Abstract
Very young children with chronic kidney disease often have difficulty maintaining adequate nutrition, which contributes to the high prevalence of short stature in this population. Characteristics of the dialysis prescription and supplemental feeding via a nasogastric (NG) tube or gastrostomy may improve growth, but this is not well understood. Here, we analyzed data from 153 children in 18 countries who commenced chronic peritoneal dialysis at <24 months of age. From diagnosis to last observation, 57 patients were fed on demand, 54 by NG tube, and 10 by gastrostomy; 26 switched from NG to gastrostomy; and 6 returned from NG to demand feeding. North American and European centers accounted for nearly all feeding by gastrostomy. Standardized body mass index (BMI) uniformly decreased during periods of demand feeding and increased during NG and gastrostomy feeding. Changes in BMI demonstrated significant regional variation: 26% of North American children were obese and 50% of Turkish children were malnourished at last observation (P < 0.005). Body length decreased sharply during the first 6 to 12 months of life and then tended to stabilize. Time fed by gastrostomy significantly associated with higher lengths over time (P < 0.001), but adjustment for baseline length attenuated this effect. In addition, the use of biocompatible peritoneal dialysate and administration of growth hormone independently associated with improved length, even after adjusting for regional factors. In summary, growth and nutritional status vary regionally in very young children treated with chronic peritoneal dialysis. The use of gastrostomy feeding, biocompatible dialysis fluid, and growth hormone therapy associate with improved linear growth.
One-third of total postnatal statural growth occurs during the first 2 years of life. Hence, early growth is crucial for realization of the growth potential of children born with chronic kidney disease (CKD). Difficulties with spontaneous feeding, vomiting, and periods of inadequate intake because of infections and surgery, along with the very rapid growth at this age and therefore high calorie and protein requirements, can rapidly lead to loss of as much as 2 height SD scores (SDS).1–4 Some centers report good growth with the use of enteral feeding, but this is by no means universal.2,3,5–11
The International Pediatric Peritoneal Dialysis Network (IPPN) was established in 2007. This prospective registry collects comprehensive patient clinical and laboratory information from 69 centers in 25 countries around the world that care for children on chronic peritoneal dialysis (CPD). All children starting CPD in each center are registered with the IPPN, and data reporting continues as long as CPD is performed. Such a database offers the potential to analyze and correlate clinical practice and outcomes in many patients.
The objective of this study was to determine associations of feeding practices and other dialysis-related factors with length and weight gain in children who commenced CPD within the first 2 years of life.
RESULTS
Study Population
All children entering the registry at age <2 years between March 2007 and November 2009 were entered into the study. This comprised 153 children (61% boys) followed at 49 pediatric nephrology centers in 18 countries. Children were from Europe (n = 67), Latin America (n = 33), North America (n = 27), Turkey (n = 20), and Asian countries (n = 6).
Detailed prospective longitudinal information was available 6, 12, 18, and 24 months after study enrollment in 84, 57, 39, and 23 subjects, respectively. Forty-five (29%) children left the study early because they died (n = 8), were transplanted (n = 19), transferred to hemodialysis (n = 12), were lost to follow-up (n = 4), or their native kidney function recovered (n = 2). Additional retrospective information on length and/or weight was available for the time of birth in 138, at peritoneal dialysis (PD) initiation in 131, and at the start of enteral nutrition in all 87 children receiving enteral feeds.
Kidney disease had been diagnosed antenatally or within the first week of life in 56%, within the first month in 9%, within the first year in 29%, and within the second year in 6% of children. CKD was diagnosed later in the six Asian children (median 5.7 months) than in those from North America and Europe (median 0 months, P < 0.05), but age at PD initiation did not differ between regions. Diagnoses were renal hypo/dysplasia with or without obstruction or reflux (n = 84), congenital and infantile nephrotic syndrome (n = 29), polycystic kidney disease (n = 12), renovascular events including hemolytic uremic syndrome (n = 16), oxalosis (n = 7), and other/unknown (n = 5).
Fifty-five percent had one or more comorbidities, including defined syndromes (Denys–Drash, Potter, Senior–Loken, Caroli, Goldenhar, Alagille, Pierre–Robin, and chromosomal abnormalities) (23%); impaired cognitive development (24%); and abnormalities of eye (10%), hearing (5%), pulmonary tract (10%), and gastrointestinal tract (3%).
Further clinical and laboratory information at the time of study entry is given in Table 1.
Table 1.
