Abstract
Leukocyte recruitment contributes to acute kidney injury (AKI), but the mechanisms by which leukocytes promote injury are not completely understood. The degranulation of mast cells releases inflammatory molecules, including TNF, but whether these cells participate in the pathogenesis of AKI is unknown. Here, we induced AKI with cisplatin in mast cell-deficient and wild-type mice. Compared with wild-type mice, deficiency of mast cells attenuated renal injury, reduced serum levels of TNF, and reduced recruitment of leukocytes to the inflamed kidney. Mast cell-deficient mice also exhibited significantly lower intrarenal expression of leukocyte chemoattractants. Mast cell-deficient mice reconstituted with mast cells from wild-type mice exhibited similar cisplastin-induced renal damage and serum levels of TNF as wild-type mice. In contrast, mast cell-deficient mice reconstituted with mast cells from TNF-deficient mice continued to demonstrate significant attenuation of cisplatin-induced renal injury. Furthermore, the mast-cell stabilizer sodium chromoglycate also significantly abrogated renal injury in this model of AKI. Taken together, these results suggest that mast cells mediate AKI through the production of TNF.
Cisplatin is a highly successful and widely used chemotherapeutic agent for the treatment of solid organ malignancies. However, cisplatin nephrotoxicity remains a major limitation to treatment and is reported to occur in approximately 30% of patients undergoing cisplatin chemotherapy.1 Although early attention focused on the direct tubular toxicity of cisplatin,2 experimental kidney injury is both associated with and mediated by significant renal inflammation.3 Administration of cisplatin reliably results in significant renal injury in an effect mediated by production of inflammatory cytokines, direct tubular injury, and vascular injury leading to renal ischemia.2–5 Inflammatory cytokines are pathogenic in cisplatin nephrotoxicity,5 and TNF in particular influences the severity of renal inflammation and injury.4 Leukocyte recruitment to the inflamed kidney plays an important role in disease progression. T lymphocyte recruitment to the kidney is known to peak early in the disease process, and CD4-deficient mice are protected from severe injury.6 Similarly, neutrophil recruitment correlates with disease severity in this model, although a pathogenic role for these cells has not been clearly established.7
Mast cells are innate immune cells traditionally believed to be involved in IgE-mediated hypersensitivity, asthma, and host defense against parasites.8 However, our understanding of mast-cell biology has increased considerably. These multifunctional pluripotent cells migrate through vascularized tissues, completing their maturation in the end organs. Mast cells are frequently located in vascular beds and epithelial surfaces where they play key roles as sentinels and first responders in host defense.9 Mast cells contain a variety of mediators that are released upon degranulation. These mediators include; cytokines, chemokines, growth factors, leukotrienes, and proteases. Among leukocytes, mast cells are unique in that they have preformed TNF which can be released immediately after degranulation.10,11 Because of their location, extensive synthetic capacities and unique surface receptor profiles, mast cells have a (potentially) diverse repertoire of phenotypes and functions. These functions include control of innate immune responses where they activate complement12 and Toll-like receptors13 and confer survival benefits.14,15 In addition, mast-cell degranulation can activate or regulate the adaptive immune system.16 In the kidney, mast cells have been shown to have both inflammatory17,18 and protective functions.19–21 Recent advances examining the role of mast cells in vivo have been facilitated by mice with genetic deletions specific for mast cells.
Signaling through the c-kit receptor is essential for mast cell differentiation. Mice with mutations in c-kit expression or signaling are deficient in mast cells. The most frequently used mouse to date is the Kit w/W-v mouse. Although these mice are profoundly mast cell deficient, they exhibit phenotypic abnormalities not related to mast cell deficiency including, sterility, anemia, and decreased numbers of lymphocytes.22 More recently, KitW-sh/W-sh mice have been used. In these mice, the mutation affecting c-kit signaling has effects more restricted to mast cell deficiency only. Although these mice are equally mast cell deficient, phenotypically they resemble C57BL/6 wild-type mice in that KitW-sh/W-sh mice are fertile, have intact immune responses, and are not anemic. KitW-sh/W-sh mice have been successfully used in many experimental models including experimental crescentic glomerulonephritis.18 Changes in outcome in disease models induced in KitW-sh/W-sh mice compared with WT (mast cell intact) mice suggest that the observed changes can be attributed to deficiency in mast cells. Strong proof for this assertion is provided when mast-cell reconstitution of KitW-sh/W-sh mice is undertaken, which restores the disease outcome to that observed in WT mice. Furthermore intravenous mast-cell reconstitution of KitW-sh/W-sh mice with bone marrow -derived mast cells from C57BL/6 wild-type (WT) mice results in normal numbers and function of mast cells in end organs.18,23
Mast cells have been implicated in a wide variety of renal diseases, by observing the infiltration of mast cells in disease where their accumulation correlates with disease severity and loss of renal function. These diseases include diabetic nephropathy, chronic inflammation, and fibrosis (reviewed in references 24–26). Although mast cells are required for regulatory T cell function and tolerance,27 it is as inflammatory mediators that they are likely to exert their effect in acute kidney injury. Mast cells are major producers of TNF15 and are also involved in the production of cytokines and chemokines essential to leukocyte recruitment and adhesion.28
In this manuscript, we have demonstrated a pivotal role for mast cells in acute kidney injury induced by cisplatin. First we found that after treatment with cisplatin, control WT mice developed severe renal injury with elevated serum blood urea nitrogen (BUN) marked tubulointerstitial injury, an influx of injurious leukocytes, and detectable levels of serum TNF. Mast cell-deficient KitW-sh/W-sh mice were protected from all of these outcomes. However, mast-cell reconstitution of KitW-sh/W-sh mice with bone marrow (BM)-derived mast cells from WT mice treated with cisplatin restored the full extent of functional and histologic renal injury and leukocyte influx. Sodium chromoglycate is a popular treatment that attenuates asthma by stabilizing mast-cell degranulation. Treatment of WT mice with sodium chromoglycate before the administration of cisplatin resulted in protection from renal injury. Finally, to assess the mechanisms of mast cell-mediated injury, we compared cisplatin-induced renal injury in KitW-sh/W-sh mice, KitW-sh/W-sh mice reconstituted with WT mast cells, and KitW-sh/W-sh mice reconstituted with mast cells from TNF−/− mice. Compared with the injury observed in KitW-sh/W-sh reconstituted with WT mast cells, renal injury and leukocyte recruitment were significantly decreased in KitW-sh/W-sh mice reconstituted with TNF−/− mast cells. These results demonstrate that in response to cisplatin treatment, mast-cell TNF mediates renal injury.
