed in the proliferating immature epithelium during intestinal development and becomes restricted to the proliferative crypt in the adult intestine |
| V$CREB (Creb) | cAMP responsive element binding protein | Binds to the cAMP responsive element in gene promoters and activates transcription |
| V$EREF (Esrrg) | Estrogen-related receptor gamma | Involved in branching morphogenesis of the mammary gland, prostate, and lung |
| V$GATA3 (Gata3) | GATA binding protein 3 | Involved in T cell development; mutation of this gene causes hypoparathyroidism, sensineural deafness, and kidney dysplasia |
| V$GFI1 (Gfi1b) | Growth factor independent 1b | Zinc finger protein required for erythroid and megakaryocytic lineages; can be a repressor or activator depending on promoter and cell type context; alters histone methylation by recruiting histone methyltransferase to target gene promoters |
| V$GLIF (Glis2) | GLIS family zinc finger 2 | Gli-related, Krüppel-like transcription factor is an activator or repressor of gene transcription, depending on the gene and promoter context and plays a role in kidney development and neurogenesis |
| V$HAML (Cbfa2t3) | Core-binding factor, runt domain, alpha subunit 2, translocated to 3 (human) | Putative breast tumor suppressor; transcriptional repressor protein containing a zinc-finger motif common to developmental proteins, suggesting a potential function in regulating differentiation and morphogenesis |
| V$HAND (Hand1) | Heart and neural crest derivatives-expressed protein 1 | Belongs to the bHLH family and plays an essential role in cardiac morphogenesis |
| V$KLFS (Klf2) | Kruppel-like transcription Factor 2 | Modulates blood vessel maturation via smooth muscle cell migration and coating of endothelial tubes |
| V$HOXC; V$HOXF (Hoxb8; Hoxd8) | Homeobox B8 and Homeobox D8 | Hoxb8: involved in the development of the sensory neuron network; knockout affects survival of spinal ganglion, causes aberrant limb reflexes and the dorsal spinal neurons are abnormally distributed Hoxd8: involved with limb and genital development; may also play a role in adult urogenital tract function and in the maturation and maintenance of lymphatic vessels |
| V$MYOD (Myod1) | Myogenic differentiation 1 | A member of the bHLH group myogenic factors subfamily of transcription factors, which regulates muscle differentiation |
| V$NR2F | Nuclear receptor subfamily 2 | Involved in Leydig cell development |
| V$RBPF (RBP-J, CBF1) | Recombination signal binding protein for immunoglobulin kappa J region | The final effector of the Notch-signaling pathway |
| V$RXRF (Vdr) | Retinoid X receptor | Ligand-activated transcription factors of the steroid hormone family of nuclear receptors, which, together with vitamin D, regulate renin gene expression |
| V$XBBF (Rfx2) | Regulatory factor X, 2 (influences HLA class II expression) | Member of the regulatory factor X gene family proteins, which contain a winged helix DNA binding domain; transcriptional activator involved in regulation of gene expression during meiosis and the early development of spermatids |
PPAR and VDR are well-known regulators of renin expression.19,20 RBPF (RBP-J) is the final effector for all Notch receptors, likely candidates for the regulation of the renin phenotype. Validating the relevance of RBP-J expression, ChIP experiments showed enrichment of binding of RBP-J to the RBP-J site in the renin promoter (Figure 4R), and conditional deletion of RBP-J in JG cells results in a significant decrease in renin gene expression and the number of renin cells.21 Similarly, V$CREB was clearly expressed in JG cells, and ChIP experiments showed its enrichment at the CRE of renin-expressing cells (Figure 4, G and Q). We have previously shown that the cAMP/CBP/p300/CREB pathway is fundamental in the control of renin synthesis and release.4,22
A similar analysis was also performed for the renin enhancer (Figure 6B) that begins at −2500 for the mouse and −11,015 for the human, and, in each case, extends about 275 bases further 5′. The presence of an estrogen response element (V$EREF) is intriguing. Estrogens are known to increase angiotensinogen and decrease plasma renin,23 explaining, in part, gender differences in the activity of the renin-angiotensin system. The molecular events that regulate the activity of the renin gene upon exposure to estrogens remain to be studied in detail. Because estrogen receptors regulate transcription of genes involved in branching morphogenesis of the mammary gland, prostate, and lung,24 and renin cells are involved in branching of the renal vasculature, it is possible that this function may be in part accomplished by the binding of estrogen receptors to the EREF of the renin gene.