Patient characteristics at study entry for total cohort and according to feeding method at baseline
| All Patients | Demand Feeding | NG Tube Feeding | Gastrostomy Feeding | |
|---|---|---|---|---|
| Number of patients | 153 | 66 | 54 | 33 |
| Age at diagnosis of renal disease (months) | 0.12 (0 to 2.7) | 0.93 (0 to 5.2)c | 0.0 (0 to 1.6)a | 0.1 (0 to 2)a |
| Age at start of EF (months) | 3.1 (0.1 to 5.8) | – | 1.73 (0.1 to 5.4) | 2.2 (0.2 to 7.96) |
| Age at start of PD (months) | 5.0 (1.4 to 0.7.3) | 7 (3.2 to 14.1)b | 3.4 (0.7 to 5.9)a | 6.3 (0.9 to 9.7)b |
| Age at study entry (months) | 10.8 (7.2 to 18) | 11.8 (7.4 to 18.6) | 9.0 (7.2 to 14.4) | 10.8 (7 to 15) |
| Length SDS at birth | −0.47 (−1.79 to 0.46) | −0.47 (−1.69 to 0.33) | −0.47 (−2.58 to 0.13) | −0.14 (−1.74 to 1.06) |
| Length SDS at start of EF | −1.69 (−2.96 to −0.28) | – | −1.77 (−3.16 to −0.25) | −1.43 (−2.58 to 0.05) |
| Length SDS at start of PD | −1.69 (−3.45 to −0.19) | −1.80 (−3.14 to −0.47 | −2.05 (−4.50 to −0.08 | −1.26 (−2.76 to 0.57) |
| Length SDS at study entry | −2.44 (−3.72 to −1.30) | −2.63 (−3.91 to −1.54)c | −2.72 (−4.12 to −1.69)c | −2.06 (−3.27 to −0.88)b |
| Length SDS at study entry excluding infants on rhGH | −2.55 (−3.77 to −1.26) | −2.63 (−3.91 to −1.54)c | −2.72 (−4.12 to −1.61)c | −1.91 (−2.83 to 0.24)b |
| BMI SDS at birth | −0.58 ± 1.6 | −0.17 ± 1.48 | −0.65 ± 1.55 | −0.68 ± 1.74 |
| BMI SDS at start of EF | – | −1.14 ± 1.56 | −1.1 ± 1.37 | |
| BMI SDS at PD start | −0.66 ± 1.8 | −0.58 ± 1.58 | −0.84 ± 2.10 | −0.62 ± 1.9 |
| BMI SDS at study entry | −0.40 ± 1.53 | −0.58 ± 1.69 | −0.47 ± 1.40 | 0.11 ± 1.36 |
| n (%) oligoanuric | 83 (54) | 43 (65)c | 30 (55) | 10 (30)a |
| n (%) with comorbidities | 84 (55) | 34 (52) | 31 (57) | 19 (57) |
| n (%) automated PD | 112 (76) | 49 (74)c | 41 (76)c | 22 (96)a,b |
| PD fluid turnover (L/m2 per day) | 5.3 (3.5 to 7.9) | 4.3 (2.8 to 6.8)c | 5.3 (3.6 to 8.3) | 6.6 (5.5 to 9.6)a |
| Dialytic glucose exposure (g/kg per day) | 4.2 (2.9 to 7.1) | 3.4 (2.3 to 5.3)b,c | 4.5 (3.2 to 7.7)a | 6.6 (4.0 to 8.1)a |
| n (%) using biocompatible PD fluid | 66 (43) | 25 (38) | 27 (50) | 14 (42) |
| n (%) using amino acid PD fluid | 8 (5.3) | 3 (4.6) | 5 (9.3) | 0 (0) |
| n (%) phosphate binders | 107 (69.9) | 54 (83.1) | 34 (62.9) | 19 (57.6) |
| n (%) NaCl supplements | 56 (36.8) | 19 (29.2)c | 22 (40.7) | 15 (45.5)a |
| n (%) bicarbonate supplements | 58 (38.2) | 29 (44.6)c | 22 (40.7) | 7 (21.2)a |
| n (%) growth hormone | 6 (4.0) | 0 (0)c | 2 (3.7) | 4 (12.1)a |
| Hemoglobin (g/dl) | 10.8 ± 1.8 | 10.8 ± 1.7 | 10.8 ± 1.7 | 10.8 ± 2.0 |
| Bicarbonate (mmol/L) | 24.3 ± 4.6 | 23.2 ± 4.4 | 24.9 ± 3.8 | 25.5 ± 5.8 |
| Albumin (g/L) | 34.0 ± 7.4 | 34.0 ± 7.2 | 34.3 ± 7.5 | 33.8 ± 7.5 |
| Urea (mg/dl) | 83 ± 45 | 92 ± 37 | 72 ± 37 | 83 ± 48 |
| Calcium (mmol/L) | 2.40 ± 0.23 | 2.40 ± 0.25 | 2.40 ± 0.23 | 2.42 ± 0.22 |
| Inorganic phosphorus (mmol/L) | 1.75 ± 0.51 | 1.82 ± 0.52 | 1.66 ± 0.46 | 1.77 ± 0.56 |
| PTH (pg/ml) | 222 (85 to 529) | 178 (74 to 510) | 247 (97 to 575) | 243 (110 to 371) |
| Total Kt/V urea | 3.06 (2.32 to 3.81) | 3.0 (2.17 to 3.57) | 3.22 (2.32 to 3.94) | 2.91 (2.3 to 3.6) |
Data are given as mean ± SD, median (interquartile range), or percent of patients. EF, enteral feeding.
Superscript letters indicate significant difference between feeding modality groups: adifferent from demand feeding; bdifferent from NG tube feeding; cdifferent from gastrostomy feeding.
Feeding Patterns
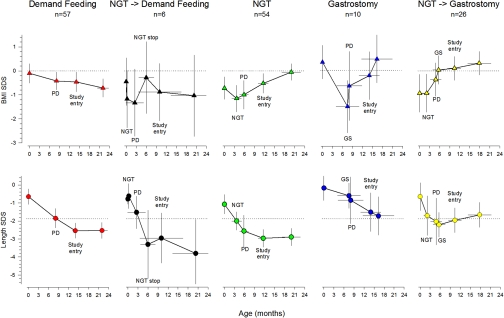
The overall time course of feeding regimens from birth to last observation is shown in Figure 1. Five feeding patterns were observed: 57 patients were fed on demand or were offered an oral dietary supplement from the time of diagnosis to the end of the observation period. Fifty-two patients received continuous nasogastric (NG) tube feeding from a median age of 2 months (interquartile range 0 to 5) to the end of the observation period. In six patients, NG tube feeding was performed for a median of 5.5 months and then discontinued. Another 26 children were switched to gastrostomy feeding after a median of 3.8 months of NG tube feeding. Primary gastrostomy feeding was instituted in ten patients at a median age of 8.2 (3.5 to 10.6) months, usually at the time of PD initiation.