RESULTS
Mast Cell-deficient, KitW-sh/W-sh Mice Are Protected from Cisplatin Nephrotoxicity
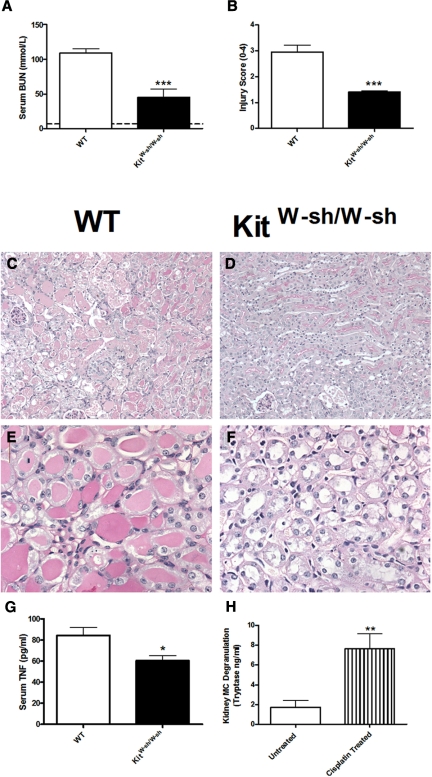
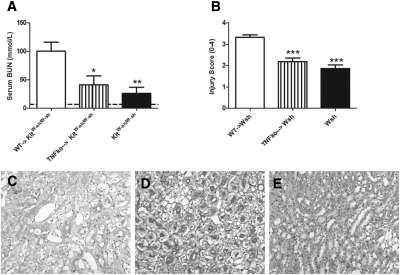
To determine the role of mast cells in cisplatin induced acute kidney injury, we administered cisplatin (12 mg/kg) to WT and KitW-sh/W-sh mice. Experiments ended 96 hours later. Compared with the renal injury observed in WT mice, injury was significantly attenuated in KitW-sh/W-sh mice. Functional injury, assessed by serum BUN (Figure 1A), was decreased in KitW-sh/W-sh mice compared with WT mice. Similarly histologic renal injury, assessed using an injury score, was decreased in KitW-sh/W-sh mice (Figure 1, B through F). No histologic injury was seen in untreated WT mice. Serum TNF levels correlate with disease severity in this model.4 Serum TNF levels were measured in WT and KitW-sh/W-sh mice 96 hours after cisplatin treatment and were decreased in KitW-sh/W-sh mice (Figure 1G). Serum TNF is not detected in normal mouse serum. To assess the effects of cisplatin on mast cell in the kidney, we measured kidney mast-cell degranulation (tryptase) in WT mice after the administration of cisplatin. Kidney mast-cell degranulation was below the assay's detection limit in four of six (untreated) control WT mice; tryptase was readily detected and significantly increased in six WT mice 4 hours after cisplatin treatment (Figure 1H). Serum mast-cell degranulation was also significantly increased 4 hours after cisplatin treatment (control 33.4 ± 2.7 versus cisplatin treated 53.2 ± 7.1 ng/ml, P < 0.05).
Figure 1.
Renal injury is attenuated in mast cell deficient mice after administration of cisplatin. We administered cisplatin (12 mg/kg) to C57BL/6 WT (n = 8) and mast cell-deficient KitW-sh/W-sh mice (n = 7). Renal injury and serum TNF levels were assessed 96 hours later. (A) Functional renal injury assessed by BUN was significantly decreased in KitW-sh/W-sh mice. (B) Similarly histologic renal injury was decreased in KitW-sh/W-sh mice. (C and D) Representative low-power images demonstrate increased interstitial injury in WT (C) compared with KitW-sh/W-sh (D) mice. (E and F) Similarly high-power representative photomicrographs demonstrate enhanced injury in WT (E) mice compared with KitW-sh/W-sh mice (F). (G) Serum TNF levels were decreased in KitW-sh/W-sh mice compared with WT mice treated with cisplatin. (H) Kidney mast-cell degranulation (tryptase) was increased in WT mice 4 hours after cisplatin treatment compared with untreated WT controls (n = 6 for both groups). Mean BUN values for untreated WT mice are represented by the dotted line. *P < 0.05, **P < 0.01, ***P < 0.001.
Leukocyte Recruitment Is Decreased in KitW-sh/W-sh Mice
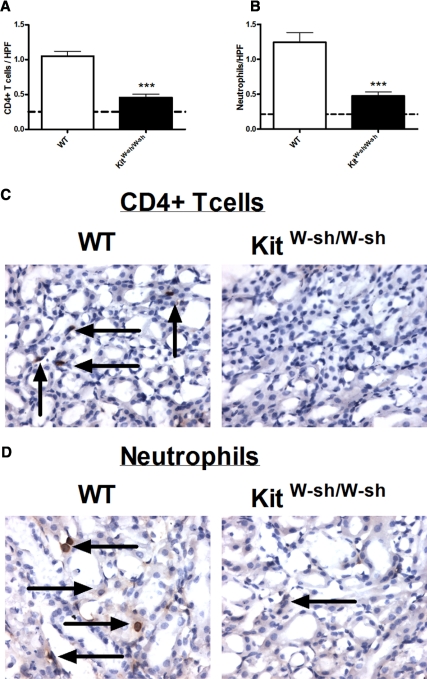
We assessed leukocyte recruitment in WT and KitW-sh/W-sh mice 96 hours after the administration of cisplatin. Numbers of CD4+ T cells (Figure 2A) and neutrophils (Figure 2B) seen per high-power field (HPF) were decreased in KitW-sh/W-sh mice compared with WT mice treated with cisplatin. High-power magnifications demonstrating CD4+ T cells (Figure 2C) and neutrophils (Figure 2D) in WT and KitW-sh/W-sh mice are shown.