As described in Results, JG cells maintain both an endocrine and a smooth muscle phenotype. Additional studies were carried out to determine the expression of a number of the JG-cell-enriched transcripts that could be important for maintenance of their smooth muscle cell characteristics and for renin expression. Among several transcription factors enriched in JG cells, Mef2c, Nkx3–1, Nr4a1 (Nur77), and Nfat are potentially involved in the maintenance of their smooth muscle character and are expressed appropriately in JG cells and vessels (Figure 4, A and D–F). The RGS proteins, which are highly expressed in JG cells and may be involved in vessel maturation, are known to inhibit sphingosine receptor signaling. S1P receptors are enriched in the JG cells arrays and localize to JG cells (Figure 4O). We found that RBP-J, the final effector of Notch signaling, is important in renin expression. Several other Notch pathway genes are also enriched in JG cells, and, among them, Notch 3 and Hey1 (Figure 4, B, I and L) were found to localize to vessels and JG cells. Thus, the aforementioned regulators are appropriately localized and may participate in development and/or maintenance of the renin cell phenotype.
DISCUSSION
Novel features of the present work include the extensive comparison of renin cells with numerous cells types from the renal cortex at different developmental points. Furthermore, we developed a single cell isolation and amplification procedure that allowed us to identify the transcriptome of individual adult JG cells. Specifically, we show that renin cells express a unique set of genes vastly different from other cell types in the kidney: They possess markers that topologically and functionally link them to arterial and interstitial pericytes, and express Akr1b7, a new and valuable marker for renin cells, independent from renin expression. Contrary to arteriolar cells distant from the glomerulus, which transiently express renin during development and/or a homeostatic threat, adult JG cells maintain a dual smooth muscle and renin phenotype, driven by a unique transcriptional network that maintains, at all cost, the cell's dual endocrine and contractile functions necessary for the maintenance of homeostasis.
The expression of Rgs5 and Rgs2 in renin cells suggests a potential lineage and/or functional relationship with pericytes. Pericytes, like renin cells, are mural cells that cover endothelial tubes and provide support to the vasculature. In the renal vasculature, renin cells are often encountered forming rings around the renal arterioles,25,26 not too dissimilar in appearance to mural cells/pericytes in other vascular territories. In addition to their location around the renal arterioles, during embryonic life or homeostatic stress, renin-expressing cells are occasionally found in the location of the renal interstitial pericytes. Although Rgs5 is involved in normal embryonic and tumoral angiogenesis,27 and it participates in the recruitment of mural cells during maturation of renal blood vessels, the role of RGS5 in kidney vascular morphogenesis seems less clear: Rgs5 –/– mice are viable and fertile, and their vasculature seems to develop normally. However, Rgs5 may play a role at the end of arteriole maturation, when branching of the renal arterioles has been completed and renin cells are confined to the classical JG localization. The factors that control the restricted localization of renin cells near the glomerulus are unknown, but RGS inhibits sphingosine-1-phosphate/S1P1 receptor(s) signaling. Inhibition of S1P1, which is expressed in JG cells, may prevent further migration of mature JG cells once they have reached their destination.