Figure 1.
Whereas both nasogastric tube (NGT) and gastrostomy (GS) feeding improve nutritional status, only GS feeding associates with stabilized linear growth in young infants undergoing CPD. The data points represent mean estimates at key time points of postnatal development, (i.e., birth, commencement of CPD, initiation and discontinuation of nasogastric tube or gastrostomy feeding, enrollment to IPPN [study entry], and last available observation). Two-dimensional error bars denote the 95% confidence intervals to mean age and SDS at the respective time point.
Body mass index (BMI) SDS and length SDS were similar in the enterally fed children and those on demand feeds at birth and at initiation of PD, and they did not differ at initiation of enteral feeding between patients starting NG tube or gastrostomy feeds. A cross-sectional description of patient characteristics according to nutritional management at the time of study entry is given in Table 1. The distribution of BMI SDS and length SDS at each time point in the children with a constant feeding modality and at least 6 months of follow-up is shown in Supplemental Figure 1.
BMI SDS uniformly decreased over time during periods without enteral feeding, whereas NG tube and gastrostomy feeding reversed the loss of BMI SDS. The median (interquartile range) annualized change in standardized BMI was −0.54 (1.91) SDS/year during periods of demand feeding versus +0.97(3.43) SDS/year during NG tube feeding (P < 0.0005) and +1.24 (3.24) SDS/year during gastrostomy feeding (P < 0.05). At last observation the children with exclusive or predominant demand feeding (Figure 1, upper panels 1 and 2) were significantly lighter than the children who received enteral feeding (Figure 1, upper panels 3 to 5) (P = 0.001).
Length SDS was significantly decreased below 0 at birth, decreased further during the first 6 to 12 months of life, and then tended to stabilize. Although growth failure was equally severe in children fed by demand and in those receiving NG tube feeds (Figure 1, lower panels 1 to 3), growth appeared to be better preserved in infants started on or switched to gastrostomy feeding (Figure 1, lower panels 4 and 5). Considering feeding periods of at least 6-month duration, the median change in standardized length was −1.35 (2.63) SDS/year during periods of demand feeding, −0.72 (1.59) SDS/year during NG tube feeding, and −0.50 (2.47) SDS/year during periods of gastrostomy feeding (P < 0.05 for gastrostomy versus demand feeds). Length SDS at last observation was significantly greater in the patients with gastrostomy feeding as compared with those with demand and/or NG tube feeding (P < 0.05).
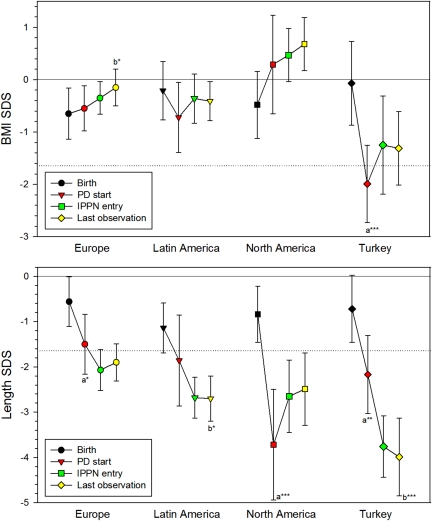
Regional Variation
Treatment practices, growth, and weight gain were also analyzed with respect to the region of residence (Figure 2, Table 2). At the time of study entry, North American children were more likely to be oligoanuric, using ambulatory PD with a higher dialytic glucose exposure, and receiving growth hormone than children in the other regions. Biocompatible PD fluids were applied more commonly in European children. Turkish children had significantly lower serum albumin and higher inorganic phosphorus levels and more often received amino-acid-containing PD fluid. Children from Latin America had the largest residual urine output, the highest rate of phosphate binder, and the lowest rate of oral sodium chloride (NaCl) supplement administration. NG tube feeding was used for some time in 74% of North American, 60% of European, 50% of Turkish, 45% of Latin American, and in one of the six Asian children. Gastrostomy tubes were used in 74% of North American, 21% of European, 6% of Latin American, and no Turkish and Asian children (P < 0.0001).
Figure 2.
Minor regional variation in linear growth despite major differences in nutritional control in infants undergoing CPD. Course of BMI SDS and length SDS by region in 67 European, 27 North American, 33 Latin American, and 20 Turkish children. Six children from different Asian countries are not represented. Data are mean ± 95% confidence intervals. aSignificant change from birth to PD start; bSignificant change from PD start to last observation. *P < 0.05; ***P < 0.001. Reference lines indicate the 50th (SDS = 0) and 5th (SDS = −1.645) percentiles.
Table 2.