Figure 2.
Leukocyte recruitment is decreased in KitW-sh/W-sh mice 96 hours after the administration of cisplatin. (A and B) Four days after the administration of cisplatin, decreased CD4+ T cells (A) and neutrophils (B) were seen per HPF in KitW-sh/W-sh mice (n = 7) compared with WT mice (n = 8). The dotted lines represent the number of leukocytes seen per HPF in untreated WT mice. (C and D) Representative photomicrographs of CD4+ T cells (C) in WT KitW-sh/W-sh mice and neutrophils (D) in WT and KitW-sh/W-sh mice are shown. The arrows represent positive leukocyte staining. ***P < 0.001.
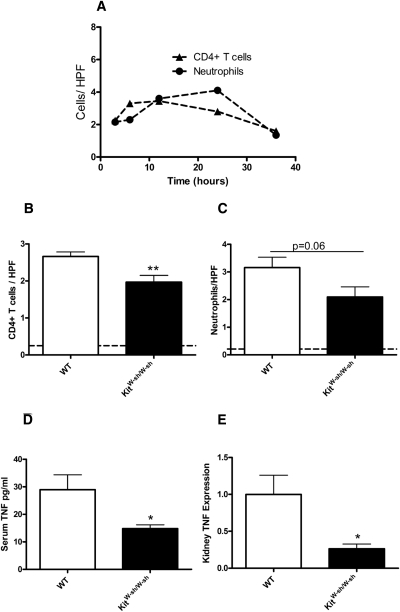
Leukocyte recruitment occurs early in cisplatin nephrotoxicity.6 We assessed CD4+ T cell and neutrophil recruitment in WT mice 3, 6, 12, 24, and 36 hours after administration of cisplatin. CD4+ T cells and neutrophil recruitment peaked within the first 24 hours (Figure 3A). Subsequently we treated WT and KitW-sh/W-sh mice with cisplatin and assessed leukocyte recruitment 24 hours after cisplatin treatment. Compared with WT mice, the number of CD4+ T cells observed per HPF was significantly decreased in KitW-sh/W-sh mice (Figure 3B). There was a trend to decreased interstitial neutrophil recruitment in KitW-sh/W-sh mice compared with WT mice (Figure 3C). Serum TNF levels and kidney TNF expression were significantly decreased in KitW-sh/W-sh compared with WT mice 24 hours after cisplatin treatment (Figure 3, D and E).
Figure 3.
Leukocyte recruitment, serum TNF production, and kidney TNF expression are decreased in KitW-sh/W-sh mice 24 hours after the administration of cisplatin. Leukocyte recruitment is known to peak early in the disease process of cisplatin nephrotoxicity. (A) We assessed CD4+ T cell and neutrophil recruitment 3, 6, 12, 24, and 36 hours after cisplatin administration to determine maximum recruitment (n = 3 for each group). (B) Recruitment of CD4+ T cells was decreased in KitW-sh/W-sh mice (n = 8) compared with WT mice (n = 8), 24 hours after cisplatin administration. (C) There was a trend to decrease in neutrophil recruitment in KitW-sh/W-sh mice. (D and E) Serum TNF levels (D) and kidney TNF expression (E) were decreased in KitW-sh/W-sh mice compared with WT mice 24 hours after cisplatin administration. *P < 0.05, **P < 0.01.
To determine the involvement of chemokines in kidney leukocyte recruitment, we measured kidney mRNA expression of pivotal T cell and neutrophil chemo-attractants in WT and KitW-sh/W-sh mice 24 hours after cisplatin treatment. Expression of the key T cell chemokines, RANTES/CXCL5, MCP1/CCL2, and IP10/CXCL10 was decreased in KitW-sh/W-sh mice compared with WT controls. Similarly, expression of KC/CXCL1 and MIP-2/CXCL2, the key murine neutrophil chemo-attractants, was decreased in KitW-sh/W-sh mice, as was kidney expression of adhesion molecules, intracellular adhesion molecule (ICAM-1) and vascular adhesion molecule (VCAM-1). These results are shown in Table 1.
Table 1.
Kidney mRNA expression of key leukocyte chemokines and adhesion molecules in wild-type and mast cell-deficient mice 24 h after treatment
| Wild-Type Mice | KitW-sh/W-sh Mice | Significance | |
|---|---|---|---|
| IP10/CXCL10 | 1.0 ± 0.2 | 0.1 ± 0.0 | P < 0.001 |
| RANTES/CXCL5 | 1.0 ± 0.1 | 0.4 ± 0.1 | P < 0.01 |
| MCP1/CCL2 | 1.0 ± 0.1 | 0.2 ± 0.0 | P < 0.001 |
| KC/CXCL1 | 1.0 ± 0.4 | 0.1 ± 0.0 | P < 0.05 |
| MIP2/CXCL2 | 1.0 ± 0.2 | 0.4 ± 0.2 | P < 0.05 |
| ICAM-1 | 1.0 ± 0.2 | 0.2 ± 0.1 | P < 0.001 |
| VCAM-1 | 1.0 ± 0.3 | 0.3 ± 0.1 | P = 0.06 |
Twenty-four hours after administration of cisplatin expression of key T cell chemokines (CXCL10, CXCL5, and CCL2) and neutrophil chemo-attractants (CXCL1 and CXCL2) were decreased in KitW-sh/W-sh mice (n = 8) compared with WT mice (n = 8). Expression of adhesion molecules demonstrated a decrease in ICAM-1 with a trend to decrease in VCAM-1 expression in KitW-sh/W-sh mice.