As mentioned above, AKR1B7 is coexpressed in renin-expressing cells throughout development and in response to physiologic challenges. Akr1b7 is re-expressed, together with renin, along the renal arterioles, suggesting that a common mechanism may regulate the expression of both enzymes. Interestingly, in animals with deletion of the renin gene, Akr1b7 expression is maintained, indicating that Akr1b7 can be used as an independent marker for cells programmed to be renin-expressing cells. Akr1b7 plays an important role in the clearance of xenobiotics as well as in the transformation of harmful aldehydes generated during hormone synthesis. Further, Akr1b7 participates in steroid synthesis in the adrenal gland, where renin is also expressed in fetal life.28 Most recently, Akr1b7 has been shown to be crucial in the synthesis of prostaglandins,29 which, in turn, are known to regulate renin synthesis and release,1 suggesting that AKR1B7 may regulate renin release by an intracrine mechanism. Further studies will be necessary to determine the role of Akr1b7 in renin cells.
As mentioned in the Results, the gene-expression profile of renin cells shares some significant similarity to that of endothelial cells. This cannot be attributed to endothelial cell contamination of the renin cells since the level of the specific endothelial cell marker Tie-2 is low. Thus, endothelial cells and JG cells may be related and share the expression of certain genes.
We have previously shown that the three major cells of the renal arteriole are present in the kidney undifferentiated mesenchyme before arterioles and nephrons are formed.14,30 Those cells have the capability to assemble the renal arterioles.14 We have shown that the differentiation, branching, and elongation of the renal arterial tree—which is maximal during the first week of postnatal development—is intimately linked to the differentiation of the renin cells: Development of each new arteriole encompasses the coating of the vessel with renin-expressing cells.31 As the vessels mature, renin cells differentiate into SMCs.3,14 This pattern is repeated with each new branch until the arteriolar tree is completed and the only remaining renin containing cells are those at the tip of the arterioles. Although a direct effect of locally produced angiotensin and/or renin is likely responsible for arteriolar differentiation, the findings of our arrays suggest that renin cells produce additional angiogeneic factors that may provide guidance cues (reelin), positional information, and cell-to-cell communication (components of the Notch pathway), matrix digestion (Timp3), endothelial elongation (angiopoietins), and recruitment of smooth muscle cells (Enpp 1 to 3, Tspan), which, in turn, govern the proper assembly of the renal arterioles. The fact that these genes are expressed at higher levels in the newborn, when the renal tree is still developing, suggests that they may play a role in renal arterial development during early life.
The results of our microarray data indicate that, contrary to aSMCs upstream from the glomerulus, JG cells maintain a dual endocrine and smooth muscle phenotype. This agrees with our immunocytochemical and qRT-PCR data showing the expression of renin and numerous markers and regulators of smooth muscle in JG cells.
The reason for the bivalent nature of the classical JG cell is unclear, but it is likely that the location of the cells within the JG apparatus continually exposes them to signals from other cells that maintain them, ready to secrete renin rapidly in acute situations, as is known to occur in acute hypotension, and simultaneously contract to regulate glomerular hemodynamics. Several in vitro and in vivo studies suggest that JG cells have the capability to contract.32,33,34 Whereas many of the signals have not yet been identified, it seems reasonable that cell-to-cell contact with other JG cells, as well as endothelial, smooth muscle, and macula densa cells, is crucial for the maintenance of the myoepithelioid JG cell phenotype. Recently, it was found that connexin 40 (Gja5), which is highly expressed in renin cells on our arrays, is fundamental for the maintenance of renin expression near the glomerulus.35 Similarly, JG cells express members of the Notch pathway (Notch 3, Jagged 1, Hey 1), known to transmit cell-to-cell signals and regulate gene expression via the transcriptional regulator RBP-J. As mentioned above, the renin gene possesses a highly conserved RBP-J (V$RBPF) binding site between its proximal promoter and the renin enhancer. Our studies show that RBP-J binds the renin promoter in vivo. In addition, and providing functional validation to the TFs identified, we recently found that conditional deletion of RBP-J in JG cells results in a marked decrease in the number of renin-expressing JG cells, reflected by decreased circulating renin and low arterial BP.36
Whereas some of the signals conveyed by other cell types are beginning to be unraveled, the intracellular events that maintain the JG cell's unique myoepithelioid phenotype were unknown until now. The results of our analyses indicate that several potential transcriptional regulators (NFAT, SFRP2, SRF, and MEF2c) may operate in conjunction with calcium signals to maintain the smooth muscle phenotype and thus keep the cell's ability to contract while still possessing the ability to synthesize and secrete renin.