Patient and treatment characteristics according to region
| Characteristics | Europe | North America | Latin America | Turkey |
|---|---|---|---|---|
| Number of patients | 67 | 27 | 33 | 20 |
| Age (months) | ||||
| at diagnosis | 0 (0 to 0.9) | 0 (0 to 0.3)c,d | 1.7 (0.2 to 6)b | 0.9 (0 to 4.1)b |
| at start of enteral feeding | 0.9 (0 to 4.7) | 0.8 (0.2 to 4.9) | 5.4 (3.2 to 9.8) | 5.4 (0.7 to 11.6) |
| at start of PD | 4.7 (0.7 to 9.7) | 5.3 (1.3 to 8.8) | 6.9 (2.9 to 12.1) | 3.3 (0.8 to 6.4) |
| Feeding modality, n (%) | ||||
| demand feeding | 22 (33)b | 3 (11)a,c,d | 17 (52)b | 10 (50)b |
| NGT → demand feeding | 1 (2)d | 1 (4) | 0 (0)d | 3 (15)a,c |
| NGT | 30 (45)b | 3 (11)a,c,d | 14 (42)b | 7 (35)b |
| gastrostomy | 5 (8) | 4 (15) | 1 (3) | 0 (0) |
| NGT → gastrostomy | 9 (13)b | 16 (59)a,c,d | 1 (3)b | 0 (0)b |
| n (%) with comorbidities | 27 (40.3)b | 19 (70.4)a | 17 (51.5) | 9 (45) |
| n (%) oligoanuric | 25 (43.1)b,c | 12 (75)a,c,d | 3 (9.4)a,b | 6 (30)b |
| n (%) automated PD | 52 (80)b | 27 (100)a,c,d | 29 (88)b,d | 13 (65)b,c |
| PD fluid turnover (L/m2 per day) | 5.3 (3.5 to 9.1)d | 6.9 (5.9 to 8.4)d | 5.0 (3.5 to 6.5) | 3.8 (2.4 to 5.0)a,b |
| Dialytic glucose exposure (g/kg per day) | 4.7 (2.8 to 7.1)b,d | 7.2 (4.1 to 9.9)a,c,d | 3.8 (2.9 to 5.1)b | 6.6 (4.0 to 8.1)a,b |
| n (%) using biocompatible PD fluids | 54 (80.6)b,c,d | 3 (11.1)a | 4 (12.1)a | 3 (15)a |
| n (%) using amino-acid PD fluid | 3 (4.5)d | 0 (0)d | 0 (0)d | 5 (25)a,b,c |
| n (%) phosphate binders | 45 (67.2)b,c | 11 (40.7)a,c,d | 31 (93.4)a,b | 15 (75)b |
| n (%) NaCl supplements | 37 (55.2)c,d | 10 (37)c | 3 (9.1)a,b | 5 (25)a |
| n (%) bicarbonate supplements | 17 (25.3)c | 5 (18.5)c | 25 (75.8)a,b,d | 8 (40)c |
| n (%) growth hormone | 2 (3)b | 4 (14.9)a,c | 0 (0)b | 0 (0) |
| Hemoglobin (g/dl) | 11.1 ± 1.7 | 10.6 ± 2.5 | 10.7 ± 1.7 | 10.1 ± 2.2 |
| Bicarbonate (mmol/L) | 25.3 ± 4.1d | 24.9 ± 6.6d | 23.3 ± 3.2 | 21.7 ± 4.1a,b |
| Albumin (g/L) | 33.7 ± 6.7d | 35.4 ± 6.8d | 37.1 ± 6.9d | 27.9 ± 7.4a,b,c |
| Urea (mg/dl) | 80 ± 44 | 100 ± 49 | 84 ± 41 | 71.2 ± 49 |
| Calcium (mmol/L) | 2.42 ± 0.23 | 2.44 ± 0.28 | 2.41 ± 0.19 | 2.30 ± 0.23 |
| Inorganic phosphorus (mmol/L) | 1.70 ± 0.36d | 1.68 ± 0.62d | 1.76 ± 0.41d | 2.05 ± 0.75a,b,c |
| PTH (pg/ml) | 140 (49 to 351) | 224 (110 to 477) | 317 (200 to 523) | 509 (97 to 1003) |
| Total Kt/V urea | 3.05 (2.62 to 3.66) | 2.36 (1.79 to 3.12) | 3.75 (2.89 to 4.14) | 3.76 (2.37 to 5.0) |
PD medication and biochemical data are given at time of entry to the IPPN study. The six infants from Asian countries are not represented. Data are given as mean ± SD, median (interquartile range), or percent of patients. NGT, nasogastric tube.
Superscript letters indicate significant difference between regions: adifferent from Europe; bdifferent from North America; cdifferent from Latin America; ddifferent from Turkey.
BMI SDS dropped from birth to PD initiation in Turkish children (P < 0.001), tended to decrease in Latin American children, and tended to increase in North American children, resulting in a significant difference among these regions at the start of PD (P < 0.05). Although these trends continued after PD initiation, European children significantly improved their BMI SDS. As a result, BMI SDS at last observation was higher in North American (P < 0.0001) and European (P < 0.005) children than in Turkish children, and BMI SDS was higher in North American than in Latin American children (P < 0.01). BMI at last observation was below the 5th percentile in 50% of the Turkish children, and above the 95th percentile in 26% of the North American infants (P < 0.005).
Whereas the postnatal loss of length SDS was more marked and the mean length SDS at PD initiation was lower in North American than in children from any other region (P < 0.05), length SDS improved significantly while on PD in North American as compared with children from the other regions (P < 0.005). It also developed better in European than in Turkish children (P < 0.05). Consequently, length SDS at IPPN entry and last observation were lowest in children from Turkey.
PD Prescription
Children treated with biocompatible PD solutions were significantly taller at study entry (length SDS −2.09 ± 1.69) than children using conventional PD fluids (−2.88 ± 1.86, P = 0.008). Among the children followed prospectively for at least 6 months, length SDS did not change in children on conventional solutions (−0.06 ± 1.96 SDS/year, NS), whereas significant catch-up growth was observed in those dialyzed with biocompatible PD fluid (+0.52 ± 1.82 SDS/year) (Supplemental Figure 2).
The children receiving amino-acid PD fluids at study entry were lighter (BMI SDS −1.61 ± 1.91 versus −0.29 ± 1.47, P = 0.006) but did not differ in length SDS, and they did not show any difference in BMI or length SDS change over time during follow-up. BMI SDS at study entry was positively correlated with dialytic glucose exposure (r = 0.31, P < 0.0001) and PD fluid turnover (r = 0.30, P < 0.0003) and inversely correlated with residual urine volume (r = −0.29, P < 0.01), whereas length SDS showed no associations with these variables.