Mast Cell TNF Mediates Renal Injury in Cisplatin Nephrotoxicity
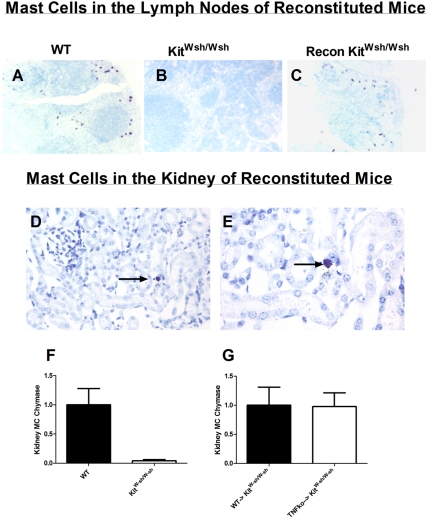
To confirm that the renal protection seen in KitW-sh/W-sh mice was a direct result of mast cell deficiency, we reconstituted KitW-sh/W-sh mice with BM-derived mast cells. To determine whether mast-cell TNF mediates renal injury in cisplatin nephrotoxicity, we reconstituted KitW-sh/W-sh mice with mast cells from TNF−/− mice. Mast cells were injected into KitW-sh/W-sh mice, and 12 weeks later, mast-cell reconstitution was assessed. We assessed reconstitution by staining for mast cells in lymph nodes and kidney and assessing kidney mast-cell chymase expression by mRNA. Although mast cells are readily identified in lymph nodes of WT mice stained with toludine blue (Figure 4A), they are not seen in KitW-sh/W-sh mice (Figure 4B). Mast cells were easily identified in lymph nodes of KitW-sh/W-sh mice reconstituted with mast cells (Figure 4C). Mast cells are seen infrequently in the kidneys of WT mice; we were able to identify mast cells in the kidney of reconstituted KitW-sh/W-sh mice (Figure 4, D and E). Mast-cell chymase is readily expressed in WT mice, whereas it is barely detectable in KitW-sh/W-sh mice (Figure 4F). We found that mast-cell chymase was readily detected and expressed at equivalent levels in KitW-sh/W-sh mice reconstituted with mast cells from WT or TNF−/− mice (Figure 4G). These data confirmed previous findings that mast-cell reconstitution of KitW-sh/W-sh mice (with WT or TNF−/− mast cells) results in a full restoration of organ-specific mast cells in recipient mice.18,23
Figure 4.
Mast cell activity is readily identified in lymph nodes and kidneys of WT mice and mast cell reconstituted KitW-sh/W-sh mice. (A and B) After toludine blue staining, mast cells (stained purple) were easily identified in lymph nodes from WT mice (A), but were absent in untreated KitW-sh/W-sh mice (B). (C) Similar to lymph nodes from WT mice, mast cells were easily identified in lymph nodes from mast cell reconstituted KitW-sh/W-sh mice. Kidneys from mast cell reconstituted KitW-sh/W-sh mice stained with toludine blue demonstrated occasional mast cells in glomeruli and renal interstitium. (D and E) An interstitial mast cell is shown at intermediate (200×) and high-power (400×) magnifications. The arrows represent positive mast cell staining. (F) Although kidney mRNA mast-cell chymase expression was easily detectable in WT mice, detection of mast-cell chymase expression in kidneys from KitW-sh/W-sh mice was barely detectable. (G) No difference in mast-cell chymase expression was seen in KitW-sh/W-sh mice reconstituted with mast cells from WT or TNF−/− mice.
Experiments began in three groups of experimental mice after mast-cell reconstitution and maturation were complete. These groups included: (1) KitW-sh/W-sh mice reconstituted with mast cells from WT mice (WT → KitW-sh/W-sh), (2) KitW-sh/W-sh mice reconstituted with BM-derived mast cells from TNF−/− mice (TNFko → KitW-sh/W-sh), and (3) nonreconstituted KitW-sh/W-sh mice, which were a negative control. All of the experimental groups received cisplatin (12 mg/kg), and experiments ended 96 hours later.
Compared with KitW-sh/W-sh, reconstitution of the mast cell repertoire in KitW-sh/W-sh mice was associated with increased cisplatin -induced renal inflammation and injury, confirming that mast cells mediate injury. Compared with renal injury seen in KitW-sh/W-sh mice reconstituted with mast cells from WT mice (WT → KitW-sh/W-sh), functional (Figure 5A), and histologic injury (Figure 5B) was decreased in TNF-deficient mast cell reconstituted KitW-sh/W-sh mice (TNFko → KitW-sh/W-sh) and nonreconstituted KitW-sh/W-sh mice. Low-power representative photomicrographs of renal injury are demonstrated (Figure 5, C–E). These results demonstrated that mast cells mediate renal injury in cisplatin nephrotoxicity and furthermore that TNF produced by mast cells is required for full expression of renal injury.
Figure 5.
Mast cells are directly pathogenic in cisplatin nephrotoxicity. To demonstrate direct pathogenicity of mast cells and mast cell-produced TNF in acute kidney injury, we reconstituted KitW-sh/W-sh mice with BM-derived mast cells. Three experimental groups were studied. The groups were: (1) WT BM mast cells injected into KitW-sh/W-sh mice (WT → KitW-sh/W-sh) (n = 8); (2) bone marrow mast cells from TNF−/− mice injected into KitW-sh/W-sh mice (TNFko → KitW-sh/W-sh) (n = 10); and (3) unreconstituted KitW-sh/W-sh mice, which were used as a negative control (n = 10). After reconstitution, the mice were administered cisplatin, and the experiments ended 4 days later. (A and B) Compared with renal injury seen in WT → KitW-sh/W-sh mice, functional (A) and histologic (B) parameters of disease were significantly decreased in TNFko → KitW-sh/W-sh and unreconstituted KitW-sh/W-sh mice. (C through E) Representative low-power magnification of tubulointerstitial injury in WT → KitW-sh/W-sh mice (C), TNFko → KitW-sh/W-sh (D), and unreconstituted KitW-sh/W-sh mice (E) are shown. Mean BUN values for untreated WT mice are represented by the dotted line. *P < 0.05, **P < 0.01, ***P < 0.001.