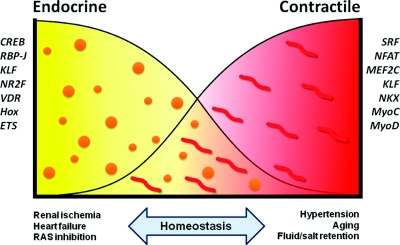
A remaining question is why JG cells retain this dual phenotype and the possible advantages accruing from it. Figure 7 depicts a potential hypothetical scenario whereby the cells could be either completely endocrine (left side), as in prolonged renal ischemia, or be completely devoid of renin granules, as in severe hypertension and salt loading. Whereas these two opposite phenotypes may temporarily accomplish the function of either producing enough renin in an attempt to reestablish BP/fluid electrolyte balance, or maintain renal blood flow constant, neither of them can be sustained for too long without leading to deterioration of renal function and the development of systemic vascular and glomerular pathology. Therefore, to be in the homeostatic range, the JG cell may need to maintain a dual endocrine-contractile phenotype, which is a myoepithelioid state (Figure 7). We anticipate that the set of 369 core genes found in the JG cells, coregulated by the transcription factors described herein, act in concert to confer the identity of this most intriguing cell type.
Figure 7.
The bivalent endocrine-contractile phenotype of JG cells and homeostatic control. See text for details.
CONCISE METHODS
Isolation of Renin Cells, RNA Purification, and Target Amplification
Renin-expressing cells were isolated by FACS from kidneys of our Ren1c-YFP mice,4 in which renin expression is marked by YFP using a high-speed digital BD FACS Aria II Cell Sorter. As a control, cells were isolated in parallel from wild-type kidneys to provide the baseline for excluding unlabeled cells. Briefly, kidneys were excised from the mouse, decapsulated, and demedullated. The remaining cortical tissue was minced in 0.3% collagenase A (Roche Applied Science, Indianapolis, IN) in phosphate buffered saline (PBS, 14190-144; Invitrogen, Carlsbad, CA) and incubated for 30 min at 37 °C, with frequent pipeting. Cells were centrifuged and the pellet resuspended in 0.05% trypsin-EDTA (25300-054; Invitrogen) and incubated at 37 °C for 5 to 10 min, with frequent pipeting, to obtain a single cell-enriched suspsension. Trypinsization was stopped with 10% FBS in PBS. The cells were filtered through a 100-μm cell strainer (08 to 771-19; Thermo Fisher Scientific Inc., Pittsburgh, PA), the red blood cells were lysed (RBC lysis buffer, R7757; Sigma, St Louis, MO), and the cells filtered through a 70-μm cell strainer (08 to 771-2; Fisher). This cell suspension was subjected to FACS to isolate YFP+ cells. The yield of YFP+ cells was routinely 0.01 to 0.02% of the cells applied to the sorter. RNA was purified and amplified as described.5 Complete protocols are available at GUDMAP.org (http://www.gudmap.org/Research/Protocols/Potter.html). Gene ontology molecular function and biologic process analysis of the JG cell gene list was conducted with ToppGene (http://toppgene.cchmc.org/).
Microarray Data Analysis
Microarray data were screened for mappable non-Y chromosome probesets, with raw expression levels of 125 or greater, yielding 12,322 probesets. These were then screened for fold change (FC) >2 compared with total adult renal cortex, giving 248, 1082, and 415 probesets, respectively, for renin cells from adult, P0, and captopril-treated adults. The pooled list, because of overlap, gave 1228 probesets, which were filtered with Welch ANOVA (P < 0.05), giving 1051 probesets, of which 226 were expressed in adult renin cells. This set of probesets was further screened, requiring at least twofold enrichment compared with a virtual renal cortex made from the individual compartment expression data, yielding 92 adult renin cell-enriched probesets.