Recombinant Growth Hormone Treatment
Length SDS was significantly greater at study entry in the six children who received recombinant human growth hormone (rhGH) at study entry. Eight children who received rhGH for at least 6 months in the prospective study showed a 1.50 ± 0.81 SDS increase in standardized length as compared with no net change (−0.05 ± 0.93 SDS) in the untreated children (P < 0.0001) (Supplemental Figure 3).
Multivariate Analysis of Factors Associated with Growth and Weight Gain
Generalized estimating equation modeling was used to explore the effect size and significance of various factors contributing to the distribution of length SDS and BMI SDS in the prospective study. When adjusting for age at PD start and age and BMI SDS at study entry, BMI increased by 0.5 SDS per year of exposure to enteral feeding. Apart from the effect of enteral feeding time, BMI SDS showed no significant trend over time. Residence in North America was associated with significantly higher BMI SDS, and residence in Turkey was associated with lower BMI SDS, independent of enteral feeding (Table 3).
Table 3.
Generalized estimating equation modeling of factors predicting BMI SDS during the observation period
| Outcome Variable | Standardized Parameter Estimate ± SEM | 95% Confidence Interval | P |
|---|---|---|---|
| BMI SDS | |||
| intercept | −0.07 ± 0.19 | −0.45 to 0.31 | 0.721 |
| observation time (years) | −0.06 ± 0.18 | −0.41 to −0.30 | 0.75 |
| age at PD start (years) | 0.23 ± 0.13 | −0.01 to 0.47 | 0.058 |
| age at study entry (years) | 0.05 ± 0.13 | −0.20 to 0.30 | 0.71 |
| BMI SDS at study entry | 0.70 ± 0.05 | 0.61 to 0.80 | <0.0001 |
| Region of residence (reference: Europe) | |||
| North America | 0.39 ± 0.18 | 0.05 to 0.74 | 0.026 |
| Latin America | −0.19 ± 0.13 | −0.44 to 0.07 | 0.153 |
| Turkey | −0.55 ± 0.20 | −0.95 to −0.16 | 0.006 |
| Asia | 0.21 ± 0.33 | −0.43 to 0.85 | 0.516 |
| Time on enteral feeding (years) | 0.50 ± 0.23 | 0.04 to 0.96 | 0.032 |
| Dialytic glucose exposure (g/kg per day) | −0.03 ± 0.02 | −0.08 to 0.02 | 0.182 |
Standardized parameter estimate indicates a unit greater BMI SDS at follow-up per unit of independent variable.
The length SDS, adjusted for age and length at study entry, age at PD start, and region of residence, did not change significantly with time, but it was significantly higher in patients receiving biocompatible PD fluid and in those receiving rhGH for at least 6 months (Table 4). The effect of enteral feeding time was dependent on the inclusion of baseline length SDS in the model. Without adjustment, length SDS increased by 0.59 ± 0.21 per year of gastrostomy feeding (P = 0.0007) whereas time on NG tube feeding had no significant effect. The effect of gastrostomy feeding time lost significance when correcting for length SDS at baseline (Table 4). Daily PD fluid turnover and the use amino-acid fluid showed no independent associations with length SDS.
Table 4.
Generalized estimating equation modeling of factors predicting length SDS during the observation period
| Outcome Variable | Standardized Parameter Estimate ± SEM | 95% Confidence Interval | P |
|---|---|---|---|
| Length SDS | |||
| intercept | −0.76 ± 0.23 | −1.98 to −0.31 | 0.0008 |
| age at PD start (years) | 0.16 ± 0.13 | −0.10 to 0.42 | 0.226 |
| age at study entry (years) | 0.05 ± 0.12 | −0.19 to 0.29 | 0.672 |
| length SDS at study entry | 0.88 ± 0.03 | 0.83 to 0.94 | <0.0001 |
| Region of residence (reference: Europe) | |||
| North America | 0.14 ± 0.20 | −0.25 to −0.54 | 0.477 |
| Latin America | 0.13 ± 0.16 | −0.17 to 0.44 | 0.393 |
| Turkey | −0.02 ± 0.17 | −0.34 to 0.31 | 0.928 |
| Asia | −0.22 ± 0.21 | −0.63 to 0.18 | 0.282 |
| Observation time (years) | −0.09 ± 0.12 | −0.33 to 0.15 | 0.463 |
| Time with NG tube (years) | 0.03 ± 0.18 | −0.32 to 0.38 | 0.862 |
| Time with gastrostomy (years) | 0.25 ± 0.22 | −0.21 to 0.73 | 0.211 |
| Growth hormone treatment | 0.57 ± 0.24 | 0.10 to 1.04 | 0.017 |
| Use of biocompatible PD fluid | 0.42 ± 0.12 | 0.19 to 0.65 | 0.0004 |
| Use of amino-acid PD fluid | −0.13 ± 0.17 | −0.36 to 0.11 | 0.296 |
| PD fluid turnover (L/m2 per day) | 0.01 ± 0.02 | −0.03 to 0.05 | 0.708 |
Standardized parameter estimate indicates a unit greater length SDS at follow-up per unit of independent variable (constant factors: presence = 1).
DISCUSSION
Although the importance of nutrition in infancy is undisputed, the role of enteral feeding and its modalities in improving growth has been a controversial topic for many years.5–11 Analysis of the worldwide IPPN database adds a novel perspective on the efficacy and global variation of current growth-promoting strategies in young infants and the relative roles of nutritional measures, dialysis prescription, and other factors. The combination of comprehensive prospective data collection with retrospective milestone analysis of length and weight at birth, PD initiation, and start and change of enteral feeding provided a complete picture of early infantile growth patterns.