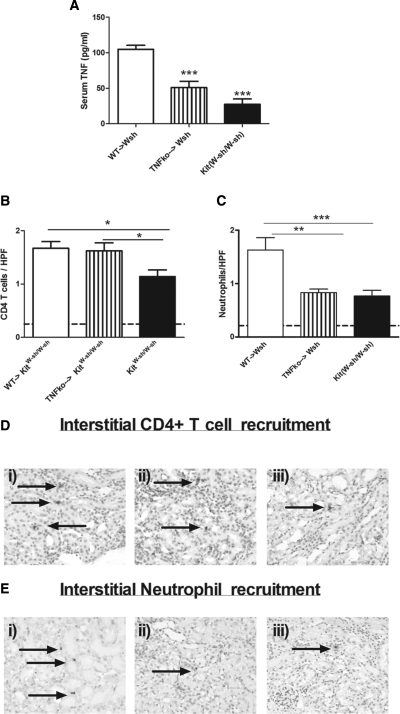
We assessed serum TNF levels in the three experimental groups. Compared with TNF levels seen in WT → KitW-sh/W-sh, levels were significantly decreased in the other experimental groups (Figure 6A). This confirmed that mast cells are major producers of serum TNF in cisplatin nephrotoxicity. Although CD4+ T infiltrates were similar in mice reconstituted with TNF-intact or TNF-deficient mast cells (Figure 6B), mast-cell TNF does play a significant role in neutrophil recruitment (Figure 6C). Representative photomicrographs of interstitial CD4+ T cell (Figure 6D) and neutrophil (Figure 6E) recruitment are shown.
Figure 6.
Mast cells recruit neutrophils to the kidney through the production of TNF. (A) Four days after cisplatin treatment, serum TNF production was decreased in TNFko → KitW-sh/W-sh (n = 10) and unreconstituted KitW-sh/W-sh mice (n = 10) compared with WT → KitW-sh/W-sh mice (n = 8). (B and C) Although CD4+ T cell recruitment was only decreased in unreconstituted KitW-sh/W-sh mice (B), neutrophil recruitment was decreased in TNFko → KitW-sh/W-sh and unreconstituted KitW-sh/W-sh mice (C). (D) Representative photomicrographs of CD4+ T cell recruitment in WT → KitW-sh/W-sh mice (panel i), TNFko → KitW-sh/W-sh (panel ii), and KitW-sh/W-sh mice (panel iii) are shown. (E) Representative photomicrographs of neutrophil recruitment in WT → KitW-sh/W-sh mice (panel i), TNFko → KitW-sh/W-sh (panel ii), and KitW-sh/W-sh mice (panel iii) are shown. The arrows represent positive leukocyte staining. *P < 0.05, **P < 0.01, ***P < 0.001.
Sodium Chromoglycate Decreases Cisplatin Induced Acute Kidney Injury
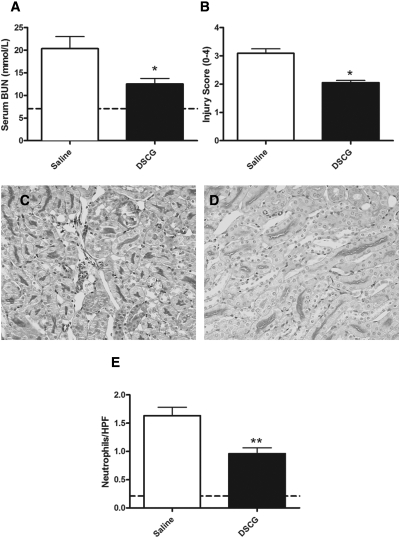
Sodium chromoglycate is used in clinical practice as a mast-cell stabilizer. To assess whether sodium chromoglycate has therapeutic potential in preventing cisplatin-induced acute kidney injury, we treated WT mice with sodium chromoglycate (10 mg/kg in 300 μl of sterile saline) or control (300 μl of sterile saline) daily for 2 days before administration of cisplatin. This treatment continued daily until the end of experiments. Compared with WT mice treated with control, treatment with sodium chromoglycate resulted in a significant decrease in both functional (Figure 7A) and histologic (Figure 7B) renal injury. Representative high-power photomicrographs are shown (Figure 7, C and D). Neutrophil recruitment was decreased in mice treated with sodium chromoglycate, compared with control mice (Figure 7E). These results confirmed the therapeutic potential of sodium chromoglycate in the prevention of acute kidney injury caused by cisplatin.
Figure 7.
Pre-emptive administration of sodium chromoglycate attenuates renal injury in cisplatin nephrotoxicity. To assess the therapeutic potential of mast-cell stabilization in cisplatin nephrotoxicity, we administered (di)sodium chromoglycate (DSCG) (n = 10) or control (sterile saline) (n = 9) to WT mice for 2 days before cisplatin administration and then daily until experiments ended. The experiments ended after 96 hours. (A and B) Functional (A) and histologic (B) renal injury was significantly decreased in WT mice treated with sodium chromoglycate. (C and D) Representative high-power images demonstrated increased renal injury in control-treated mice (C) compared with sodium chromoglycate-treated mice (D). (E) Neutrophil recruitment was significantly decreased in mice treated with sodium chromoglycate. The mean values for untreated WT mice are represented by the dotted line. *P < 0.05; **P < 0.01.
DISCUSSION
Mast cells have been implicated in many forms of kidney disease, including primary and secondary glomerulonephritis and tubulointerstitial injury.24 Renal mast-cell accumulation has been semiquantitatively correlated with fibrosis, decreased glomerular filtration rates, and poorer renal outcomes.29–31 Interestingly, most of these studies concentrated on chronic forms of kidney injury, where renal injury was a consequence of mast-cell degranulation and the release of profibrotic mediators, including TGF-β32 and MMP-9.33 However, mast-cell degranulation results in the release of mediators, which can influence acute inflammation, and surprisingly the role of mast cells in acute renal failure is not well studied. In these experiments, we define a pathogenic role for mast cells in acute kidney injury induced by cisplatin. Mast cell-deficient KitW-sh/W-sh mice were protected from the full extent of cisplatin-induced injury seen in WT control mice. Restoration of the full extent of injury by reconstitution of KitW-sh/W-sh mice with mast cells confirmed that the protection observed in KitW-sh/W-sh mice could be attributed specifically to mast cells. Renal injury was dependent on mast-cell TNF, and mast cell-deficient mice reconstituted with TNF−/− mast cells were protected from injury. Lower serum TNF levels consistently correlated with decreased neutrophil recruitment to interstitium after cisplatin treatment. We suggest that mast-cell TNF links leukocyte recruitment to the inflamed kidney with subsequent renal injury.