Single Cell Amplification Procedure (SCAMP)
We optimized a PCR target amplification protocol37 previously used by Cepko and colleagues.38 We added a random primer to the initial reverse transcription (RT) step to give improved full-length representation of transcripts and modified the reverse transcription reaction by adding T4 gene 32 protein to increase the yield of RT-PCR products.39,40 An exonuclease treatment step after the RT reaction was added to remove excess primers, which can contribute to nonspecific products. Finally, we optimized the fragmentation and labeling procedure to produce target properly sized for Affymetrix oligonucleotide array hybridization. See Supplemental Methods for the detailed protocol and validation of SCAMP.
RT-PCR
RNA extraction, reverse transcription, and PCR were performed as described previously.41
Immunostaining
Mice were anesthetized with tribromoethanol. The kidneys were removed, weighed, and either frozen for in situ hybridization, placed in RNAlater for RNA extraction, or fixed in Bouin's fixative. Immunostaining was performed on 5-μm thick paraffin sections using the following primary antibodies and the appropriate Vectastain ABC kit (Vector Laboratories, Burlingame, CA). The primary antibodies used were rabbit anti-mouse renin polyclonal, 1:500, α-SMA, 1:10,000 (A2547; Sigma, St. Louis, MO), Akr1b7, 1:200 (sc-27763; Santa Cruz, Santa Cruz, CA), NFATc4, 1:500 (62613; Abcam, Inc., Cambridge, MA), MEF2C, 1:500 (79436; Abcam, Inc.), Crip1, 1:200 (sc-131473; Santa Cruz), Notch3, 1:100 (1308-NT; R&D Systems, Inc., Minneapolis, MN), S1pR, 1:100 (LS-c11168 Lifespan Biosciences, Seattle, WA) and Nkx3.1, 1:1000 (gift of Dr. Chuck Bieberich, University of Maryland, Baltimore, MD).
Chromatin Immunoprecipitation (ChIP)
ChIP was performed as described previously4 using chromatin from adult kidney cortex and skeletal muscle for the phopsho-Creb ChIP, and from arterial smooth muscle cells of the renin lineage4 in the RBP-J studies. The antibodies used were Phospho-Creb (Ser 133, 9198S; Cell Signaling Technology, Inc., Danvers, MA) and RBP-J (AB5790; Millipore, Billerica, MA).
In situ Hybridization
In situ hybridization in kidneys from newborn mice was performed as described previously.42
Public Data Availability
All data are available on the public GUDMAP website (GUDMAP.org), which includes links to GEO, where the array data are also available.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The support of the GUDMAP program is greatly appreciated. SP and BA are supported by 3UO1DK70251. RAG is supported by R37HL066242 and RO1HL096735. MLSSL is supported by KO8DK75481. JY is supported by 1R01DK085080. We thank Niloofar Latifi, Yan Hu, and Cristina Monteagudo for immunostainings; Ruth Castellanos-Rivera for Notch3 immunostaining and the RBP-J PCR in YFP+ cells; William H. Wilson IV for initial bioinformatics discussions; Magali Cordaillat for the ChIP data; and Yanru Dou (School of Life Science, Peking University, Beijing, China) for the in situ hybridizations. We are grateful to Ken Gross (Roswell Park Cancer Institute) for helpful discussions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Keeton TK, Campbell WB: The pharmacologic alteration of renin release. Pharmacol Rev 32: 81–227, 1980 [PubMed] [Google Scholar]
- 2. Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ: Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA: Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Pentz ES, Sequeira Lopez ML, Cordaillat M, Gomez RA: Identity of the renin cell is mediated by cAMP and chromatin remodeling: An in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol 294: H699–H707, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS: Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15: 781–791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM: Renin and kallikrein in connecting tubule of mouse. Kidney Int 64: 2155–2162, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG: Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O: Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH: Pericyte-specific expression of Rgs5: Implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gaengel K, Genove G, Armulik A, Betsholtz C: Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630–638, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C: Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latonen L, Jarvinen PM, Laiho M: Cytoskeleton-interacting LIM-domain protein CRP1 suppresses cell proliferation and protects from stress-induced cell death. Exp Cell Res 314: 738–747, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Pomies P, Louis HA, Beckerle MC: CRP1, a LIM domain protein implicated in muscle differentiation, interacts with alpha-actinin. J Cell Biol 139: 157–168, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA: Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Li