We observed a high prevalence of early growth failure across different regions of the world. Notably, length was already slightly reduced at birth, compatible with a link between intrauterine growth conditions and renal development. Severe growth failure occurred before and early after initiation of dialysis, followed by stabilization of growth rates in the second year of life. In view of the excellent growth reported for this age group in single-center studies, the observed outcomes show a large potential for improvement at the global population level.
Although this observational study cannot prove causalities, it identified several factors and conditions consistently associated with early infantile growth patterns that deserve attention. Nutrition is generally considered to be the most important determinant of body growth in infancy. Calorie and protein requirements are proportionately greater at this age than at any other time of life. Significant protein losses occur in the peritoneal dialysate.4,12 Infants on CPD are often difficult to feed and are prone to gastroesophageal reflux and vomiting. This is attributed to uremic anorexia, raised intra-abdominal pressure due to PD,13 and the need to concentrate the feed in oliguric infants. Severe vomiting may in itself compromise growth because of the ensuing fluid and electrolyte imbalances.
Enteral feeding by NG or gastrostomy tube is regarded as a key measure to optimize nutrition and growth in young infants.14 An additional benefit of tube feeding beyond the provision of energy, protein, and fluid is the ability to use it to administer medications such as NaCl supplements, which are commonly required both in polyuric infants with structural renal abnormalities and in anuric infants with high ultrafiltration-related losses4,12; salt depletion is known to limit growth.10 Provision of NaCl by tube is much easier and causes less vomiting than administration by mouth.
The recent Kidney Disease Outcomes Quality Initiative (KDOQI) pediatric nutritional guidelines recommend early institution of enteral feeding.12 However, only 41% of the infants in this study were enterally fed even after the start of dialysis, reflecting marked global variation in feeding strategies and, possibly, a perceived lower need for intensified nutritional management in infants less affected by feeding problems.
Our analysis clearly demonstrates that NG tube and gastrostomy feeding are effective in improving the nutritional status, as indicated by BMI SDS, in young infants with advanced and end-stage CKD. However, the relationship between enteral feeding and growth seems to be more complex. The restitution of a normal BMI distribution by NG tube feeding did not prevent further growth failure, at least within the given time window of observation. Infants initiated on or switched to gastrostomy feeding showed somewhat better preservation of growth as compared with demand-fed children. The beneficial effect of gastrostomy feeding prevailed when correcting for confounding factors such as region of residence of the prospective study, but it lost significance when correcting for length SDS at first observation, probably because length had already normalized in some children who had started gastrostomy feeding before entering the IPPN registry. We can only speculate why gastrostomy use was associated with superior growth. The most likely explanation for the difference between NG and gastrostomy feeding is a reduction in vomiting: the presence of a NG tube may act as a stent passing through the gastroesophageal junction. This will usually improve with a gastrostomy.14 Also, a slightly higher fraction of gastrostomy patients received oral sodium supplements, although the difference was significant only when compared with children fed by demand.
The marked regional variation and overall low rate of gastrostomy use would suggest that the findings in this paper should prompt a reconsideration of enteral feeding policies for young children on CPD. We do not know why so few children had a gastrostomy; one reason may be a relative reluctance to perform an open gastrostomy procedure, which is recommended instead of percutaneous placement when patients are already established on PD.14,15
The lack of detailed data on the prescription and actual intake of nutrients is a limitation of our study. These types of data are difficult to collect and assess accurately. Reassuringly, a designated renal dietician was available in all but 2 of the 49 contributing centers. Also, the KDOQI pediatric nutrition guidelines12 were made available through the IPPN website and were consulted regularly by the centers. Nevertheless, we observed marked regional variation in nutritional outcomes, with a 0.6 SD negative and 0.4 SD positive mean BMI difference attributable to children living in Turkey and the United States as compared with Europe, respectively, independent of feeding modalities. These differences may be explained by substantial global variation regarding the contents and/or effectuation of dietary prescriptions. Of course, we cannot rule out other unidentified confounding factors contributing to the regional differences in BMI SDS.
It is remarkable that the regional differences in nutritional status appeared to align with linear growth patterns only in the “milestone” analysis from birth to study entry, but not in the multivariate analysis of the prospective study. Although worsening malnutrition coincided with growth failure in the Turkish children, infants in North America, while continuously gaining BMI SDS also grew significantly less well than European children while followed longitudinally. In Europe major malnutrition was prevented throughout the disease process, but growth retardation still developed, albeit at a milder degree than in the other regions. These findings are compatible with the view that growth in infancy is a multifactorial process, with adequate nutrition being just one of several prerequisites.
Unexpectedly, the use of biocompatible PD fluids low in toxic glucose degradation products was consistently associated with better body growth. The association prevailed even after correction for nutritional status and region and was quantitatively stronger than the effect of gastrostomy feeding. Although the causality postulate would require testing by controlled studies, one might speculate about a growth-preserving effect of attenuated local and systemic inflammatory processes and carbonyl stress accomplished with reduced GDP exposure.
In contrast, the use of amino-acid PD solutions was not associated with improved growth and was actually a negative predictor of BMI SDS by univariate analysis, most likely because of bias by indication related to the selective use of amino acid solutions in the most malnourished children.
Increasing evidence from uncontrolled and controlled trials supports the efficacy of rhGH in very young children with CKD.16–18 Although provided to only eight children for at least 6 months, rhGH therapy was independently associated with improved growth in this study. The severe overall growth retardation observed even in the European and North American centers and the fact that even gastrostomy feeding only mitigated but did not readily reverse growth failure should be reason to consider early institution of rhGH in young infants who are failing to grow adequately despite provision of an adequate dietary intake.