TNF is stored in a preformed state in mast cells and is released upon degranulation.10,11 We measured kidney and serum tryptase levels, and tryptase is a major constituent of mast cells and is stored almost exclusively in mast cells.34 In the kidney, mast cells are seen infrequently both before and after cisplatin treatment. However, we found a statistically significant increase in kidney mast-cell degranulation and TNF expression in mice treated with cisplatin. In addition, we found an increase in serum tryptase and TNF production after cisplatin treatment. Systemic mast-cell degranulation could result in the activation of secondary lymphoid tissues, with an up-regulation of inflammatory molecules that subsequently localize to the kidney. Therefore we believe that both systemic and local (kidney) mast-cell degranulation with TNF production contributes to the renal injury seen in this model.
Several studies have explored the role of TNF in cisplatin nephrotoxicity. After cisplatin treatment, levels of TNF in serum, urine, and kidney mRNA expression increase. Injury is attenuated in TNF−/− mice,4,35,36 and pharmacologic inhibitors of TNF attenuate renal injury in cisplatin nephrotoxicity.37 There is a strong association between mast cell accumulation and TNF production in response to infection, injury, and allergy.14–15,38,39 However, mast cells have numerous effects on surrounding cells, and potentially increased TNF production could occur indirectly, after stimulation of neighboring cells. We found that both serum TNF levels and kidney TNF expression was decreased in KitW-sh/W-sh mice after cisplatin treatment, and renal injury was attenuated. To demonstrate that mast cells were the major source of the pathogenic TNF, we reconstituted KitW-sh/W-sh mice with WT and TNF−/− mast cells before inducing injury. Four days later when experiments ended, serum levels of TNF were significantly decreased in KitW-sh/W-sh mice that had been reconstituted with TNF−/− mast cells, demonstrating that mast-cell TNF was a major contributor to serum levels of TNF. Consistent with decreased serum TNF levels, renal injury was significantly attenuated after reconstitution with TNF−/− mast cells. Compared with KitW-sh/W-sh mice reconstituted with WT mast cells, reconstitution with TNF−/− mast cells resulted in decreased neutrophil recruitment and significant protection from histologic and functional renal injury.
It is well described that in response to bacterial infections, mast cells increase leukocyte recruitment to areas of inflammation, where they are involved in host defense.14,15,40–42 We found that T cell and neutrophil recruitment was significantly decreased in KitW-sh/W-sh mice compared with WT mice 96 hours after cisplatin treatment. Similarly after 24 hours, T cell recruitment was decreased, with a trend to decreased interstitial neutrophil recruitment. Consistent with decreased leukocyte recruitment, production of several key T cell and neutrophil chemo-attractants were significantly decreased in KitW-sh/W-sh mice.
It is likely that neutrophil recruitment is largely driven by TNF produced by mast cells. In experimental allergic encephalomyelitis, TNF produced by mast cells is required for the recruitment of T cells and neutrophils across the meningeal barrier.43 In addition to T cell recruitment, it has been suggested that mast cell-produced TNF is a requirement for T cell activation.44 Similarly in a model of bacterial peritonitis, mast-cell TNF mediates peritoneal neutrophil influx,45 whereas in experimental arthritis mast cell-related cytokines recruit neutrophils to the inflamed joint.46 We found that neutrophil but not T cell recruitment was significantly attenuated in TNF−/− reconstituted mice. We hypothesize that TNF produced by mast cells is responsible for neutrophil recruitment, which then facilitate the kidney injury seen after administration of cisplatin. A pathophysiological role for T cells in cisplatin nephrotoxicity is well established.6 This study shows that whereas CD4 recruitment is mast cell dependent, CD4+ cell recruitment is not dependent on mast-cell TNF production. Mast cells have the capacity to produce a range of chemokines and cytokines capable of recruitment of CD4+ cells, independently of TNF.47 Mast-cell TNF may attenuate the capacity of CD4+ cells to contribute to renal injury because mast-cell TNF has been shown to be a requirement for CD4+ cell activation.44
The mast-cell stabilizer (di)sodium chromoglycate has been successfully used in clinical practice for over four decades.48 The drug has proven efficacy in limiting allergic symptoms with an excellent side-effect profile.49 Sodium chromoglycate prevents increases in membrane permeability to Ca2+ preventing mast-cell degranulation.50 More recently, it has been shown that in experimental endotoxaemic sepsis, sodium chromoglycate significantly decreased serum TNF levels and survival in WT mice and not in mast cell-deficient mice.51 We found that administering sodium chromoglycate before cisplatin and for the duration of the experiment resulted in a significant decrease in functional and histologic renal injury, with decreased neutrophil recruitment. Potentially, sodium chromoglycate could be used in the prevention of cisplatin-induced acute kidney injury. By extension of these observations, it is likely that this proposed prophylactic and/or therapeutic use of chromoglycate could be useful in other important forms of acute renal injury associated with demonstrated mast cell-associated inflammation.
In these studies, we have demonstrated a pathogenic role for mast cells, through the production of TNF, and subsequent leukocyte recruitment in acute kidney injury induced by cisplatin. After reconstituting mast cell-deficient mice, we identified mast cells as a major source of injurious TNF. Mast-cell stabilization offered a potential therapeutic option because it significantly decreased renal injury.