S, Wang DZ, Wang Z, Richardson JA, Olson EN: The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 100: 9366–9370, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones ME, Kondo M, Zhuang Y: A tamoxifen inducible knock-in allele for investigation of E2A function. BMC Dev Biol 9: 51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aronheim A, Shiran R, Rosen A, Walker MD: The E2A gene product contains two separable and functionally distinct transcription activation domains. Proc Natl Acad Sci U S A 90: 8063–8067, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graef IA, Chen F, Chen L, Kuo A, Crabtree GR: Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kong J, Qiao G, Zhang Z, Liu SQ, Li YC: Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int 74: 1577–1581, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Desch M, Schreiber A, Schweda F, Madsen K, Friis UG, Weatherford ET, Sigmund CD, Sequeira Lopez ML, Gomez RA, Todorov VT: Increased renin production in mice with deletion of peroxisome proliferator-activated receptor-gamma in juxtaglomerular cells. Hypertension 55: 660–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA: The transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 2011. July 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez ML, Gomez RA: The renin phenotype: roles and regulation in the kidney. Curr Opin Nephrol Hypertens 19: 366–371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komukai K, Mochizuki S, Yoshimura M: Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 24: 687–698, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA: Estrogen receptors: How do they signal and what are their targets. Physiol Rev 87: 905–931, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA: Ren1d and Ren2 cooperate to preserve homeostasis: Evidence from mice expressing GFP in place of Ren1d. Physiol Genomics 6: 45–55, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC: Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol 265: F151–F156, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R: Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 105: 1094–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Barski OA, Tipparaju SM, Bhatnagar A: The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev 40: 553–624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambert-Langlais S, Pointud JC, Lefrancois-Martinez AM, Volat F, Manin M, Coudore F, Val P, Sahut-Barnola I, Ragazzon B, Louiset E, Delarue C, Lefebvre H, Urade Y, Martinez A: Aldo keto reductase 1B7 and prostaglandin F2alpha are regulators of adrenal endocrine functions. PLoS ONE 4: e7309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomez RA, Norwood VF: Recent advances in renal development. Curr Opin Pediatr 11: 135–140, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Reddi V, Zaglul A, Pentz ES, Gomez RA: Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Peti-Peterdi J: Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol 288: F1079–F1083, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Peti-Peterdi J, Toma I, Sipos A, Vargas SL: Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 24: 88–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takenaka T, Suzuki H, Okada H, Hayashi K, Kanno Y, Saruta T: Mechanosensitive cation channels mediate afferent arteriolar myogenic constriction in the isolated rat kidney. J Physiol 511(Pt 1):245–253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurtz L, Schweda F, de WC, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C: Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA: Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 43: 1021–1028, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brady G, Iscove NN: Construction of cDNA libraries from single cells. Methods Enzymol 225: 611–623, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Cherry TJ, Trimarchi JM, Stadler MB, Cepko CL: Development and diversification of retinal amacrine interneurons at single cell resolution. Proc Natl Acad Sci U S A 106: 9495–9500, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villalva C, Touriol C, Seurat P, Trempat P, Delsol G, Brousset P: Increased yield of PCR products by addition of T4 gene 32 protein to the SMART PCR cDNA synthesis system. Biotechniques 31:81–83, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Jefferies D, Farquharson C: Effects of choice of reverse-transcriptase enzyme and use of T4 gene 32 protein on banding patterns in agarose gel differential display. Anal Biochem 308: 192–194, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML: CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296: H1255–H1262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP: A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.