In conclusion, the IPPN database is unique in its ability to study and compare protocols throughout the world and, by virtue of the numbers of patients for study, to be able to ascertain the best practice. Because complete case reporting is achieved, information bias is kept to a minimum. Interrogation of the database has shown severe early growth failure in infants on CPD and an important association between early gastrostomy feeding and better preservation of growth rates at this crucial time. It has also generated interesting hypotheses regarding additional dialysis-related factors of influence to be tested in future clinical trials.
CONCISE METHODS
Data Collection
The following data were collected at entry to the registry: patient characteristics (age, sex, diagnosis and comorbidity, age at diagnosis of CKD, age at start of enteral feeding and CPD, and the presence of residual urine), feeding method (demand feeding, NG tube or gastrostomy), medications (use of erythropoietin, iron, sodium or bicarbonate supplements, vitamin D, phosphate binders, and/or rhGH), blood levels (hemoglobin, bicarbonate, calcium, phosphate, parathyroid hormone, and albumin), dialysis program (PD modality, dialysate turnover, average dialysate glucose concentration, type of fluid used, Kt/V urea, and creatinine clearance), and supine length weight and BMI (length was measured to the nearest 5 mm by infantometers in 12 centers and by tape in 28 centers). The above observations were collected prospectively at 6-month intervals. In addition, anthropometric data at birth, at the start of PD, and at the start and at any change of enteral feeding modalities were collected retrospectively. The prospective analysis was terminated on November 30, 2009 or alternatively if the patient died, was transplanted, or transferred to chronic hemodialysis.
Statistics
Length and BMI were normalized to SDS using the World Health Organization 2006 normative values for children up to age 5 years.19 Data were checked for normal distribution by the Kolmogorov–Smirnoff test. Data are expressed by mean ± SD for normally distributed and by median and interquartile range for non-normally distributed variables. Between-group differences in group means (log-transformed in case of non-Gaussian distribution) were assessed by t test for two-group comparisons and by ANOVA followed by Newman–Keuls testing for multiple comparisons. Differences in proportions were assessed using χ2 tests.
Generalized estimating equation analysis was performed on the prospective part of the study, which started with entry to the IPPN registry. Associations of time-invariant factors (region of residence, ≥6 months of growth hormone treatment, and use of biocompatible or amino-acid PD fluid) and time-variant covariates (cumulative observation time with NG tube or gastrostomy, PD fluid turnover, and dialytic glucose exposure) related to nutritional and dialytic management with the evolution of length and BMI SDS were explored using the “Repeated” statement of the SAS GENMOD procedure (time variable: observation time in prospective study). An autoregressive correlation matrix structure was assumed. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the International Society for Peritoneal Dialysis, Baxter Health Care, Fresenius Medical Care, Ipsen, Pfizer, IBM, and Sandoz. We also appreciate the continued dedicated support of IPPN by the medical and nursing staff in all collaborating centers.
The following principal investigators are contributing to the IPPN registry:
Argentina: E. Sojo, Hospital de Pediatria Garrahan, Buenos Aires; P.A Coccia, Hospital Italiano de Buenos Aires; A. Suarez, Hospital de Niños Sor. Maria Ludovica La Plata; P.G. Valles, Hospital Pediatrico Humberto Notti, Mendoza; R. Salim, Rennius S.A. Salta. Belgium: K. van Hoeck, University Hospital Antwerp, Edegem. Brazil: V. Koch, Instituto da Criança— Hospital das Clinicas FMUSP, Sao Paulo. Canada: J. Feber, Children's Hospital of Eastern Ontario, Ottawa; D.A. Geary, Hospital for Sick Children, Toronto; C. White, BC Children's Hospital, Vancouver. Chile: M. Valenzuela, Hospital Guillermo Grant Benavente, Concepcion; J. Villagra, Hospital Base, Osorno; F. Cano, Hospital Luis Calvo Mackenna, Santiago, M.A. Contreras, Roberto del Rio Hospital, Santiago; A. Vogel, Pontivicia Universidad Catolica de Chile, Santiago; P. Zambrano, Hospital Dr. Gonzales Cortes, Santiago, P. Berrocal Hospital Sotero del Rio, Santiago. China: M.C. Chiu, Department of Pediatric & Adolescent Medicine, Hong Kong, H. Xu, Children's Hospital of Fudan University, Shanghai. Czech Republic: K. Vondrak, University Hospital Motol, Prague. Finland: K. Rönnholm, Hospital for Children and Adolescents, Helsinki; France: B. Ranchin, Hôpital Femme Mère Enfant, Lyon; T. Ulinski, Armand Trousseau Hospital, Paris; M. Fischbach, Children's Dialysis Center, Strasbourg. Germany: R. Büscher, Children's Hospital Essen; M. Kemper, University Medical Center, Hamburg; L. Pape, Medical School, Hannover; F. Schaefer, D. Borzych, Center for Pediatrics and Adolescent Medicine, Heidelberg; J. Misselwitz, Kidney Center for Children and Adolescent; G. Klaus, University Hospital, Marburg; D. Haffner, University Children's Hospital, Rostock. Greece: F. Papachristou, Aristoteles University, Thessaloniki. India: A. Bagga, All India Institute of Medical Sciences, New Delhi; M. Kanitkar, Armed Forces Medical College, Pune. Italy: E. Verrina, G. Gasini Institute, Genova; A. Edefonti, Fondazione Ospedale Maggiore Policlinico, Milano; G. Leozappa, Dipartimento Nefrologia-Urologia, Rome. Israel: D. Landau, Soroka Medical Center, Beer-Sheva. Korea: I. S. Ha, Dialysis Center for Children and Adolescents, Seoul; K. H. Paik, Samsung Medical Center, Seoul. Macedonia: E. Sahpazova Pediatric Clinic, Skopje. The Netherlands: J.W. Groothoff, Academic Medical Center, Amsterdam. Nicaragua: Y. Silva, Hospital Infantil de Nicaragua, Managua. Poland: A.M. Zurowska, D. Borzych, Medical University, Gdansk; D. Drozdz, University Children's Hospital, Krakow; M. Lipka, Children's Memorial Health Institute, Warsaw, M. Sczepanska, Dialysis Division for Children, Zabrze. Romania: O. Brumariu, St. Maria Children's Hospital, Iasi. Singapore: H.K. Yap, Shaw-NKF-NUH Children's Kidney Center. Spain: G. Ariceta, Hospital de Cruces, Baracaldo. Turkey: A.S. Bakkaloglu, Hacettepe University, Ankara; S. Bakkaloglu, Gazi University, Ankara; I. Bilge, Department of Pediatric Nephrology, Çapa-Istanbul; E. Serdaroglu, Dr. Behcet Children Research and Educational Hospital, Izmir; A. Bal, Tepecik Children and Research Hospital, Izmir; S. Mir, Ege University Faculty of Medicine, Izmir-Bornova. United Kingdom: L. Rees, Great Ormond Street Hospital, London. A.R. Watson, Children & Young People's Kidney Unit, Notthingham. Uruguay: J. Grünberg, SE.N.NI.AD, Montevideo. United States: L. Greenbaum, Children's Healthcare Pediatric Dialysis Unit, Atlanta; A. Neu, Johns Hopkins Hospital, Baltimore; D. Askenazi, Children's Hospital of Alabama, Birmingham; D. Gipson, University of North Carolina, Chapel Hill; H. Patel, Children's Hospital, Columbus; S. Pottoore, Children's Medical Center, Dallas; V. Dharnidharka, Division of Pediatric Nephrology, Gainesville; T. Bunchman, Helen DeVos Children's Hospital; A. Chua, Texas Children's Hospital, Houston; B.A. Warady, Children's Mercy Hospital, Kansas City; J. Zaritsky, UCLA Medical Center, Los Angeles.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Karlberg J, Schaefer F, Hennicke M, Wingen AM, Rigden S, Mehls O: Early age-dependent growth impairment in chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10: 283–287, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Kari J, Gonzalez C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with chronic renal failure. Kidney Int 57: 1681–1687, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Mekahli D, Shaw V, Ledermann SE, Rees L: Long term outcome of infants with severe chronic kidney disease. Clin J Am Soc Nephrol 5: 10–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rees L, Shaw V: Nutrition in children with CRF and on dialysis. Pediatr Nephrol 22: 1689–1702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strife CF, Quinlan M, Mears K, Davey ML, Clardy C: Improved growth of three uremic children by nocturnal nasogastric feedings. Am J Dis Child 140: 438–443, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Guillot M, Broyer M, Cathelineau L, Boulegue D, Dartois AM, Folio D, Guimbaud P: Continuous enteral feeding in pediatric nephrology. Long-term results in children with congenital nephrotic syndrome, severe cystinosis and renal failure. Arch Fr Pediatr 37: 497–505, 1980 [PubMed] [Google Scholar]
- 7. Rees L, Rigden SPA, Ward GM: Chronic renal failure and growth. Arch Dis Child 64: 573–577, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coleman JE, Watson AR, Rance CH, Moore E: Gastrostomy buttons for nutritional support on chronic dialysis. Nephrol Dial Transplant 13: 2041–2046, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS: Long-term outcome of peritoneal dialysis in infants. J Pediatr 136: 24–29, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Parekh RS, Flynn JT, Smoyer WE, Milne JL, Kershaw DB, Bunchman TE, Sedman AB: Improved growth in young children with severe chronic renal insufficiency who use specified nutritional therapy. J Am Soc Nephrol 12: 2418–2426, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ramage IJ, Geary DF, Harvey E, Secker DJ, Balfe JA, Balfe JW: Efficacy of gastrostomy feeding in infants and older children receiving chronic peritoneal dialysis. Perit Dial Int 19: 231–236, 1999 [PubMed] [Google Scholar]
- 12. KDOQI Work Group: KDOQI Clinical Practice Guideline for Nutrition in Children with Chronic Kidney Disease: 2008 Update. Am J Kidney Dis 53: S11–104, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Fischbach M, Warady BA: Peritoneal dialysis prescription in children: Bedside principles for optimal practice. Pediatr Nephrol 24: 1633–1642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rees L, Brandt M: Tube feeding in children with CKD: Technical and practical issues. Pediatr Nephrol 25: 699–704, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Ledermann SE, Spitz L, Moloney J, Rees L, Trompeter RS: Gastrostomy feeding in infants and children on peritoneal dialysis. Pediatr Nephrol 17: 246–250, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fine RN, Attie KM, Kuntze J, Brown DF, Kohaut EC. for the Genentech Collaborative Study Group: Recombinant human growth hormone in infants and young children with chronic renal insufficiency. Pediatr Nephrol 9:452–457, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Maxwell H, Rees L. on behalf of the British Association for Paediatric Nephrology: Recombinant human growth hormone treatment in infants with chronic renal failure. Arch Dis Child 74: 40–44, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mencarelli F, Kiepe D, Leozappa G, Stringini G, Cappa M, Emma F: Growth hormone treatment started in the first year of life in infants with chronic renal failure. Pediatr Nephrol 24: 1039–1046, 2009 [DOI] [PubMed] [Google Scholar]
- 19. WHO Child Growth Standards: Methods and Development. WHO Multicentre Growth Reference Study Group Geneva, Switzerland: World Health Organization, 2006. Available at: http://www.who.int/childgrowth/standards/technical_report/en/index.html Accession date: February 1, 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.