CONCISE METHODS
Experimental Design and Statistical Analyses
Eight- to ten-week-old male WT (C57BL/6), TNF−/−, and KitW-sh/W-sh mice were used for all experiments. WT mice were from Monash University Animal Services (Melbourne, Australia), and KitW-sh/W-sh mice were from Jackson Laboratories (Bar Harbor, ME) and were bred at Monash University. TNF-deficient mice were as described previously.52 Studies adhered to the National Health and Medical Research Council of Australia guidelines for animal experimentation. 12 mg/kg cisplatin (Sigma-Aldrich) was injected intraperitoneally. Sodium chromoglycate (Sigma-Aldrich) at a dose of 10 mg/kg was dissolved in sterile saline and injected at day −2 and then daily for the duration of experiments. Control mice were injected with equivalent volumes of sterile saline. Experiments ended at either 24 or 96 hours. The data are expressed as the means ± SEM. Groups of data were analyzed using t test for analysis of two groups. and one-way ANOVA (Tukey's post test) for more than two groups of data using GraphPad Prism (GraphPad Software, San Diego, CA). A P value of <0.05 was considered significant.
Renal Injury
Kidney sections were fixed in buffered formalin for 24 hours, processed, and embedded in paraffin wax. Tubular injury was assessed on periodic acid-Schiff-stained sections, and slides were coded so that the person scoring the slides was unaware of from which group the sections originated. Scoring was performed using minor modifications from previously described protocols.6 The tubular injury score, determined by assessing tubular epithelial cell loss, tubular necrosis, accumulation of cellular debris, and tubular cast formation, was scored according to the percentage of affected tubules under high-power microscopy. The percentage of tubules affected was assigned a score: 0 = normal, 1 = <10 to 25%, 2 = 26 to 50%, 3 = 51 to 75%, and 4 = >75%. BUN was measured from sera collected at the end of experiments and assessed using an auto-analyzer; BUN is recorded in mmol/L.
Interstitial CD4+ and Neutrophil Staining
Kidney sections were fixed in periodate lysine paraformaldehyde for 4 hours, then washed with 20% sucrose solution, and frozen in liquid nitrogen. Tissue sections were cut, and a three-layered immunoperoxidase technique as described previously was used to stain for T cells and neutrophils.53 The primary antibodies used were GK1.5 (anti-mouse CD4; American Type Culture Collection, Manassas, VA) and RB6-8C5 (anti-Gr-1; DNAX, Palo Alto, CA) for neutrophils. The secondary antibody used was rabbit anti-rat biotin (BD Bioscience, North Ryde, Australia). A minimum of 20 consecutively interstitial sections were assessed per animal, and the results are expressed as cells per HPF.
Intrarenal Cytokine mRNA Expression
For measurement of CCL5/RANTES, MCP1/CCL2, IP10/CXCL10, KC/CXCL1, MIP2/CXCL2, TNF, ICAM-1, and VCAM-1, RNA was extracted from whole kidney and measured by reverse transcription-PCR, and 500 ng of RNA was treated with 1 unit of amplification grade DNase I (Invitrogen, Melbourne, Australia), primed with random primers (Applied Biosystems, Foster City, CA), and reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems,). Gene-specific oligonucleotide primers designed using the Primer 3 software (Whitehead Institute for Biomedical Research, Cambridge, MA) were synthesized by Invitrogen as described previously.54 A Rotor Gene RG-3000 (Corbett Research, Mortlake, Australia) using Power SYBR Green PCR master mix (Applied Biosystems) was used to perform reverse transcription-PCR. PCR products were confirmed using melt-curve analysis, whereas mRNA expression was quantified using serial dilutions of an exogenous standard. Primer sequences used were as described previously,54 whereas expression was standardized to 18 S (housekeeping gene) before being expressed as a fold increase (or decrease) relative to WT mice treated with cisplatin.
Mast-Cell Reconstitution
Bone marrow was harvested from 6- to 8-week-old mice. The cells were filtered and spun, and the supernatant discarded and after red blood cells lysis were cultured in RPMI (Sigma-Aldrich) using 15% fetal calf serum, 1% penicillin/streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 μM 2-Mercaptoethanol. IL-3 (from WEHI3 cell culture supernatants) and 12.5 ng/ml recombinant mouse stem cell factor (R & D Systems, Minneapolis, MN) were added. Culture medium was changed every 3 days for 6 to 8 weeks. Mast cell purity (>98%) was assessed using a cyto-spinner after toludine blue staining. 5 × 106 cells were injected intravenously into mice, and 12 weeks was allowed for full reconstitution to develop.23 Reconstitution was confirmed by staining for mast cells with toludine blue in peripheral tissues and kidneys, 12 weeks after reconstitution.
Measurement of Serum TNF, Serum, and Kidney Mast-Cell Degranulation
Measurement of serum TNF was by ELISA as described previously, with a detection ranging from 7.8 to 4,000 pg/ml, with antibodies from R&D Systems.55 Mast-cell degranulation was measured by ELISA using a mast-cell degranulation assay kit (Millipore catalogue number IMM001) according to the manufacturer's specifications. Mast-cell degranulation is measured as detectable tryptase, the range of the assay was 1.6 to 100ng/ml. WT mice were injected with cisplatin, and the experiments ended 4 hours later. Serum and kidneys were collected. Kidneys were placed in clear FCS (Sigma-Aldrich) and digested with collagenase and DNAse and incubated for 25 minutes at 37°C. Kidney digests were centrifuged at 13,000 rpm for 10 minutes. Serum and kidney supernatants were evaluated using the degranulation assay kit according to the manufacturer's specifications.
DISCLOSURES
None.
Acknowledgments
S.A.S. is supported by a Jacquot Research Establishment Award administered by the Royal Australasian College of Physicians. S.R.H. is supported by a National Medical Research Council of Australia project grant.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Beyer J, Rick O, Weinknecht S, Kingreen D, Lenz K, Siegert W: Nephrotoxicity after high-dose carboplatin, etoposide and ifosfamide in germ-cell tumors: Incidence and implications for hematologic recovery and clinical outcome. Bone Marrow Transplant 20: 813–819, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Ries F, Klastersky J: Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 8: 368–379, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramesh G, Reeves WB: Inflammatory cytokines in acute renal failure. Kidney Int Suppl S56–S61, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H: A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17: 765–774, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL: Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322: 8–15, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Marshall JS: Mast-cell responses to pathogens. Nat Rev Immunol 4: 787–799, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Kitamura Y: Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol 7: 59–76, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Beil WJ, Login GR, Galli SJ, Dvorak AM: Ultrastructural immunogold localization of tumor necrosis factor-alpha to the cytoplasmic granules of rat peritoneal mast cells with rapid microwave fixation. J Allergy Clin Immunol 94: 531–536, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Olszewski MB, Groot AJ, Dastych J, Knol EF: TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J Immunol 178: 5701–5709, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC: Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390: 172–175, 1997 [DOI] [PubMed] [Google Scholar]
- 13. McCurdy JD, Lin TJ, Marshall JS: Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol 70: 977–984, 2001 [PubMed] [Google Scholar]
- 14. Echtenacher B, Mannel DN, Hultner L: Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381: 75–77, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Malaviya R, Ikeda T, Ross E, Abraham SN: Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381: 77–80, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Bischoff SC: Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat Rev Immunol 7: 93–104, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR: A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol 17: 150–159, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Scandiuzzi L, Beghdadi W, Daugas E, Abrink M, Tiwari N, Brochetta C, Claver J, Arouche N, Zang X, Pretolani M, Monteiro RC, Pejler G, Blank U: Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol 185: 624–633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, Maurer M, Rosenkranz AR: Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol 35: 3074–3082, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kanamaru Y, Scandiuzzi L, Essig M, Brochetta C, Guérin-Marchand C, Tomino Y, Monteiro RC, Peuchmaur M, Blank U: Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J Immunol 176: 5607–5615, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM: IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 186: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai M, Tam SY, Wedemeyer J, Galli SJ: Mast cells derived from embryonic stem cells: A model system for studying the effects of genetic manipulations on mast cell development, phenotype, and function in vitro and in vivo. Int J Hematol 75: 345–349, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ: Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 19: 2254–2261, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Mack M, Rosenkranz AR: Basophils and mast cells in renal injury. Kidney Int 76: 1142–1147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y: Mast cells and inflammatory kidney disease. Immunol Rev 217: 79–95, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ: Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442: 997–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Meng H, Tonnesen MG, Marchese MJ, Clark RA, Bahou WF, Gruber BL: Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. J Cell Physiol 165: 40–53, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Otsubo S, Nitta K, Uchida K, Yumura W, Nihei H: Mast cells and tubulointerstitial fibrosis in patients with ANCA-associated glomerulonephritis. Clin Exp Nephrol 7: 41–47, 2003 [DOI] [PubMed] [Google Scholar]
- 30. El-Koraie AF, Baddour NM, Adam AG, El Kashef EH, El Nahas AM: Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int 60: 167–172, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Hiromura K, Kurosawa M, Yano S, Naruse T: Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kidney Dis 32: 593–599, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT: Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol 147: 564–573, 1995 [PMC free article] [PubMed] [Google Scholar]
- 33. Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA: Human mast cells release metalloproteinase-9 on contact with activated T cells: Juxtacrine regulation by TNF-alpha. J Immunol 167: 4008–4016, 2001 [DOI] [PubMed] [Google Scholar]
- 34. He S, Aslam A, Gaca MD, Gaça MD, He Y, Buckley MG, Hollenberg MD, Walls AF: Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: Effects of tryptase and other agonists of proteinase-activated receptor 2 on histamine release. J Pharmacol Exp Ther 309: 119–126, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Zhang B, Ramesh G, Norbury CC, Reeves WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int 72: 37–44, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Ramesh G, Zhang B, Uematsu S, Akira S, Reeves WB: Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol 293: F325–F332, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Ramesh G, Reeves WB: Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int 65: 490–499, 2004 [DOI] [PubMed] [Google Scholar]
- 38. McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN: Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol 4: 1199–1205, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H: Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest 109: 1351–1359, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R: The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem 282: 20809–20815, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Malaviya R, Abraham SN: Mast cell modulation of immune responses to bacteria. Immunol Rev 179: 16–24, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Dawicki W, Marshall JS: New and emerging roles for mast cells in host defence. Curr Opin Immunol 19: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Sayed BA, Christy AL, Walker ME, Brown MA: Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol 184: 6891–6900, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ: Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176: 2238–2248, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Ramos BF, Jakschik BA: Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 258: 1957–1959, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Verri WA, Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, Oliveira FR, Teixeira MM, Silva JS, McInnes IB, Ferreira SH, Louzada-Junior P, Liew FY, Cunha FQ: IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis 69: 1697–1703, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Kalesnikoff J, Galli SJ: New developments in mast cell biology. Nat Immunol 9: 1215–1223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernstein IL, Siegel SC, Brandon ML, Brown EB, Evans RR, Feinberg AR, Friedlaender S, Krumholz RA, Hadley RA, Handelman NI, Thurston D, Yamate M: A controlled study of cromolyn sodium sponsored by the Drug Committee of the American Academy of Allergy. J Allergy Clin Immunol 50: 235–245, 1972 [DOI] [PubMed] [Google Scholar]
- 49. Storms WW: Pharmacologic approaches to daytime and nighttime symptoms of allergic rhinitis. J Allergy Clin Immunol 114: S146–S153, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Spataro AC, Bosmann HB: Mechanism of action of disodium cromoglycate: Mast cell calcium ion influx after a histamine-releasing stimulus. Biochem Pharmacol 25: 505–510, 1976 [DOI] [PubMed] [Google Scholar]
- 51. Ramos L, Pena G, Cai B, Deitch EA, Ulloa L: Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol 185: 709–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG: Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol 14: 1785–1793, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O'Sullivan KM, Tipping PG, Takeda K, Akira S, Holdsworth SR: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, Borza DB, Braley H, Holdsworth SR, Kitching AR: Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol 20: 2518–2524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG: Contributions of IL-1beta and IL-1alpha to crescentic glomerulonephritis in mice. J Am Soc Nephrol 15: 910–918, 2004 [DOI] [PubMed] [Google Scholar]